Abstract
An enhanced expression of the inflammatory mediators in the perimeniscal synovium in knee osteoarthritis (OA) has been suggested to contribute to progressive cartilage degeneration. However, whether the expression levels of these molecules correlated with the severity of OA still remained unclear. Medial perimeniscal synovial samples were obtained from 23 patients with Kellgren-Lawrence (K/L) grades 2 to 4 of medial knee OA. Immunohistochemical analysis of the synovium revealed that the MMP-1, COX-2 and IL-1β expression of the patients with K/L 4 to be significantly reduced in comparison to those with either K/L 2 or 3, while the TGF-β expression showed the opposite. The synovial expression of MMP-1 and IL-1β showed a significant negative correlation with the severity of OA, while that of TGF-β again showed the opposite. In conclusion, although synovial inflammation remained active, the MMP-1, COX-2 and IL-1β expression in synovium decreased depending upon the severity of OA, while the TGF-β expression increased.
Introduction
Most research in osteoarthritis (OA) has so far concentrated on understanding the events within the degenerated articular cartilage, and changes in synovial tissues in OA have been ignored [1]. While OA has traditionally been regarded as a non-inflammatory arthritis, recent studies have shown that OA is associated with the signs and symptoms of inflammation [2], synovitis and an enhancement of an expression of inflammatory mediators are common in OA. Therefore, the importance of synovial inflammation in the pathophysiology of OA has increasingly been recognised.
Synovitis in OA may be a secondary phenomenon related to cartilage and bone alteration induced by the release of degenerative compounds from the extracellular matrix of hyaline cartilage. This could further stimulate cartilage damage [3]. Therefore, it was thought that inflammation in the synovial tissue observed in knee OA was a focal phenomenon [4]. As synovial inflammation in OA occurs locally in areas of cartilage loss, the inflammation of the medial perimeniscal synovium commonly occurs in medial femoro-tibial OA [5]. In addition, the synovial inflammation in the early stages of knee OA is enhanced in comparison to that of late stage OA [5].
Based on these data, we examined whether the expression levels of the inflammatory mediators and growth factor in the medial perimeniscal synovium correlated with the severity of OA assessed by radiographic parameters and a patient-oriented outcome measures in medial knee OA.
Materials and methods
Patient profiles This study was approved by the institutional ethics committee of the university and written informed consent was obtained from all patients enrolled. Twenty-three patients (21 female and two male) who had medial knee OA and underwent either joint replacement (n = 19) or arthroscopic surgery (n = 4) in the hospital were recruited as the subjects according to the criteria of knee OA defined by the American College of Rheumatology criteria in addition to standard exclusion criteria [6]. The characteristics of the patients are described in Table 1.
Table 1.
Basal characteristics of the patients in this study
| Characteristic | Value |
|---|---|
| Number of patients | 23 |
| K/L grading | Two grade 2 patients |
| Two grade 3 patients | |
| 19 grade 4 patients | |
| Age (y) | 74.4 (64.0–86.0) (6.3, 71.9–77.0) |
| Gender (F/M) | 21/2 |
| BMI (kg/m2) | 24.0 (19.6–28.8) (2.5,22.3–25.2) |
| Disease duration (month) | 140.6 (10.0–324.0) (80.8,107.2–173.9) |
| JSW (mm) | 1.16 (0–5.0) (1.52, 0.50–1.81) |
| FTA (º) | 186.3 (175.0–197.0) (6.0, 183.7–188.9) |
| JKOM | 52.3 (7–81) (21.5, 43.0–61.6) |
BMI body mass index, K/L Kellgren-Lawrence grade, JSW joint space width, FTA femoro-tibial angle, JKOM Japanese Knee Osteoarthritis measure
Data are presented as <upper lane> the mean (minimum–maximum) and <lower lane> (standard deviation [SD], 95% confidence interval (95%CI) of the mean)
* indicates that p value was less than 0.05
Sample preparation Antero-medial perimeniscal synovial tissue samples were obtained from the patients at the time of the operation. The synovial tissue samples were fixed in 10% neutral buffered formalin, and subsequently processed by standard histological techniques and mounted in paraffin blocks for sectioning (5 µm).
Immunohistochemical analysis An immunohistochemical analysis was conducted by the method as described previously [7]. Antigen retrieval with proteinase K (Dako, Glostrup, Denmark) digestion was applied in the case of CD31. The following primary antibodies were added to the serial sections: CD31 (JC70A; Dako, Glostrup, Denmark; 1:50 dilution) [8], NF-κB (p105/50) (ab7971; Abcam, Cambridge, UK; 1:100 dilution) [9], MMP-1 (41-1E5; Fuji Chemical Industries, Toyama, Japan; 1:3000 dilution) [10], COX-2 (C22420; BD Biosciences, Franklinlakes, USA; 1:50 dilution) [11], IL-1β (MAB601; R&D Systems, Wiesbaden, Germany; 1:20 dilution), TGF-β (TB21, AbD Serotec, Kidlington; 1:5000 dilution) [12]. For control sections, the primary antibodies were omitted or irrelevant isotype-matched mouse antibodies instead of primary antibody were applied.For quantitative analysis, 23 sections from 23 patients were stained at the same time. This process was conducted three times and then semi-quantitative or quantitative analyses of these sections were conducted. Some control section was always included in each process for comparison in (semi-)quantitative analysis.
Semi-quantitative and quantitative analysis of the stained sections A semi-quantitative analysis of the stained sections was conducted using the method by Soden et al. [13]. The number of positive staining cells was estimated in ten high-powered fields (400 x) chosen at random. In each high-powered field, three histological findings of synovium, such as vascular, synovial sublining layer and synovial lining layer, were scored separately based on the scoring system. Each section was scored on a scale from 0–3 to reflect the degree of specific staining as follows: 0 represented 0–5% positive staining, 1 was 6 –29%, 2 indicated 30 –59%, and 3 was >60%. The cumulative staining scores were calculated by summing the mean score of these four parameters for each case. The area and perimeter and the number of blood vessels detected by CD31 expression were measured per mm2 [14].Intra-observer reproducibility (L.N.) of the histological scores was measured at separate times for ten sections (interclass correlation coefficient [ICC] 0.97; 95% CI 0.93–0.99). The interobserver reproducibility was measured by two observers (L.N. and A.Y.) who conducted ten examinations (ICC 0.93; 95% CI 0.61–0.99).
Measurement of clinical manifestations Standing, extended and antero-posterior view radiographs were taken at the first visit of the hospital according to the method reported previously [15]. The staging of a knee OA on radiograph was assessed using the Kellgren-Lawrence (K/L) grade [16]. The joint space width (JSW) was determined at the centre point of the medial femoro-tibial compartment on a radiograph using a 0.1-mm graduated magnifying lens [17].A patient-oriented outcome measure was evaluated by the Japanese Knee Osteoarthritis measure (JKOM) [18]. JKOM is higher in patients with more pain and physical disabilities and this evaluation modality is considered to have sufficient reliability and validity for studies of the clinical outcomes of Japanese people with knee OA.
Statistical analysis SPSS version 17 (SPSS Inc., Chicago, IL) was used for statistical analysis. A comparison of the means between two groups was conducted using the Mann-Whitney U test. Correlations were determined using Spearman’s correlation coefficient. Values of less than 0.05 were considered to be statistically significant.
Results
Comparison of the expression levels of the inflammatory mediators and growth factor in the medial perimeniscal synovial tissues between OA patients with either K/L 2 or 3 and those with K/L 4
The patients were divided into two groups according to the K/L classification, and the expression levels of the inflammatory mediators and growth factor were compared between these two groups (Table 2, Fig. 1).
Table 2.
Semi-quantitative analysis of the expression levels of the mediators for inflammation in lining and sublining layer of synovial tissue in the patients
| Mediator | Layer | K/L 2 or 3 (n = 4) | K/L 4 (n = 19) |
p |
|---|---|---|---|---|
| MMP-1 | Lining | 2.0 (0.8, 0.7–3.3) | 1.0 (0.6, 0.7–1.3) | 0.03* |
| Sublining | 2.1 (0.6, 1.1–3.1) | 1.0 (0.5, 0.7–1.2) | 0.01* | |
| COX-2 | Lining | 1.4 (0.7, 0.2–2.5) | 0.5 (0.3, 0.3–0.7) | 0.01* |
| Sublining | 0.9 (0.4, 0.3–1.4) | 0.3 (0.3, 0.1–0.4) | 0.01* | |
| IL-1β | Lining | 2.1 (0.7, 1.0–3.2) | 1.1 (0.6, 0.9–1.4) | 0.02* |
| Sublining | 2.0 (0.5, 1.1–2.8) | 1.2 (0.4, 1.0–1.4) | 0.02* | |
| NF-κB1 | Lining | 1.5 (0.4, 0.9–2.2) | 1.1 (0.5, 0.8–1.3) | 0.08 |
| Sublining | 1.3 (0.4, 0.7–2.0) | 1.2 (0.6, 0.79–1.5) | 0.51 | |
| TGF-β | Sublining | 0.1 (0.1, −0.1 to 0.2) | 0.6 (0.3, 0.5–0.8) | <0.01* |
K/L Kellgren-Lawrence grade
Data are presented as the mean (standard deviation [SD], 95% confidence interval [CI] of the mean)
* p value is significant
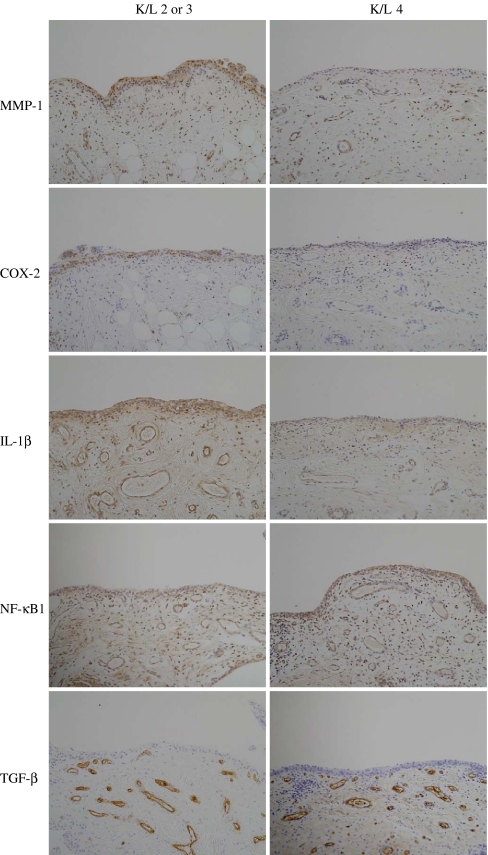
Fig. 1.
Expression patterns of the inflammatory mediators and growth factor in the medial perimeniscal synovial tissue of the patients
An immunohistochemical analysis in both the lining and sublining layer of the synovial tissue revealed that the MMP-1 expression of the patients with K/L 4 was significantly reduced in comparison to those with either K/L 2 or 3. The COX-2 and IL-1β expression in both the lining and sublining layer of the synovial tissue of the patients with K/L 4 also significantly decreased in comparison to those with either K/L 2 or 3. No significant difference in the NF-κB expression in both the lining and sublining layer of the synovium was observed between the patients with K/L 4 and those with K/L 2 or 3. In contrast, the TGF-β expression in the sublining layer of the synovial tissues of the patients with K/L 4 significantly increased in comparison to that observed in those with either K/L 2 or 3.
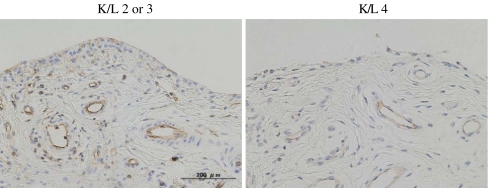
Vascular proliferation in synovial tissue is one of the characteristics of inflammation [19]. Cartilage breakdown products are believed to result in vascular proliferation [20]. CD31 expressing endothelial cells were recognised in the sublining layer of the synovium in the patients (Fig. 2). A significant reduction of both the vascular density and vascular perimeter in patients with K/L 4 were observed in comparison to those with either K/L 2 or 3 (Table 3).
Fig. 2.
Vascular proliferation detected by CD31 expression in medial perimeniscal synovial tissues of the patients
Table 3.
Quantitative analysis of vascular proliferation detected by CD31 expression of the synovial tissue of the patients
| Parameter | K/L 2 or 3 | K/L 4 | p |
|---|---|---|---|
| Density (blood vessel number/mm2) | 79.0 (25.4, 38.7–119.3) | 46.6 (16.7, 38.6–54.7) | <0.01* |
| Perimeter (total perimeter of blood vessel in μm/mm2) | 7970.5 (2458.4, 4058.7–11882.3) | 5339.9 (1468.6, 4632.1–6047.7) | 0.04* |
Data are presented as the mean (standard deviation [SD], 95% confidence interval [CI] of the mean)
* p value is significant
Correlation of the expression levels of the inflammatory mediators and growth factor in the medial perimeniscal synovial tissues with the severity of medial knee OA
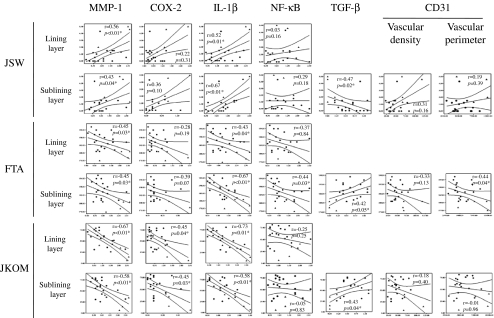
As the K/L classification is a categorical and subjective radiographic parameter to evaluate the severity of OA, we next used continuous and objective radiographic parameters, JSW and femoro-tibial angle (FTA), and examined whether any correlations existed between the expression levels of the molecules and these parameters in all the patients in this study (Fig. 3).
Fig. 3.
Correlation between the expression levels of inflammatory mediators and growth factor in the medial perimeniscal synovial tissue with both the radiographic parameters and patient-oriented outcome measures for knee OA of the patients. Circles and triangles indicate the data from patients with K/L 4 and those with K/L 2 or 3, respectively (n = 23). JSW joint space width, FTA femoro-tibial angle, JKOM Japanese Knee Osteoarthritis measure
The expression levels of MMP-1 and IL-1β in both the lining and sublining layer of the medial perimeniscal synovial tissue showed significant correlation with the radiographic severities, such as JSW of the medial tibio-femoral joint, positively, and FTA, negatively, of the patients with medial knee OA. On the other hand, the COX-2 expression levels in both the lining and sublining layer of the synovial tissue did not correlate with either the JSW of the medial tibio-femoral joint or FTA. The NF-κB expression levels in both the lining and sublining layer of the synovial tissue did not correlate with the JSW of the medial tibio-femoral joint. While the NF-κB expression levels in the sublining layer of the synovial tissue correlated with the FTA, those in the lining layer did not. The expression levels of TGF-β in the sublining layer of the medial perimeniscal synovial tissue in the patients showed a significantly negative correlation with the JSW, and a significantly positive correlation with the FTA. The vascular density in the sublining layer of the medial perimeniscal synovial tissue did not correlate with either the JSW of the medial tibio-femoral joint or FTA. While the vascular perimeter in the sublining layer of the medial perimeniscal synovial tissue significantly correlated with FTA, it did not correlate with the JSW of the medial tibio-femoral joint.
Concerning the K/L classification, JSW and FTA reflect an historical view of the disease, we therefore examined whether the expression levels of the inflammatory mediators and growth factor also correlated with the current physical disability of the patients as evaluated by the JKOM score. The expression levels of MMP-1, COX-2 and IL-1β in both the lining and sublining layers of the medial perimeniscal synovial tissue showed a significantly negative correlation with the JKOM score of the patients. On the other hand, the NF-κB expression in both the lining and sublining layer of the medial perimeniscal synovial tissues of the patients did not correlate with the JKOM score of the patients. The expression levels of TGF-β in the sublining layer of the medial perimeniscal synovial tissue in the patients showed a significantly positive correlation with the JKOM score. However, neither the vascular density nor the vascular perimeter in the sublining layer of the medial perimeniscal synovial tissue correlated with the JKOM score of the patients.
Discussion
This study revealed that, while the synovial inflammation remained active, the expression levels of MMP-1, COX-2 and IL-1β in the medial perimeniscal synovium were decreased and those of TGF-β were increased depending upon the severity of the disease in patients with medial knee OA. The progression of the disease was assessed not only by the classical K/L radiographic classification but also by other radiographic parameters, such as FTA and JSW, and a patient-oriented outcome measure for OA—the JKOM score—thereby confirming the results of this study.
Synovial cells, in addition to chondrocytes, of OA patients produce increased levels of inflammatory cytokines, such as IL-1β and TNF-α, which in turn decrease anabolic collagen synthesis and increase catabolic mediators, such as MMP1 and MMP-13, via their subsequent intracellular signalling through NF-κB and COX-2 [21]. Therefore, the expression levels of these molecules, which play important roles in the inflammation observed in OA, were examined as representative molecules of this phenomenon in this study. As the expression of MMP-1, COX2 and IL-1β in synovial tissues is thought to be activated by degenerated articular cartilage, the expression of these inflammatory mediators might be enhanced in synovial tissues of the patients with either K/L 2 or 3 of knee OA in comparison to those with K/L 4 of knee OA (Table 2). This phenomenon may confirm the occurrence of synovitis in OA as a secondary phenomenon related to cartilage alteration. The results, in which the COX2 expression in perimeniscal synovium correlated with the JKOM score, while it did not correlate with the radiographic parameters, such as JSW and FTA, may be related to the differences of the COX-2 metabolism in comparison to that of other molecules, such as MMP-1, and IL-1β.
TGF-β in synovial tissue is thought to be involved in osteophyte formation and fibrosis of the joint capsule in OA, while TGF-β in cartilage has a protective role for chondrocytes [22]. An osteophyte is a bony outgrowth at the margins of the joint. It is generally accepted that the formation of an osteophyte is one of the signs of severe OA, although it is not known whether this structure is good or bad; or if it can help to stabilise the joint or promote the degenerative process [23]. The results of this study for the TGF-β expression of the synovium in OA suggest that synovial TGF-β signalling plays some role in the progression of OA, especially in the later stage of the disease.
NF-κB is one of the transcription factors that induces COX-2 expression via IL-1β in chondrocytes and other cells including synovial cells and is involved in general stress- and inflammation-induced signalling, including angiogenesis [3]. The results of NF-κB expression in OA synovium of this study suggest that stress- and inflammation-induced signalling had been activated in synovial tissue in patients with OA.
Synovitis has been associated with progressive OA, and synovial vascular turnover may either mediate this effect or exacerbate it. Vascular densities have been reported to increase within the synovium in OA. Vascular perimeter into a synovium is thought to reflect chronic inflammation. In this study, both the vascular density and vascular perimeter in the synovium of the patients with K/L 4 decreased in comparison to those observed in patients with either K/L 2 or 3. However, no significant correlation of the vascular parameters except for the correlation between the vascular perimeter and FTA was observed between both the radiographic parameters and JKOM score. This result therefore suggests that the metabolism of the synovial tissue in the patients with K/L 4 was still active, although its production of inflammatory mediators had decreased in comparison to that in the patients with either K/L 2 or 3. An active metabolism may reflect the increased TGF-β and NF-κB production which help to create capsular fibrosis and/or bony spur formation.
This study had some limitations. The interpretation of the results is limited by the small number of the patients. The number of patients with either K/L 2 or 3, especially, was small in comparison to those with K/L 4. The expression levels of inflammatory mediators were only examined by immunohistochemical analysis in this study. Other molecules, in addition to those examined in this study, have already been reported to play important roles in synovial inflammation in OA. Only a few molecules, on the other hand, were examined in this study. Based on these limitations, this study could introduce certain bias into the results. However, the results did show statistical significance.
This study focussed on the synovial tissues in the medial compartment of tibio-femoral joints of patients with medial knee OA. The results of this study revealed that, while the synovial inflammation remained active, the expression levels of inflammatory mediators, such as MMP-1, COX-2 and IL-1β, and growth factor, TGF-β, in the medial perimeniscal synovial tissues were all observed to change depending on the severity of knee OA.
Acknowledgement
We wish to thank Dr. Takayuki Kawasaki, Dr. Hiroshi Ikeda, Dr. Yuji Takazawa, Dr. Yoshitomo Saita, and Dr. Yuta Kimura for their helpful comments.
This study was partially supported by grants from the High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Takeda Science Foundation (to M.I.).
Conflict of interest All authors report no conflicts of interest.
Footnotes
Liang Ning, Muneaki Ishijima and Haruka Kaneko contributed equally to this study.
References
- 1.Roach HI, Aigner T, Soder S, Haag J, Welkerling H. Pathobiology of osteoarthritis: pathomechanisms and potential therapeutic targets. Curr Drug Targets. 2007;8:271–282. doi: 10.2174/138945007779940160. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 3.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis—an untreatable disease? Nat Rev Drug Discov. 2005;4:331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 4.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr Cartil. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Benito MJ, Veale DJ, FitzGerald O, Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell M, Kaplan D, Koopman W, Longley S, Mankin H, McShane D, Medsger T, Jr, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 7.Shinosaki T, Notoya M, Nomura Y, Miyai I, Kobayashi T, Kurihara H. Glomerular epithelial cell injury accelerates the progression of antibody-induced mesangial proliferative nephritis. Exp Nephrol. 2002;10:245–258. doi: 10.1159/000063699. [DOI] [PubMed] [Google Scholar]
- 8.Korkusuz P, Dagdeviren A, Eksioglu F, Ors U. Immunohistological analysis of normal and osteoarthritic human synovial tissue. Bull Hosp Joint Dis. 2005;63:63–69. [PubMed] [Google Scholar]
- 9.Kanbe K, Inoue K, Inoue Y, Suzuki Y. Histological analysis of synovium in cases of effect attenuation associated with infliximab therapy in rheumatoid arthritis. Clin Rheumatol. 2008;27:777–781. doi: 10.1007/s10067-008-0850-z. [DOI] [PubMed] [Google Scholar]
- 10.Catrina AI, Lampa J, Ernestam S, af Klint E, Bratt J, Klareskog L, Ulfgren AK. Anti-tumour necrosis factor (TNF)-alpha therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology (Oxford) 2002;41:484–489. doi: 10.1093/rheumatology/41.5.484. [DOI] [PubMed] [Google Scholar]
- 11.Crofford LJ. COX-2 in synovial tissues. Osteoarthr Cartil. 1999;7:406–408. doi: 10.1053/joca.1999.0226. [DOI] [PubMed] [Google Scholar]
- 12.Mussener A, Funa K, Kleinau S, Klareskog L. Dynamic expression of transforming growth factor-betas (TGF-beta) and their type I and type II receptors in the synovial tissue of arthritic rats. Clin Exp Immunol. 1997;107:112–119. doi: 10.1046/j.1365-2249.1997.d01-896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soden M, Rooney M, Cullen A, Whelan A, Feighery C, Bresnihan B. Immunohistological features in the synovium obtained from clinically uninvolved knee joints of patients with rheumatoid arthritis. Br J Rheumatol. 1989;28:287–292. doi: 10.1093/rheumatology/28.4.287. [DOI] [PubMed] [Google Scholar]
- 14.Chantrain CF, DeClerck YA, Groshen S, McNamara G. Computerized quantification of tissue vascularization using high-resolution slide scanning of whole tumor sections. J Histochem Cytochem. 2003;51:151–158. doi: 10.1177/002215540305100203. [DOI] [PubMed] [Google Scholar]
- 15.Ravaud P, Auleley GR, Chastang C, Rousselin B, Paolozzi L, Amor B, Dougados M. Knee joint space width measurement: an experimental study of the influence of radiographic procedure and joint positioning. Br J Rheumatol. 1996;35:761–766. doi: 10.1093/rheumatology/35.8.761. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawasaki T, Kurosawa H, Ikeda H, Kim SG, Osawa A, Takazawa Y, Kubota M, Ishijima M. Additive effects of glucosamine or risedronate for the treatment of osteoarthritis of the knee combined with home exercise: a prospective randomized 18-month trial. J Bone Miner Metab. 2008;26:279–287. doi: 10.1007/s00774-007-0813-5. [DOI] [PubMed] [Google Scholar]
- 18.Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol. 2005;32:1524–1532. [PubMed] [Google Scholar]
- 19.Abramson SB. Inflammation in osteoarthritis. J Rheumatol Suppl. 2004;70:70–76. [PubMed] [Google Scholar]
- 20.Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11:227. doi: 10.1186/ar2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthr Cartil. 2008;16(Suppl 3):S1–S3. doi: 10.1016/j.joca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Lent PL, Blom AB, Kraan P, Holthuysen AE, Vitters E, Rooijen N, Smeets RL, Nabbe KC, Berg WB. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 23.Brandt KD. Osteophytes in osteoarthritis. Clinical aspects. Osteoarthr Cartil. 1999;7:334–335. doi: 10.1053/joca.1998.0187. [DOI] [PubMed] [Google Scholar]