Abstract
We retrospectively reviewed 107 patients with 108 malignant or locally aggressive bone tumours treated between 1978 and 2009 by extracorporeal irradiation with 300 Gy to eradicate the tumour, and reimplantation of the bone as an orthotopic autograft. Patient subgroups were defined according to resection type. We describe the local recurrence rate, the graft infection rate and the factors affecting graft healing and graft survival. No local recurrences were detected in the irradiated grafts. At five-year follow-up, graft healing had occurred in 64% of patients, providing a stable and lasting reconstruction. For various reasons, 11% of grafts were removed, although no single factor was predictive of failure. All patient subgroups had comparable results. Early infection predicted the development of pseudarthrosis. Pelvic reconstructions had a worse graft survival. Rigid fixation and bridging of the graft appeared to be important technical points.
Introduction
Primary bone tumours are an indication for (radical) surgical treatment, but limb salvage is currently the treatment of choice if it does not compromise wide en bloc resection [1]. Because most patients are young and active, a given treatment must not only preserve the affected limb, but also maintain function without major complications or re-operations over the long term.
Reconstruction of the resected bone is necessary, either with a bone graft or with a prosthetic implant. Prosthetic reconstruction allows rapid restoration of mobility and weightbearing, but complications have been described in 25–92% of cases [2, 3]. A 57% cumulative five-year survival for knee prostheses has been reported [4]. Stress shielding and prosthetic loosening remain major concerns and may require major revision surgery, resulting in further bone loss [5–7].
The use of a bone graft has the theoretical advantage of preserving bone stock, particularly in younger patients [8–10]. Furthermore, these grafts provide an anchor point for tendons and muscles [11]. Either an allograft or an autograft can be used. However, the use of allografts requires large bone banks and a sufficient number of quality bone donors [12]. In Asian countries, bone allografts are often avoided for religious reasons. In addition, allograft strength decreases with time after implantation [13], presumably due to an accumulation of unremodelled microfractures that decrease the torsional properties of bone, and a decreased bone mineral density within the allograft cortex [14]. An orthotopic bone autograft should overcome all the problems of availability, size and shape adaptation, risk of disease transmission associated with allografts, and loss of bone stock and ligamentous tissue when prostheses are used [15].
Extracorporeal irradiation aims at eradicating all tumour cells, as this is the primary goal of treatment, while preserving bone stock [16, 17]. In smaller, selected patient series, a low recurrence rate and a favourable graft survival have been reported, with an acceptable functional outcome in the short- to medium-term follow-up. Our findings confirm these results. We have overcome many pitfalls, including insufficient fixation and graft fracture, and the experience we acquired has led to many useful tips and tricks.
Patients and methods
We retrospectively reviewed 107 patients with 108 locally aggressive or malignant bone tumours treated with tumour resection, extracorporeal irradiation, and reimplantation of the orthotopic autograft between 1978 and 2009. If limb salvage was not possible, or if severe osteolysis of the bone was present, this procedure was contraindicated.
Our population included 63 males and 44 females, ranging in age from nine to 79 years (Table 1). Osteosarcoma (43%), chondrosarcoma (25%), and Ewing sarcoma (11%) were the most common tumours, consistent with known frequencies in the literature [18]. Two patients had a giant-cell tumour of bone. Thirty-one patients died of their disease during the study period, with a minimum follow-up of three months (median 25 months; range 3–292 months). Seventy-two patients survived, with a minimum follow-up of 31 months (median 124 months; range, 31–383 months). Five patients were lost to follow-up.
Table 1.
Tumour incidence according to age at diagnosis (decade)
| Decade | Number |
|---|---|
| 1 | 3 |
| 2 | 45 |
| 3 | 17 |
| 4 | 6 |
| 5 | 17 |
| 6 | 8 |
| 7 | 8 |
| 8 | 4 |
| Total | 108 |
Full oncological staging of each patient was performed before any surgical procedure, including biopsy. The timing of tumour resection was determined according to the multidisciplinary treatment protocol. For osteosarcoma and Ewing sarcoma, adjuvant and neoadjuvant chemotherapy was administered according to contemporary protocols.
All but one patient underwent treatment of a single bone. One patient with Ollier’s disease developed chondrosarcoma at two different enchondroma sites. The long bones of the limbs were most frequently affected (femur, 31%; humerus, 26%; tibia, 18%; Table 2), followed by the pelvic bone (16%). The length of the reimplanted graft varied between 2.7 and 35.0 cm, with a mean of 13.7 cm (median, 14.0 cm).
Table 2.
Distribution of reconstruction type according to bone localisation
| Anatomical site | Intercalary | Composite | Osteoarticular | Total |
|---|---|---|---|---|
| Femur | 8 | 15 | 8 | 31 |
| Humerus | 9 | 8 | 8 | 25 |
| Tibia | 11 | 3 | 5 | 19 |
| Pelvis | 8 | 7 | 8 | 23 |
| Radius | 0 | 0 | 3 | 3 |
| Ulna | 1 | 0 | 3 | 4 |
| Clavicle | 1 | 0 | 0 | 1 |
| Scapula | 0 | 0 | 1 | 1 |
| Fibula | 0 | 0 | 1 | 1 |
| Total | 38 | 33 | 37 | 108 |
The resection plane was established by analysing images obtained by radiography, arteriography, CT scanning, dynamic contrast MRI, and Tc total body scanning. Wide en bloc resection of the affected area was performed. The proximal and/or distal medullary content of the host bone were biopsied.
The resected specimen was wrapped in three sterile plastic bags and one sterile drape for transport to the linear accelerator at the Department of Subatomic and Radiation Physics. On arrival, the specimen was placed in a rice-filled container, selected because its homogeneous, specimen-surrounding, dose buildup properties are adaptable to all bone geometries. Next, extracorporeal irradiation of the resected specimen was performed using a refined technique in which a dose of 300 Gy was delivered with a flattened 10 MeV bremsstrahlung photon beam at a dose rate of 1 Gy/s, uniform within 10%, in a 25 × 25-cm2 field [19, 20]. The whole irradiation procedure took about half an hour, transport included (range 25–40 minutes). After irradiation, the extraosseous bulk of the lesion and the endomedullary content were removed before reimplantation, allowing histological and tumour response assessment.
Meanwhile, the resection site was prepared for fixation of the irradiated graft. The irradiated bone was reimplanted and rigidly fixed to the remaining bone. Our technique of fixation has evolved over time and depends on whether the grafts are intercalary, osteoarticular, or pelvic. A pelvic graft was considered to be both intercalary and osteoarticular when the acetabulum was involved.
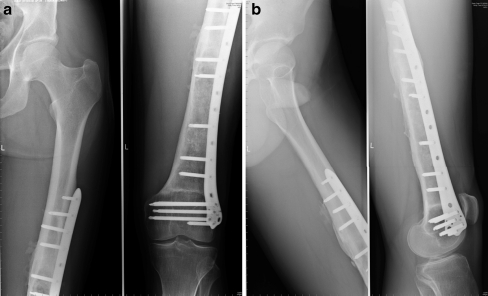
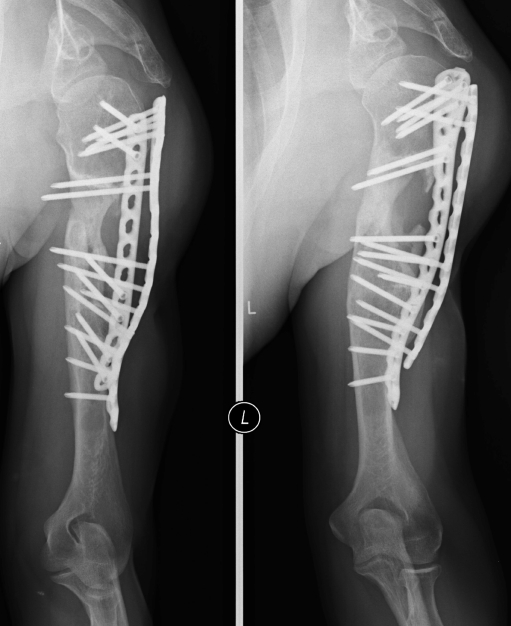
In the intercalary procedure, only the diaphyseal part of a long bone was treated, and the adjacent joints were spared. We fixed intercalary grafts with single plating for the lower limb (Fig. 1a, b) and double plating for the upper limb (Fig. 2). We prefer locking plates and recommend that both bridge the graft. In tibial reconstructions, fixation of the proximal and the distal tibiofibular joints was added to classic plating. In the postoperative period, weightbearing or limb loading was individually determined according to radiographic healing.
Fig. 1.
Intercalary reconstruction of the femoral diaphysis with a bridging locking plate. a Anterior view. b Side view
Fig. 2.
Anterior view of intercalary reconstruction of the humerus with double plate fixation
A true osteoarticular procedure implies reimplantation of the affected bone, including the articular surface. However, we observed disintegration of articular cartilage and resorption of the subchondral bone with this type of reconstruction. Therefore, after resections of the shoulder, hip and knee, we used a hybrid reconstruction that combined a tumour joint arthroplasty with an intercalary diaphyseal irradiated autograft, fixed by the prosthetic stem. Loading or weightbearing was allowed after healing of the soft tissues, usually at six weeks. At the wrist and elbow, the use of osteoarticular irradiated autografts remains a valuable option that obviates the need for prosthetic implants. We rigidly fixed the wrist grafts by a bridging plate. In our experience, Kirschner fixation was inadequate, causing displacement of the hand with subsequent functional loss. In patients with unicondylar involvement of the femur or tibia, osteoarticular irradiated autografts are a valuable alternative. After irradiation, all ligaments must be reinserted to minimise knee instability. We restricted weightbearing for three to six months.
For pelvic tumours with hip involvement, the hip was resected en bloc with the affected part of the pelvis, and only the pelvic part was reinserted. The hip was reconstructed with a hip prosthesis. Postoperatively, we instructed the patients to wear an abduction brace for three months. We advised nonweightbearing for six months, followed by progressive weightbearing until nine months postoperatively.
By resection type, 38 intercalary, 37 true osteoarticular and 33 composite reconstructions were performed. During the initial reconstruction, 12 hip prostheses, 13 rotating hinge knee prostheses, and eight shoulder prostheses (inverted Delta shoulder prosthesis) were implanted.
Local recurrence was assessed by clinical examination, ultrasonography, bone scintigraphy and local MRI. These investigations were performed every three months during the first three years, every six months during the subsequent two years, and then yearly for five years. Graft infection was diagnosed according to clinical signs and positive inflammatory parameters on laboratory tests. Data related to factors that determine graft healing, graft survival, and infection were retrieved retrospectively from medical files and radiographic material.
All subsequent radiographs were reviewed, and graft healing and osteosynthesis integrity were assessed according to the Musculoskeletal Tumor Society graft evaluation system [21]. The most recent radiographs of individual patients were used for assessing current graft status.
Kaplan-Meier survival analysis was performed to determine graft survival, with graft resection as an endpoint. A Cox regression model was used to determine the role of chemotherapy and infection in predicting graft healing. Data were analysed using SPSS statistical software (version 17.0; SPSS Inc, Chicago, IL).
Results
One patient had a local recurrence in surrounding soft tissue, most likely resulting from inadequate resection. No other local recurrences were observed, indicating complete tumour cell eradication.
Normal fracture healing times were not applicable to the irradiated grafts. At the two- and five-year follow-ups, 31 out of 50 (62%) and 27 out of 42 (64%) grafts had healed, respectively. Fifteen grafts developed nonunions. The follow-up period for most of the patients who died was insufficient to achieve healing; two of the 31 patients who died had developed a nonunion. Chemotherapy adversely affected graft healing (p = 0.007). The presence of a prosthesis (eight patients) had no effect on graft healing (p = 0.142) or graft resection rates (p = 0.342). Nine out of the 15 patients with nonunion underwent revision surgery; one required an amputation.
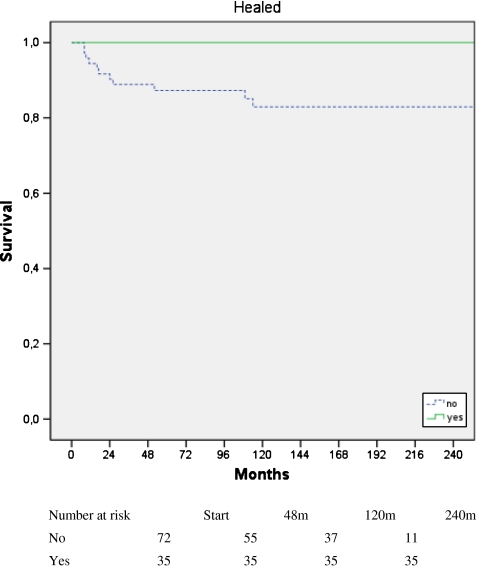
Graft resection was performed in 12 of the 107 patients (11%), and none was considered healed. Only one had a true pseudarthrosis. Of the 12 resected grafts, six showed signs of infection. Patients who underwent chemotherapy had graft removal earlier than those not receiving chemotherapy, but the difference in final graft retention was not significant (p = 0.061). According to resection type, graft resection was performed in three intercalary, three osteoarticular and six composite reconstructions. The survival curve demonstrated that grafts considered healed provided stable reconstructions (p = 0.019; Fig. 3).
Fig. 3.
Cumulative graft survival. Once the graft had healed, it remained stable
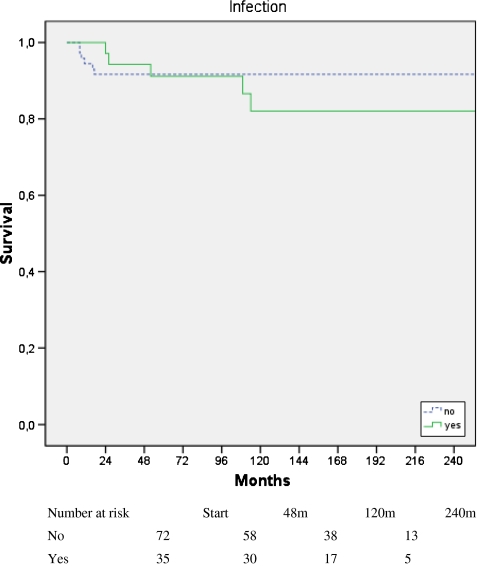
Infection occurred in 35 of the 108 grafts in the healed and nonunion groups. Seventeen patients presented with early infection, and late infection was detected in 18 cases. Chemotherapy had no effect on infection rate. Early or late infections did not influence graft resection (p = 0.436 for early infection; p = 0.459 for late infection) (Fig. 4). The development of pseudarthrosis was associated with early infection (p = 0.023), but revision surgery had no effect on graft resection (p = 0.732). Nine of the infected grafts showed graft healing, three in the early and six in the late infection group.
Fig. 4.
Graft survival rate in the presence and absence of infection. Survival time refers to time of graft survival
Two grafts were retrieved because of complications (one infection, one fracture) and were thus available for histological evaluation. Active bone remodelling was observed with viable osteocytes in the lacunae (Fig. 5a, b). Bone apposition and regeneration were observed at the bone surface and within the bone structure.
Fig. 5.
Retrieval of a graft in the femur. The retrieved graft (a), and an illustration of new bone formation in the existing graft (b)
Discussion
Treatment for bone tumour may preserve the affected limb, but to be completely successful it must also maintain function without major complications or reoperations over the long term. In selected patients, biological reconstruction with an irradiated orthotopic autograft can provide a strong and viable alternative to massive allografts and prosthetic implants with preservation of bone stock, but to be curative, all tumour cells must be eradicated as this is the primary goal of treatment.
In the literature, an irradiation dose between 40 and 300 Gy has been reported. For complete eradication, bone tumours require an irradiation dose of at least 80 Gy with a higher dose required under anoxic conditions. Doses above 27,000 Gy affect the biomechanical properties of a bone graft [22]. The frequency of delayed unions for irradiated autografts at 12 weeks in rats has been related to the irradiation dose: 16% at 1 kGy, 24% at 5 kGy, and 100% at 25 kGy [23]. At 50 kGy, all grafts were fractured, and the series was interrupted. At 1 and 5 kGy, irradiated autogenic bone grafts retained most of their biological potential. Another study showed that an irradiation dose of 25 Gy affected graft reintegration after 24 weeks, but reintegration at 12 weeks was equal to that of nonirradiated reimplanted autografts [24]. To achieve complete control of the tumour without affecting graft performance, we chose to use an irradiation dose of 300 Gy [16, 20]. We observed complete eradication of tumour cells in all cases of wide excision, and viable osteocytes, bone apposition, and regeneration throughout the available samples of the irradiated bone, indicating a viable graft. However, we found systematic joint degeneration in osteoarticular grafts resulting from subchondral bone disintegration, forcing us to reorient our technique towards a composite reconstruction in the shoulder, hip, and knee.
Our main outcome measure was recurrence. We identified only one local recurrence in surrounding soft tissue, most likely attributable to inadequate resection. In all other cases, we found no local recurrence, indicating successful eradication of tumour cells.
Normal fracture healing times did not apply to irradiated autografts. Massive bone grafts typically took one to two years to heal. Chemotherapy prolonged the graft healing time. The presence of a prosthesis did not affect graft healing time or graft resection incidence. We did not observe any graft weakening as had previously been observed with allografts [13]. Once healed, the graft behaved like normal bone. If too much osteolysis is present because of tumour invasion, this technique is prone to failure.
Infection was demonstrated in one third of the patients. However, six of the 12 resected grafts were considered infected. The infection rate was related to the number of interventions, which is consistent with published findings [25]. Therefore, we no longer add bone grafts or osteoinductive substances in a second intervention to accelerate graft healing. In order to prevent early infection, intravenous antibiotics were administered for five days postoperatively. Also, during chemotherapy, intravenous or oral antibiotics were started in case of fever or neutropenia. Furthermore, patients were instructed to take prophylactic antibiotics in case of dental care or in case of bacterial infections or abscesses.
Over time, we have developed a number of guidelines that are helpful in preventing certain pitfalls associated with this technique. For graft survival, rigid fixation of the graft is of utmost importance. During the study period, the quality of osteosynthesis material improved substantially, and the new material allowed rigid bridging fixation of the graft. This improvement was reflected in better fusion rates over time and the observation that pseudarthrosis no longer led to automatic loosening of the osteosynthesis material. For composite reconstructions, an intercalary piece, which was fixed by the prosthetic stem, was reinserted to add bone stock. However, we observed fractures adjacent to the stem, and we assumed that slow graft healing might have caused inefficient stem fixation, resulting in accelerated prosthetic loosening, cortical erosion, and eventually fracture of the cortical bone at the tip of the stem. Stress shielding may also cause graft resorption. When no stable joint replacement is available, a true osteoarticular graft replacement can be a valuable option. However, because joint degeneration can be expected over the long term, revision surgery may be necessary.
For pelvic reconstructions with hip involvement, the whole resection specimen was irradiated, followed by reinsertion of the pelvic part. The hip was reconstructed with a hip prosthesis. Because a large part of the stabilising soft-tissue envelope was resected in most cases, we preferred to use an antidislocation cup. However, these cups permitted force transfer toward the bone graft, which sometimes caused disruption of the osteosynthesis and nonunion of the graft. Recently, larger-diameter components have been developed; these allow greater range of motion after hip arthroplasty and prevent force transfer to the irradiated acetabular region. In exceptional cases, this method can also be used in palliative settings to alleviate local problems, including tumour-induced fungal growth or neurovascular compression by tumour tissue, and offers excellent pain control.
Patient selection should be meticulous, and patients should be provided with a detailed preoperative explanation of the functional result they can expect with this treatment.
Acknowledgments
The authors wish to thank Githa Gillis for record keeping.
Conflict of interest The authors declare that they have no conflict of interest.
Footnotes
The Departments of Orthopaedic Surgery and Pathology are partners of the EuroBoNet consortium, a European Commission-granted Network of Excellence for studying the pathology and genetics of bone tumours.
This study was approved on 27 November 2007 by the Ethics Committee of the Ghent University Hospital. Work was performed at the Ghent University Hospital and Ghent University.
References
- 1.Pakos EE, Nearchou AD, Grimer RJ, Koumoullis HD, Abudu A, et al. Prognostic factors and outcomes for osteosarcoma: An international collaboration. Eur J Cancer. 2009;45:2367–2375. doi: 10.1016/j.ejca.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Langlais F, Belot N, Ropars M, Lambotte JC, Thomazeau H. The long-term results of press-fit cemented stems in total knee prostheses. J Bone Joint Surg Br. 2006;88:1022–1026. doi: 10.1302/0301-620X.88B8.17722. [DOI] [PubMed] [Google Scholar]
- 3.Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526. doi: 10.1302/0301-620X.89B4.18631. [DOI] [PubMed] [Google Scholar]
- 4.Kinkel S, Lehner B, Kleinhans JA, Jakubowitz E, Ewerbeck V, Heisel C. Medium to long-term results after reconstruction of bone defects at the knee with tumor endoprostheses. J Surg Oncol. 2010;101:166–169. doi: 10.1002/jso.21441. [DOI] [PubMed] [Google Scholar]
- 5.Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79. doi: 10.1097/01.blo.0000180050.27961.8a. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007;459:54–59. doi: 10.1097/BLO.0b013e3180514c8e. [DOI] [PubMed] [Google Scholar]
- 7.Shin DS, Weber KL, Chao EYS, An KN, Sim FH. Reoperation for failed prosthetic replacement used for limb salvage. Clin Orthop Relat Res. 1999;358:53–63. doi: 10.1097/00003086-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000;375:214–217. doi: 10.1097/00003086-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Mankin HJ, Doppelt S, Tomford W. Clinical experience with allograft implantation. The first 10 years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed] [Google Scholar]
- 10.Sanjay BKS, Moreau PG, Younge DA. Reimplantation of autoclaved tumour bone in limb salvage surgery. Int Orthop. 1997;21:291–297. doi: 10.1007/s002640050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asada N, Tsuchiya H, Kitaoka K, Mori Y, Tomita K. Massive autoclaved allografts and autografts for limb salvage surgery. A 1–8 year follow-up of 23 patients. Acta Orthop Scand. 1997;68:392–395. doi: 10.3109/17453679708996184. [DOI] [PubMed] [Google Scholar]
- 12.Harrington KD, Johnston JO, Kaufer HN, Luck JV, Moore TM. Limb salvage and prosthetic joint reconstruction for low-grade and selected high-grade sarcomas of bone after wide resection and replacement by autoclaved autogeneic grafts. Clin Orthop Relat Res. 1986;211:180–214. [PubMed] [Google Scholar]
- 13.Wheeler DL, Enneking WF. Allograft bone decreases in strength in vivo over time. Clin Orthop Relat Res. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 14.Friedlaender GE. Bone allografts: the biological consequences of immunological events. J Bone Joint Surg Am. 1991;73:1119–1122. [PubMed] [Google Scholar]
- 15.Tomford WW, Thongphasuk J, Mankin HJ, Ferraro MJ. Frozen musculoskeletal allografts: a study of the clinical incidence and causes of infection associated with their use. J Bone Joint Surg Am. 1990;72:1137–1143. [PubMed] [Google Scholar]
- 16.Sys G, Uyttendaele D, Poffyn B, Verdonk R, Verstraete KL. Extracorporeally irradiated autografts in pelvic reconstruction after malignant tumour resection. Int Orthop. 2002;26:174–178. doi: 10.1007/s00264-002-0352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uyttendaele D, De SA, Claessens H, Roels H, Berkvens P, Mondelaers W. Limb conservation in primary bone tumours by resection, extracorporeal irradiation and re-implantation. J Bone Joint Surg Br. 1988;70:348–353. doi: 10.1302/0301-620X.70B3.3163694. [DOI] [PubMed] [Google Scholar]
- 18.Unni KK. Dahlin’s bone tumors: General aspects and data on 11,087 cases. 5. Philadelphia: Lippincott Williams & Wilkins; 1996. [Google Scholar]
- 19.Mondelaers W, VanLaere K, Goedefroot A, VandenBossche K. The Ghent University 15 MeV high-current linear electron accelerator facility. Nucl Instr Methods Phys Res A. 1996;368:278–282. doi: 10.1016/0168-9002(95)00666-4. [DOI] [Google Scholar]
- 20.Mondelaers W, VanLaere K, Uyttendaele D. Treatment of primary tumors of bone and cartilage by extracorporeal irradiation with a low-energy high-power electron linac. Nucl Instr Methods Phys Res B. 1993;79:898–900. doi: 10.1016/0168-583X(93)95494-P. [DOI] [Google Scholar]
- 21.Poffyn BA, Sys G, Maele G, Hoorebeke L, Forsyth R, Verstraete K, Uyttendaele D. Radiographic analysis of extracorporeally irradiated autografts. Skelet Radiol. 2010 doi: 10.1007/s00256-010-0889-1. [DOI] [PubMed] [Google Scholar]
- 22.Sabo D, Brocai DR, Eble M, Wannenmacher M, Ewerbeck V. Influence of extracorporeal irradiation on the reintegration of autologous grafts of bone and joint. Study in a canine model. J Bone Joint Surg Br. 2000;82:276–782. doi: 10.1302/0301-620X.82B2 .9447. [DOI] [PubMed] [Google Scholar]
- 23.Voggenreiter G, Ascherl R, Blumel G, Schmit-Neuerburg KP. Extracorporeal irradiation and incorporation of bone grafts: autogeneic cortical grafts studied in rats. Acta Orthop Scand. 1996;67:583–588. doi: 10.3109/17453679608997761. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi S, Sugimoto M, Kotoura Y, Nishimatsu H, Shibamoto Y, Abe M, Yamamuro T. The effects of intraoperative radiotherapy on bone-healing ability in relation to different doses and postradiotherapy intervals. Int J Radiat Oncol Biol Phys. 1994;30:1147–1152. doi: 10.1016/0360-3016(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 25.Anacak Y, Sabah D, Demirci S, Kamer S. Intraoperative extracorporeal irradiation and re-implantation of involved bone for the treatment of musculoskeletal tumors. J Exp Cancer Res. 2007;26:571–574. [PubMed] [Google Scholar]