Abstract
The World Health Organisation (WHO) recommends routine use of a surgical safety checklist prior to all surgical operations. The aim of this study was to prospectively audit checklist use in orthopaedic patients before and after implementation of an educational programme designed to increase use and correlate this with early complications, mortality and staff perceptions. Data was collected on 480 patients before the educational program and 485 patients after. Pre-training checklist use was 7.9%. The rates of early complications and mortality were 8.5% and 1.9%, respectively. Forty-seven percent thought the checklist improved team communication. Following an educational program, checklist use significantly increased to 96.9% (RR12.2; 95% CI 9.0–16.6). The rate of early complications and mortality was 7.6% (RR 0.89; 95% CI 0.58–1.37) and 1.6% (RR 0.88; 95% CI 0.34–2.26), respectively. Seventy-seven percent thought the checklist improved team communication. Checklist use was not associated with a significant reduction in early complications and mortality in patients undergoing orthopaedic surgery. Education programs can significantly increase accurate use and staff perceptions following implementation.
Introduction
With an estimated 234 million surgical operations performed each year around the world, a system to implement and maintain practices designed to improve patient safety is a necessity [1, 2]. Two retrospective reviews have suggested that at least 50% of all surgical adverse events are preventable [3, 4]. The majority of these are not caused by technical problems but a failure of teamwork skills, leadership, communication, decision-making and situational awareness [5, 6]. This prompted the World Health Organisation (WHO) to identify multiple recommended practices to ensure the safety of surgical patients worldwide [7]. Based on these guidelines, the Surgical Safety Checklist (SSC) was designed [8]. This is a 19-item checklist designed to improve communication between the operating team and provide a minimum standard of care that reduces complications and deaths associated with surgery. Members of the Safe Surgery Saves Lives Study Group prospectively analysed the effects of the SSC on a global scale in eight hospitals in both developed and non-developed countries [9]. Data was collected prospectively on patients >16 years undergoing non-cardiac surgery before and after implementation of the SSC. Primary end points were death and complication rate as defined by the American College of Surgeons’ National Surgical Quality Improvement Program [10] occurring in the first 30 days after operation or before hospital discharge. Mortality significantly declined from 1.5% before the SSC to 0.8% afterwards and inpatient complications fell from 11% to 7%.
The SSC has been modified in England and Wales [11] and has been mandated for use for surgical procedures in several countries including the United Kingdom, Canada, America and Jordan. Many institutions within these countries already have policies to reduce surgical risk for patients undergoing orthopaedic surgery [12–18]. The SSC has not been exclusively studied in trauma and orthopaedic patients and previous studies have shown non-orthopaedic operations to be the most significant cause of preventable surgical adverse events [3]. The aim of this study was to prospectively audit use of the SSC in orthopaedic patients before and after an educational program designed to increase accurate use and correlate this with early complications, mortality and staff perceptions about the checklist.
Methods and materials
Between February and May 2009, 480 patients undergoing both elective and emergency orthopaedic operations at a single institution were prospectively reviewed. Four separate clinicians were involved in data collection. Information was collected from hospital notes and clinical review. There were 253 females and 227 males. Mean age of the patients was 56.7 years (range 2–98). Patient and procedure demographics are shown in Table 1.
Table 1.
Patient demographics
| Demographic | Audit 1—pre-training | Audit 2—post-training |
|---|---|---|
| Patient number | 480 | 485 |
| Female gender (%) | 52.7 | 48.9 |
| Mean age (years) | 56.7 | 53.2 |
| Urgent casesa (%) | 27.3 | 30.1 |
| General anaesthetic cases (%) | 79.2 | 75.1 |
| Daycase proceduresb (%) | 8.5 | 9.7 |
a Urgent cases defined as those ideally requiring surgery within 24 hours
b Daycase procedures defined as those going home the same day as surgery
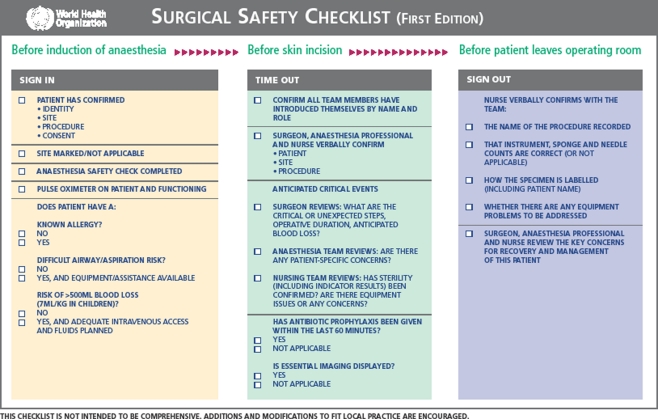
Accurate use of the 19-item checklist (Fig. 1) was audited. Process measures for assessing adherence and correct completion of the checklist have been previously reported (Table 2) [9]. Complication rate and mortality in the first 30 days or until hospital discharge were recorded. The American College of Surgeons’ National Surgical Quality Improvement Program definition of complications was used [10] which included: acute renal failure, bleeding requiring the transfusion of four or more units of red cells within the first 72 hours after surgery, cardiac arrest requiring cardiopulmonary resuscitation, coma of 24 hours’ duration or more, deep vein thrombosis, myocardial infarction, unplanned intubation, ventilator use for 48 hours or more, pneumonia, pulmonary embolism, stroke, major disruption of wound, infection of surgical site, sepsis, septic shock, the systemic inflammatory response syndrome, unplanned return to the operating room, graft failure, and death. One hundred members of the operating room team, including surgeons, anaesthetists, nurses and allied health care professionals, were asked four questions relating to their perceptions of checklist use: whether they believed the checklist incurred an unnecessary time delay, whether the checklist improved patient safety, whether the checklist improved team communication and teamwork and whether they would want the checklist used if they were having an operation. Each response was recorded as yes, not sure or no.
Fig. 1.
Surgical Safety Checklist (reproduced with permission of the WHO [8])
Table 2.
Process measures of the checklist
| Measures |
|---|
| Objective airway evaluation made |
| Pulse oximeter used |
| Two peripheral or one central venous catheter sited when EBL >500 ml |
| Preoperative prophylactic antibiotics given when appropriate |
| Oral confirmation of patient identity and operative site |
| Sponge count completed |
EBL estimated blood loss
After the first audit measures were taken to improve uptake and practise. Surgical safety checklist forms were placed in operating rooms so that staff members could become familiar with the details. A compulsory training video was produced detailing the correct and incorrect way to perform the checklist. Emphasis was placed on all team members being responsible and accountable for a certain part of the checklist. A ‘tick and flick’ approach whereby each checklist item is acknowledged but not really considered or acted upon was discouraged. Large and small group educational sessions were given to the operating room team—surgeons, theatre staff and anaesthetists. Educational sessions discussed the common causes of surgical adverse events, how the checklist could be used to prevent them and provided a forum for questions. The fundamental premise aimed to promote a culture change away from a hierarchical system whereby one or two individuals were responsible for patient safety to one that relied upon a team approach with all team members being equally responsible.
Following this, between June and October 2009, a second audit was undertaken using the same methodology. Four hundred eighty-five patients were identified and data were collected prospectively. There were 248 males and 237 females with a mean age of 53.2 years (range 1–94). Accurate and correct use of the checklist was audited and early complications and mortality recorded. The same 100 operating room team members were each asked the initial four questions relating to staff perceptions of checklist use. Relative risk (RR) statistical analyses were performed with 95% confidence intervals (CI) to ascertain risk of the event occurring relative to the exposure (educational program and infrastructure changes).
Results
Pre-training audit
Four hundred eighty patients were identified. Correct use of the checklist was performed in 38 patients (7.9%). Nine patients died (1.9%). Eight of these patients had sustained a proximal femoral fracture and died of medical complications following surgery. The other patient died of systemic infection secondary to severe soft tissue infection. Forty-one patients developed a major, early complication (8.5%). Three patients were anaesthetised without appropriate orthopaedic instrumentation being available.
Post-training audit
Four hundred eighty-five patients were identified. Correct use of the checklist was performed in 470 patients (96.9%) which was a significant increase (RR12.2; 95% CI 9.0–16.6). Eight patients died (1.6%). Seven of these had sustained a proximal femoral fracture and died of postoperative medical complications. The other patient died of cardiac arrhythmia. Thirty-seven patients developed a major, early complication (7.6%). No patients were anaesthetised without appropriate orthopaedic instrumentation being available. The results of both audits are summarised in Table 3.
Table 3.
Pre- and post-training audits
| Results | Audit 1—pre-training | Audit 2—post-training |
|---|---|---|
| Use of WHO SSC (%) | 7.9 | 96.9 |
| Mortality rate (%) | 1.9 | 1.6 |
| Overall complication rate (%) | 8.5 | 7.6 |
| LRTI (%) | 2.1 | 2.5 |
| Surgical site infection (%) | 4.4 | 3.5 |
| Unplanned return to operating theatre (%) | 1.0 | 1.0 |
SSC surgical safety checklist, LRTI lower respiratory tract infection
There was no significant difference in mortality (RR 0.88; 95% CI 0.34–2.26) or early major complications (RR 0.89; 95% CI 0.58–1.37) between the two audits. It took a mean time of two minutes to complete the 19-item checklist. Staff perceptions about the checklist both before and after the educational program are shown in Table 4.
Table 4.
Staff perceptions about the checklist
| Question | Audit 1—pre-training | Audit 2—post-training |
|---|---|---|
| 1. Do you think the checklist causes an unnecessary time delay? | Yes 55% | Yes 20% |
| Not sure 21% | Not sure 12% | |
| No 24% | No 68% | |
| 2. Do you think the checklist improves patient safety? | Yes 28% | Yes 68% |
| Not sure 47% | Not sure 20% | |
| No 25% | No 12% | |
| 3. Do you think the checklist improves team communication and teamwork? | Yes 47% | Yes 77% |
| Not sure 35% | Not sure 15% | |
| No 18% | No 8% | |
| 4. Would you want the checklist used if you were having an operation? | Yes 64% | Yes 80% |
| Not sure 26% | Not sure 14% | |
| No 10% | No 6% |
Discussion
Use of the WHO SSC in emergency and elective orthopaedic patients at our institution was not associated with a significant reduction in early major complications or mortality. Education and infrastructure changes were associated with a significant increase in accurate checklist use and improved perceptions from operating room team members about its value. Use of a checklist to safeguard patient care is not novel. A simple central line insertion checklist has been shown to reduce infections in critical care medicine [19]. The SSC is concerned with activity within the operating room. It requires a formal pause preoperatively for introductions and briefings followed by a second pause postoperatively for team de-briefings. These practises are known to be associated with improved safety measures and interpersonal communication [20–22]. The underlying philosophy of the checklist is that a true team approach with good communication between operating team members is safer and more efficient than a hierarchical system that relies on individuals [23].
The initial implementation of the checklist was met with resistance by some operating room team members as there was a belief that many of the points were already in practise and 55% of those questioned thought the checklist conferred an unnecessary time delay. This was manifest by a low initial uptake of 7.9%. Education on the background of the checklist, the benefits of its use, and importance of a team-based approach significantly improved uptake to 96.9%. Twenty percent of those questioned still considered it conferred an unnecessary time delay; however, 80% requested the checklist be used if they were having an operation. We advocate an educational program following implementation of such a checklist in hospital trusts to optimise staff perceptions, correct use and team communication.
Preventable adverse events are common in surgery [24] with communication failures being the most common cause [6]. Seventy-seven percent of those questioned thought the checklist improved communication between team members and 68% thought this improved patient safety. In situations where theatre teams are unaware of other team members, such as emergency surgery at night, the checklist may confer benefits in terms of improved communication and team function. In this study despite a high percentage of urgent cases (28.7%), over 95% of patients had operations performed on scheduled operating lists where theatre staff knew each other preoperatively and were used to working together. This may be one reason why checklist use was not associated with a significant reduction in early major complications or mortality. Policies already in place [12–18] for reducing surgical risk to patients undergoing orthopaedic surgery may have also nullified potential benefits conferred by the checklist at our institution.
We did note a modest nonsignificant reduction in adverse surgical events associated with correct use of the checklist and no patients were anaesthetised without correct orthopaedic instrumentation being available. This may be because of improved communication between team members and the culture of safety the checklist instils, which begins on the ward with preoperative marking and continues postoperatively with antibiotic and anti-thrombosis prophylaxis. Such measures are known to reduce risks associated with surgery [12–17, 25]. However, temporal effects may have led to this outcome, such as bed occupancy levels, changing staffing levels and junior doctor working hours.
There are limitations to this study. This is a prospective design without randomisation in a single hospital setting. There is always the risk of confounding. Lack of statistical study power may have failed to demonstrate a significant difference between the two groups. Only complications defined by the American College of Surgeons’ National Surgical Quality Improvement Program [10] in the first 30 days or until hospital discharge were recorded which will result in an underestimation of overall complications. Patient attendance at other institutions with early complications may also have resulted in an overall underestimation.
This study has shown that education and infrastructure changes can significantly increase accurate use and improve staff perceptions of the WHO SSC; however, this was not associated with a significant reduction in early major complications or mortality in orthopaedic patients at our institution. Patient safety is and must remain central to every aspect of surgical care and a team-based approach is thought to be fundamental in reducing risk [23]. With the advent of the new 48-hour working week and European Working Time Directive, continuity of patient care and interpersonal communication may become compromised by multiple handovers and different healthcare professionals caring for patients. In such a system, use of a checklist may provide a means to safeguard patient care and reduce preventable surgical adverse events through improved team communication and function; however, this should augment, not replace, an environment which is centred around safety for the entire patient journey.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Amalberti R, Auroy Y, Berwick D, Barach P. Five system barriers to achieving ultrasafe health care. Ann Intern Med. 2005;142:756–764. doi: 10.7326/0003-4819-142-9-200505030-00012. [DOI] [PubMed] [Google Scholar]
- 3.Gawande AA, Thomas EJ, Zinner MJ, Brennan TA. The incidence and nature of surgical adverse events in Colorado and Utah in 1992. Surgery. 1999;126:66–75. doi: 10.1067/msy.1999.98664. [DOI] [PubMed] [Google Scholar]
- 4.Kable AK, Gibberd RW, Spigelman AD. Adverse events in surgical patients in Australia. Int J Qual Health Care. 2002;14:269–276. doi: 10.1093/intqhc/14.4.269. [DOI] [PubMed] [Google Scholar]
- 5.Catchpole K, Mishra A, Handa A, McCulloch P. Teamwork and error in the operating room: analysis of skills and roles. Ann Surg. 2008;247:699–706. doi: 10.1097/SLA.0b013e3181642ec8. [DOI] [PubMed] [Google Scholar]
- 6.Lingard L, Espin S, Whyte S, et al. Communication failures in the operating room: an observational classification of recurrent types and effects. Qual Saf Health Care. 2004;13:330–334. doi: 10.1136/qshc.2003.008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO guidelines for safe surgery. Geneva: World Health Organisation; 2008. [Google Scholar]
- 8.WHO (2008) The Surgical Safety Checklist. World Health Organisation, Geneva. http://www.who.int/patientsafety/safesurgery/tools_resources/SSSL_Checklist_finalJun08.pdf. Accessed 5 August 2010
- 9.Haynes AB, Weiser TG, Berry WR, et al. A Surgical Safety Checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 10.Khuri SF, Daley J, Henderson W, et al. The national veterans administration surgical risk study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180:519–531. [PubMed] [Google Scholar]
- 11.National Patient Safety Agency. (2009) Patient Safety Alert UPDATE. NPSA, London. http://www.npsa.nhs.uk/nrls/alerts-and-directives/alerts/safer-surgery-alert/. Accessed 5 August 2010
- 12.Torholm C, Broeng L, Jorgensen PS, et al. Thromboprophylaxis by low-molecular weight heparin in elective hip surgery. A placebo controlled study. J Bone Joint Surg Br. 1991;73:434–438. doi: 10.1302/0301-620X.73B3.1670445. [DOI] [PubMed] [Google Scholar]
- 13.Pitto RP, Hamer H, Heiss-Dunlop W, Kuehle J. Mechanical prophylaxis of deep-vein thrombosis after total hip replacement: a randomised clinical trial. J Bone Joint Surg Br. 2004;86:639–642. doi: 10.1302/0301-620X.86B5.14763. [DOI] [PubMed] [Google Scholar]
- 14.Warwick D, Friedman RJ, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the global orthopaedic registry. J Bone Joint Surg Br. 2007;89:799–807. doi: 10.1302/0301-620X.89B6.18844. [DOI] [PubMed] [Google Scholar]
- 15.Blanco M, Clarke JR, Martindell D. Wrong site surgery near misses and actual occurrences. AORN J. 2009;90:215–222. doi: 10.1016/j.aorn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Reuther F. Avoidance of wrong site surgery. Experiences by the introduction of measures for quality control and patient safety in a surgical casualty hospital. Unfallchirurg. 2009;112:675–678. doi: 10.1007/s00113-009-1635-9. [DOI] [PubMed] [Google Scholar]
- 17.Prokuski L. Prophylactic antibiotics in orthopaedic surgery. J Am Acad Orthop Surg. 2008;16:283–293. doi: 10.5435/00124635-200805000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Phillips JE, Crane TP, Noy M, et al. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 19.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020. doi: 10.1097/01.CCM.0000142399.70913.2F. [DOI] [PubMed] [Google Scholar]
- 20.Lingard L, Regehr G, Orser B, et al. Evaluation of a preoperative checklist and team briefing among surgeons, nurses, and anaesthesiologists to reduce failures in communication. Arch Surg. 2008;143:12–18. doi: 10.1001/archsurg.2007.21. [DOI] [PubMed] [Google Scholar]
- 21.Sexton JB, Makary MA, Tersigni AR, et al. Teamwork in the operating room: frontline perspectives among hospitals and operating room personnel. Anaesthesiology. 2006;105:877–884. doi: 10.1097/00000542-200611000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Makary MA, Sexton JB, Freischlag JA, et al. Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder. J Am Coll Surg. 2006;202:746–752. doi: 10.1016/j.jamcollsurg.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Taylor B, Slater A, Reznick R. The surgical safety checklist effects are sustained, and team culture is strengthened. Surgeon. 2010;8:1–4. doi: 10.1016/j.surge.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Sarker SK, Vincent C. Errors in surgery. Int J Surg. 2005;31:75–81. doi: 10.1016/j.ijsu.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Pitto RP, Young S. Foot pumps without graduated compression stockings for prevention of deep-vein thrombosis in total joint replacement: efficacy, safety and patient compliance. Int Orthop. 2008;32:331–336. doi: 10.1007/s00264-007-0326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]