Abstract
Background: Many patients show modulation of tinnitus by gaze, jaw or neck movements, reflecting abnormal sensorimotor integration, and interaction between various inputs. Postural control is based on multi-sensory integration (visual, vestibular, somatosensory, and oculomotor) and indeed there is now evidence that posture can also be influenced by sound. Perhaps tinnitus influences posture similarly to external sound. This study examines the quality of postural performance in quiet stance in patients with modulated tinnitus. Methods: Twenty-three patients with highly modulated tinnitus were selected in the ENT service. Twelve reported exclusively or predominately left tinnitus, eight right, and three bilateral. Eighteen control subjects were also tested. Subjects were asked to fixate a target at 40 cm for 51 s; posturography was performed with the platform (Technoconcept, 40 Hz) for both the eyes open and eyes closed conditions. Results: For both conditions, tinnitus subjects showed abnormally high lateral body sway (SDx). This was corroborated by fast Fourrier Transformation (FFTx) and wavelet analysis. For patients with left tinnitus only, medio-lateral sway increased significantly when looking away from the center. Conclusion: Similarly to external sound stimulation, tinnitus could influence lateral sway by activating attention shift, and perhaps vestibular responses. Poor integration of sensorimotor signals is another possibility. Such abnormalities would be accentuated in left tinnitus because of the importance of the right cerebral cortex in processing both auditory–tinnitus eye position and attention.
Keywords: lateral body sway, Romberg test, tinnitus, gaze position
Introduction
Tinnitus is an auditory perception, experienced in the absence of any external or internal auditory stimulus (perception of a sound without a recordable source). Subjective tinnitus must be differentiated from objective tinnitus which is induced by the perception of an internal, vascular, or muscular noise that can actually be recorded. Subjective tinnitus is commonly described in terms of a hissing or buzzing sound. It can be heard in one ear or both or perceived in a central position (Shulman, 1991). Subjective tinnitus is a very common condition (10% of general population). When chronic, it can lead to severe impairment of quality of life (Vio and Holme, 2005).
The physiopathology of subjective tinnitus perception remains incompletely understood. Nevertheless, subjective tinnitus is thought to result from hyperactivity and neuroplastic reorganization of cortico-subcortical auditory and non-auditory networks following acoustic deafferentation induced by cochlear or auditory nerve damage (Eggermont and Roberts, 2004). Thus, even though hearing loss is frequently associated with tinnitus perception, subjective tinnitus can be triggered or modulated by non-auditory events. This is certainly the case in stressful situations or states of anxiety/depression (Heinecke et al., 2008) which probably reflects the complex intermodulation between limbic and prefrontal networks and auditory pathways (Jastreboff, 1990).
On the other hand, the somatosensory system, especially when involving head and neck regions, also seems to be strongly interrelated to tinnitus. Many somatosensory disorders of this area affecting the temporomandibular joint or the upper spine or whiplash injuries have been described as frequently associated with subjective tinnitus (de Flicio et al., 2008; Tranter and Graham, 2009). Somatosensory modulation of subjective tinnitus is also frequent. Fluctuations in perceived intensity or pitch after voluntary or induced head and neck movements or isometric contractions is spontaneously described by many individuals presenting subjective tinnitus. As demonstrated by Levine et al. (2003), when systematically screened, fluctuations in perceived intensity or pitch occur in up to 80% of a tinnitus population but also in patients previously unaware of the presence of tinnitus and even in normal subjects among which 20% are able to elicit a transient tinnitus perception during muscular systemic testing. Other movements such as lateral gaze, finger shoulder or hip flexion have also been described as tinnitus modulators (Levine et al., 2007).
Postural stabilization is based on multiple sources of information namely, somatosensory, proprioceptive, vestibular, visual, oculomotor, and auditory. External sound stimulation has been shown to influence body sway particularly in the medial–lateral axis (Tanaka et al., 2001; Priplata et al., 2002; Deviterne et al., 2005; Alessandrini et al., 2006; Dozza et al., 2007). Such influence may be mediated via the vestibular system causing shifts in the lower limbs and in the body. The question addressed in this study is whether or not tinnitus (a fantom sound) could act in a similar fashion on postural control. The study examines postural control as maintained during quiet stance in tinnitus subjects when their eyes are converged at near distance (at 40 cm), a condition where controls exhibit the greatest stability (Kapoula and Le, 2006). For healthy subjects an eccentric gaze position influences the postural stability: lateral gaze beyond 40° induces higher postural instability compared with straight ahead gaze (Brandt, 1999). Also, elevation of the eyes by 15° provokes postural instability while down gaze provides higher stability relative to a straight ahead gaze (Kapoula and Le, 2006). This study also examines the quality of postural control with lateral gaze direction in subjects with left versus right side tinnitus.
Materials and Methods
Subjects
A total of 23 patients (49.0 ± 12.5 years) attending our tinnitus clinic (ENT department Georges Pompidou European Hospital Paris, France) were studied; they were selected because of their ability to modulate their tinnitus by somatic stimuli (movements, muscle pressure). Complete otologic and neurologic testing was done (audiometry, tympanometry, stapedial reflexes, auditory evoked potential, and/or MRI). The first posture study (Romberg test) was conducted using 16 of them (49.3 ± 12.5 years); their data were compared with those from 16 age-matched controls (49.5 ± 11.9 years, nine females). Participants in the second experiment examining gaze position consisted in 21 patients who perceived their tinnitus exclusively in one ear (48.1 ± 12.7 years). Tinnitus severity and distress, and perceived laterality were evaluated with standard questionnaires.
The investigation adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional human experimentation committee. Written informed consent was obtained from all subjects after the nature of the procedure had been explained.
Clinical data
Clinical data are summarized in Table 1. The mean duration of tinnitus was 5.6 years (SD = 7). High pitch tone tinnitus was perceived by 13 patients, broad band noise (white noise) by 5, and a mix of both types by 5. Thirteen patients perceived their tinnitus more so (T5, T17) or totally (11) in their left ear, eight of the subjects more so (6) or totally (T19, T21) in their right ear, and the two remaining patients had bilateral tinnitus equally perceived between the two ears.
Table 1.
Clinical characteristic of tinnitus patients.
| Subjects | Sex | Age | Etiology | Side tinnitus | Pitch | Duration (years) | Audiogram | Movements modulation | Tinnitus severity (16) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | F | 21 | Idiopathic | L | High | 3 | Normal | Effort | 7 |
| T2 | M | 29 | Stress | L | High | 1 | Normal | Jaw | ND |
| T3 | M | 40 | Stress | L | High | 1 | Normal | Head jaw | 10 |
| T4 | M | 43 | Idiopathic | L | High | 1 | Normal | Jaw | 14 |
| T5 | M | 43 | Serous otitis media (right), stress | L > R | High WN | 10 | High fHz | Head | 5 |
| T6 | F | 46 | Left otosclerosis surgery, stress | L | High WN | 5 | High fHz SNHL | Jaw, muscular pressure | 12 |
| T7 | M | 47 | Stress | L | High | 2 | High fHz SNHL | Jaw, muscular pressure, effort | 14 |
| T8 | M | 51 | Bilateral noise induced HL | R > L | High | 4 | High fHz SNHL | Jaw, muscular pressure | 11 |
| T9 | M | 54 | Right acoustic neuroma | R > L | High WN | 13 | Right cophosis | Head eye | 8 |
| T10 | F | 57 | Left head trauma, TMJ luxation | L | High | 30 | Normal | Jaw | 11 |
| T11 | F | 57 | Meningitis, stress | L = R | WN | 5 | Normal | Head | 8 |
| T12 | F | 58 | Stress | R > L | High WN | 1 | High fHz SNHL | Jaw, head, muscular pressure | 12 |
| T13 | M | 59 | Bilateral congenital SNHL | R > L | WN | 20 | Middle fHz | Head | 8 |
| T14 | F | 60 | Abd surgery, stress | L = R | High | 4 | High fHz SNHL | Head, muscular pressure | 7 |
| T15 | M | 61 | Orthopedic surgery; stress | L | WN | 2 | High fHz SNHL | Jaw, muscular pressure | 13 |
| T16 | F | 62 | Cervicalgia | L | High | 3 | High fHz | Head jaw | 15 |
| T17 | F | 58 | Stress, Diplopia | L > R | High | 8 | High fHz SNHL | Eye | 10 |
| T18 | F | 36 | Left acoustic neuroma | L | High WN | 6 | Left cophosis | Eye | 7 |
| T19 | M | 42 | Idiopathic | R | High | 2 | Normal | Jaw | 12 |
| T20 | F | 42 | Stress | L | WN | 2 | Middle fHz | Jaw, head | 11 |
| T21 | M | 43 | Right otitis media, stress | R | High | 1 | Normal | Muscular pressure | 14 |
| T22 | F | 41 | Stress | R > L | WN | 2 | High fHz SNHL | Jaw muscular pressure | 12 |
| T23 | F | 78 | Right meningioma | R > L | High | 3 | High fHz SNHL | Head Jaw muscular pressure | 13 |
Tinnitus severity was evaluated with the Subjective Tinnitus Severity Scale (Halford and Anderson, 1991; Meric and Chery-Croze, 1996); Score >8 indicates moderate tinnitus, Score >12 severe tinnitus. Tinnitus stress was evaluated with the Tinnitus Reaction Questionnaire (Wilson et al., 1991; Meric and Chery-Croze, 1997); Scores >30 <60 show moderate distress, Scores >60 show marked distress. Anxiety and depression were evaluated with the Hospital Anxiety and Depression Scale (Zigmond and Snaith, 1983); Scores >8 <10 show suspect anxiety or depression, Scores >10 show certain anxiety or depression.
Tinnitus was modulated by jaw movements in 14 patients, head movements in 10 patients, muscle pressure in 9 patients, eye movements in 3 patients, and global muscular effort in 2 of the patients. One condition elicited tinnitus modulation in 11 patients, two conditions in 9 patients, and three in the remaining 3 patients.
The pathological conditions being present at the onset of tinnitus were either otological (right otitis media (2), left otosclerosis surgery, bilateral noise induced hearing loss, sensorineural hearing loss) or neuro-muscular (acoustic neuroma – left for subject T18 and right for subject T9, right meningioma, meningitis, left head trauma with TMJ luxation, cervicalgia, abdominal, or orthopedic surgery). The causal link between these medical conditions and tinnitus is only putative. Unusually stressful circumstances were indicated by 13 patients; these patients had a mean score of 171 on the Holmes and Rahe stress questionnaire (Miller and Rahe, 1997). Even if stress is not the direct cause of tinnitus it can be considered as a trigger of tinnitus intrusiveness (Andersson and Westin, 2008; Hesser and Andersson, 2009). Tinnitus was considered as idiopathic in the remaining three patients, i.e., no specific condition was associated with the onset of tinnitus. There was no difference between right sided tinnitus patients (5/8, i.e., 63%), and left sided tinnitus patients (7/13, i.e., 54%) regarding the presence of an organic disorder linked to tinnitus onset.
Audiometry thresholds were normal in 7 patients, and demonstrated high frequency sensorineural hearing loss in 12 subjects, middle frequency sensory neural hearing loss in 2 subjects, and total unilateral cophosis in 2 patients (acoustic neuroma patients). All patients had stable hearing levels at the time of testing with no recent impairment. This is in keeping with previous reports of the high prevalence of hearing impairment in tinnitus (Nicolas-Puel et al., 2002). Eight patients, among whom were included the two patients with a history of surgically treated acoustic neuroma, experienced dizziness at some point during the tinnitus time course. But none of them had acute clinical vestibular dysfunction at the time of testing as attested by the absence of vertigo or dizziness or spontaneous nystagmus.
In summary, the pathophysiological mechanisms underlying the tinnitus percept probably vary among patients. Yet, this group of patients is homogeneous because of their spontaneously modulated tinnitus following different types of movements.
Posturography platform
We used a posturography apparatus that consisted of two dynamometric soles; one for each foot (produced by TechnoConcept, Céreste, France). The excursions of the center of pressure (CoP) were measured during 51.2 s; the equipment contained an Analogical–Digital converter of 16 bits. The sampling frequency of the CoP was 40 Hz.
Procedure
During all posturography tests, subjects were required to fixate a target placed at eye level. Subjects maintain an upright and standardized Romberg position (feet placed side-by-side forming an angle of 30° with both heels separated by 4 cm). Subjects were required to maintain a quiet stance (i.e., arms are held side-by-side while breathing normally, not to speak and not to clench their teeth) during 51.2 s.
For the Romberg test, subjects performed two conditions: eyes open versus eyes closed. The distance between the subject and the target was 40 cm. Such close distance requires convergence of the eyes and is known to produce optimal postural stability in controls (Kapoula and Le, 2006; Le and Kapoula, 2006). Participants in this experiment included patients 1 through 16 shown in Table 1 and the controls. For the second experiment, patients were required to fixate the target at the median–sagittal plane (centered gaze) or at 30° of eccentricity with respect to either the tinnitus side or to the non-tinnitus side (based on the diagnosis from the ENT physician). In the experiment dealing with gaze position, patients were asked after each condition to evaluate the intensity of their tinnitus on a scale from 0 to 10. Participants in this experiment included all those patients indicated in Table 1 with the exception of T11 and T14 both of whom presented bilateral tinnitus.
Posturography parameters
Basic parameters
Postural stability was measured by the size of the surface area of CoP that contains 90% of the CoP positions closest from the central ones, the standard deviation of the lateral and of the antero-posterior sway (respectively SDx, and SDy), and the variance of speed of the CoP excursions (Gagey and Weber, 2005). These parameters were described in previous studies (Kapoula and Le, 2006; Le and Kapoula, 2006, 2008). In terms of physiologic significance, body sway is believed to be controlled by two distinct muscular strategies involving the ankle for the anterior–posterior sway, and the hip for lateral sway (Day et al., 1993; Winter et al., 1996, 2003; Gatev et al., 1999). The variance of speed is related to the energy released by the leg muscle activity in order to stabilize posture (see Amiridis et al., 2003; Jonsson et al., 2005).
Frequency analysis
Fast Fourrier Transform was applied to determine the power spectrum of the whole frequency band or for three frequency bands (i.e., 0–0.5 Hz; 0.5–2 Hz; higher than 2 Hz) for the lateral (FFTx) and for the antero-posterior sway (FFTy). The high, the intermediate, and the low frequency bands correspond respectively to the involvement of the reflexive loop (Golomer et al., 1994; Kohen-Raz et al., 1996; Paillard et al., 2002), the cerebellum (Paillard et al., 2002), and the visual and the vestibular system (Nashner, 1979; Kohen-Raz et al., 1996; Paillard et al., 2002).
Another analysis applied on the data is the wavelet transform. Applied to CoP displacements, the wavelet transform elaborates a time–frequency chart of body sway, i.e., a non-linear analysis (Dumitrescu and Lacour, 2006; Bernard-Demanze et al., 2009). Similarly to the Fast Fourier Transform, the spectral power was calculated for the frequency bands 0–0.5 Hz (F1), 0.5–1.5 Hz (F2), higher than 1.5 Hz (F3) on the antero-posterior and medio-lateral sway as power indices (PIy and PIx, respectively). The physiological significance of the spectral power of different bands is the same as for the FFT, i.e., 0–0.5 Hz visual/vestibular, 0.5–1.5 Hz cerebellar, >1.5 Hz reflexive loops (Lacour et al., 2008). Moreover, the cancelling time (CT) for each of the three frequency bands was also calculated on the antero-posterior (CTy) and medio-lateral (CTx) sway, i.e., the total time during which the spectral power of the body in a given frequency band was canceled by the posture control mechanisms. The longer the CT for a frequency band, the better the posture control (Dumitrescu and Lacour, 2006; Bernard-Demanze et al., 2009).
The postural instability index (PII) which quantifies the postural performance by taking into account the two PI and CT indices, was also calculated (Dumitrescu and Lacour, 2006; Bernard-Demanze et al., 2009) as following:
PII = ΣxΣyPI(F1, F2, F3)/CT(F1, F2, F3).
The wavelet analysis was done with the software PosturoPro (Framiral, Cannes, France).
Statistical analysis
Statistical analysis was run on individual mean values for each parameter. For the Romberg experiment, a two-way ANOVA was run. The factors were the group (tinnitus versus controls) and the vision condition (eyes open versus eyes closed). For the second experiment, a two-way ANOVA was done. The factors were the group (left or left dominant versus right or right dominant tinnitus) and the gaze position (center, lateral gaze to tinnitus, and non-tinnitus side). Post hoc analysis was made with the Fischer's PLSD test. The factors effect was significant when the associated p value was below to 0.05.
Results
Romberg test: eye open/eyes closed
Results are shown in the Table 2. During the experiment, 10 of the subjects with tinnitus reported hearing the sound. The intensity of their tinnitus was mildly higher under the eyes closed condition (2.4 ± 3.1) than under the eyes open condition (1.8 ± 2.2).
Table 2.
Group mean values for each parameter of posturography for the control and tinnitus groups (16) matched for sex and age.
| Eyes open | Eyes closed | |
|---|---|---|
| Tinnitus intensity | 1.8 ± 2.2 | 2.4 ± 3.1 |
| Surface | ||
| Tinnitus | 81 ± 66 | 202 ± 176 |
| Controls | 97 ± 98 | 167 ± 123 |
| SDx | ||
| Tinnitus* | 2.1 ± 0.6 | 3.1 ± 1.3 |
| Controls | 1.7 ± 1.1 | 2.4 ± 1.1 |
| SDy | ||
| Tinnitus | 3.9 ± 1.7 | 5.2 ± 2.7 |
| Controls | 3.6 ± 1.2 | 4.8 ± 1.8 |
| Speed variance | ||
| Tinnitus | 22 ± 13 | 61 ± 31 |
| Controls | 28 ± 22 | 81 ± 130 |
| FFTx 0–0.5 Hz | ||
| Tinnitus | 5.8 ± 1.9 | 10.0 ± 5.9 |
| Controls | 4.8 ± 2.4 | 6.5 ± 3.0 |
| 0.5–2 Hz | ||
| Tinnitus* | 3.1 ± 1.3 | 4.9 ± 2.4 |
| Controls | 2.5 ± 1.0 | 3.3 ± 1.4 |
| >2 Hz | ||
| Tinnitus | 1.7 ± 0.4 | 2.5 ± 0.7 |
| Controls | 1.7 ± 0.7 | 2.1 ± 0.9 |
| Total | ||
| Tinnitus* | 10.6 ± 3.3 | 17.4 ± 8.5 |
| Controls | 8.9 ± 3.4 | 11.9 ± 4.6 |
| FFTy 0–0.5 Hz | ||
| Tinnitus | 11.4 ± 6.1 | 17.1 ± 9.4 |
| Controls | 9.2 ± 2.8 | 12.0 ± 3.0 |
| 0.5–2 Hz | ||
| Tinnitus | 4.5 ± 1.4 | 8.0 ± 3.3 |
| Controls | 3.8 ± 1.0 | 6.7 ± 2.7 |
| >2 Hz | ||
| Tinnitus | 2.0 ± 0.5 | 3.4 ± 1.0 |
| Controls | 2.5 ± 1.0 | 3.3 ± 1.0 |
| Total | ||
| Tinnitus | 17.8 ± 7.0 | 28.6 ± 12.1 |
| Controls | 15.6 ± 3.5 | 22.0 ± 3.9 |
| Wavelets PII | ||
| Tinnitus | 0.9 ± 0.4 | 1.6 ± 0.5 |
| Controls | 0.8 ± 0.5 | 1.4 ± 0.5 |
| Wavelets CTx 0–0.5 Hz | ||
| Tinnitus | 2.2 ± 1.7 | 1.6 ± 2.6 |
| Controls | 3.1 ± 2.4 | 1.9 ± 1.6 |
| 0.5–1.5 Hz | ||
| Tinnitus | 0.5 ± 0.4 | 0.8 ± 0.6 |
| Controls | 0.3 ± 0.5 | 0.5 ± 0.6 |
| >1.5 Hz | ||
| Tinnitus | 0 ± 0 | 0 ± 0 |
| Controls | 0 ± 0 | 0 ± 0 |
| Wavelets CTy 0–0.5 Hz | ||
| Tinnitus | 1.2 ± 0.9 | 0.4 ± 0.3 |
| Controls | 1.3 ± 0.5 | 0.6 ± 0.3 |
| 0.5–1.5 Hz | ||
| Tinnitus | 0.6 ± 0.6 | 1.2 ± 0.7 |
| Controls | 0.8 ± 0.6 | 1.2 ± 0.6 |
| >1.5 Hz | ||
| Tinnitus | 0 ± 0 | 0 ± 0 |
| Controls | 0 ± 0 | 0 ± 0 |
| Wavelets Px 0–0.5 Hz | ||
| Tinnitus* | 57.5 ± 5.7 | 64.9 ± 7.9 |
| Controls | 52.8 ± 6.9 | 59.1 ± 7.0 |
| 0.5–1.5 Hz | ||
| Tinnitus* | 47.2± 4.4 | 53.4 ± 6.5 |
| Controls | 43.3 ± 6.0 | 48.0 ± 6.8 |
| >1.5 Hz | ||
| Tinnitus * | 28.8± 5.3 | 35.5 ± 5.7 |
| Controls | 24.7 ± 7.2 | 29.1 ± 7.5 |
| Wavelets Py 0–0.5 Hz | ||
| Tinnitus | 67.6 ± 7.4 | 74.0 ± 7.4 |
| Controls | 64.9 ± 5.3 | 71.4 ± 5.7 |
| 0.5–1.5 Hz | ||
| Tinnitus | 55.6 ± 5.4 | 62.0 ± 6.4 |
| Controls | 53.6 ± 4.4 | 59.5 ± 5.0 |
| >1.5 Hz | ||
| Tinnitus | 36.8 ± 5.7 | 44.8 ± 5.9 |
| Controls | 36.2 ± 5.3 | 42.5 ± 5.7 |
All comparisons between eyes open and eyes closed are statistically significant (p at <0.05). Asterisks indicate the parameters for which significant group effect was observed (p < 0.05); values were higher for the tinnitus group.
The ANOVA run on posture measures showed that all basic parameters are higher under the eyes closed condition than under the eyes open conditions and indeed all differences were significant (p < 0.05, in Table 2).
The ANOVA run on FFT data showed that all postural parameters are significantly higher under the eyes closed condition than under the eyes open condition (p < 0.05). The ANOVA run on data for the wavelet analysis showed that the PII and the power spectrum of the antero-posterior (PIy) and medio-lateral sway (PIx) for all frequency bands were significantly higher under the eyes closed condition than under the eyes open condition. There was also a significant effect on the cancelling time of the antero-posterior (CTy) and medio-lateral sway (CTx). However, the effect varied according to the frequency band. The CTy and CTx were higher under the eyes open condition than under the eyes closed condition for the frequency band 0–0.5 Hz and the opposite result was observed for the frequency bands 0.5–2 Hz as well as frequency bands higher than 2 Hz.
The interaction between the group versus tinnitus and the two conditions (eyes open and eyes closed) was significant for the power spectrum of FFTx for high frequency (>2 Hz); The FFTx was only significantly higher for tinnitus in the eyes closed condition (Figure 1). Thus, with the exception of the FFTx >2 Hz, tinnitus patients behave normally in the Romberg test, i.e., they showed an increase in postural instability under the eyes closed condition by a similar amount as controls.
Figure 1.
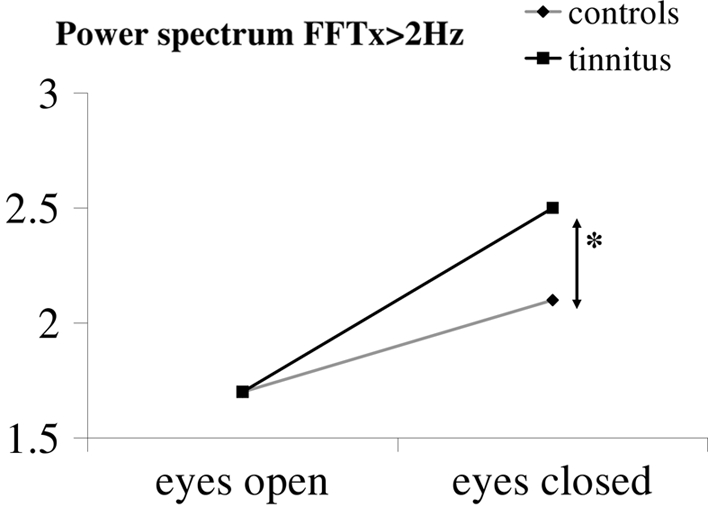
Interaction between group and vision condition. The power index for high frequencies of the medio-lateral body sway is only significantly higher in tinnitus patients under the eyes closed condition.
Group effects
There was a main effect of the group only on the SDx [F(1,28) = 6.76; p < 0.0148]. Subjects with tinnitus showed higher SDx than the controls (see asterisks in Table 2).
There was a main effect of the group on the FFTx for the whole frequency band [F(1,28) = 4.6; p < 0.041] and for the 0.5- to 2-Hz frequency band [F(1,28) = 4.7; p < 0.038]. The values from these parameters were significantly higher for the tinnitus group than for controls (see asterisks in Table 2).
For the wavelet analysis, there was a significant effect for the group on the power spectrum of the medio-lateral sway (PIx) for all frequency bands (F(1,28) = 6.9; p < 0.013) for the 0- to 0.5-Hz band; (F(1,28) = 6.6; p < 0.016) for the 05- to 1.5-Hz band and (F(1,28) = 6.0; p < 0.021) for the >1.5 Hz band. Post hoc analysis revealed higher values for these parameters in tinnitus subjects than in controls (see asterisks in Table 2).
Tinnitus versus non-tinnitus side
Recall that for this analysis, we regrouped the patients shown in Table 1 as follows: one group contained unilateral left and bilateral but left dominant tinnitus (e.g., 13 patients), the other group contained right tinnitus and bilateral but right dominant tinnitus (e.g., 8 patients). Note that this classification is based on the clinical examination based on every day life, not on what patients reported during our laboratory testing.
Posture results are shown in Table 3. Subjects with left tinnitus evaluated their tinnitus intensity as being mildly higher for the lateral gaze condition (3.2 ± 2.6 for the tinnitus side condition, and 3.2 ± 2.7 for the non-tinnitus side condition) than for the center gaze condition (2.4 ± 2.1). In contrast, subjects with right tinnitus rated their tinnitus intensity as being lower for the lateral gaze condition (3.8 ± 3.4 for the tinnitus and non-tinnitus side conditions) than for the straight ahead gaze condition (4.3 ± 3.0).
Table 3.
Group mean values for each parameter of posturography according to gaze position.
| Center gaze | Non-tinnitus side | Tinnitus side | |
|---|---|---|---|
| Tinnitus intensity | |||
| Left | 2.4 ± 2.1 | 3.2 ± 2.7 | 3.2 ± 2.6 |
| Right | 4.3 ± 3.0 | 3.8 ± 3.4 | 3.8 ± 3.4 |
| Surface | |||
| Left | 70 ± 64 | 139 ± 209 | 171 ± 229 |
| Right | 97 ± 59 | 110 ± 100 | 86 ± 53 |
| SDx | |||
| Left | 2.0 ± 0.6 | 2.8 ± 1.8 | 3.0 ± 1.5 |
| Right | 2.8 ± 1.8 | 2.5 ± 1.0 | 2.1 ± 1.1 |
| SDy | |||
| Left | 3.2 ± 1.3 | 4.1 ± 2.5 | 4.8 ± 4.0 |
| Right | 4.3 ± 1.4 | 4.1 ± 1.2 | 4.0 ± 1.1 |
| Speed variance | |||
| Left | 21 ± 14 | 18 ± 11 | 20 ± 14 |
| Right | 25 ± 18 | 22 ± 12 | 20 ± 8 |
| Limb load asymmetry | |||
| Left | −3.1 ± 14.8 | 1.4 ± 13.8 | −1.4 ± 10.1 |
| Right | −1.6 ± 4.8 | 3.1 ± 7.6 | −2.4 ± 7.0 |
| FFTx 0–0.5 Hz | |||
| Left | 5.9 ± 2.2 | 6.7 ± 2.9 | 7.4 ± 4.2 |
| Right | 7.3 ± 4.2 | 6.8 ± 3.4 | 6.0 ± 3.3 |
| 0.5–2 Hz | |||
| Left | 3.2 ± 1.5 | 2.6 ± 1.1 | 2.8 ± 1.0 |
| Right | 3.3 ± 0.6 | 3.0 ± 0.9 | 3.3 ± 0.7 |
| >2 Hz | |||
| Left | 1.8 ± 0.5 | 1.7 ± 0.6 | 1.7 ± 0.4 |
| Right | 1.9 ± 0.3 | 1.9 ± 0.4 | 1.9 ± 0.5 |
| Total | |||
| Left | 10.9 ± 3.9 | 11.1 ± 3.7 | 12.0 ± 5.0 |
| Right | 12.5 ± 4.1 | 11.7 ± 4.3 | 11.2 ± 3.0 |
| FFTy 0–0.5 Hz | |||
| Left | 9.5 ± 4.3 | 10.0 ± 4.1 | 10.3 ± 5.2 |
| Right | 11.2 ± 3.0 | 11.2 ± 6.8 | 9.5 ± 3.4 |
| 0.5–2 Hz | |||
| Left | 4.0 ± 1.5 | 3.9 ± 1.5 | 4.1 ± 1.2 |
| Right | 5.4 ± 2.9 | 4.2 ± 1.0 | 4.4 ± 1.8 |
| >2 Hz | |||
| Left | 1.9 ± 0.5 | 2.1 ± 0.8 | 2.0 ± 0.7 |
| Right | 2.2 ± 0.5 | 2.1 ± 0.3 | 2.1 ± 0.5 |
| Total | |||
| Left | 15.2 ± 6.7 | 16 ± 5.5 | 16.4 ± 6.4 |
| Right | 18.8 ± 3.3 | 17.4 ± 7.5 | 16.1 ± 4.2 |
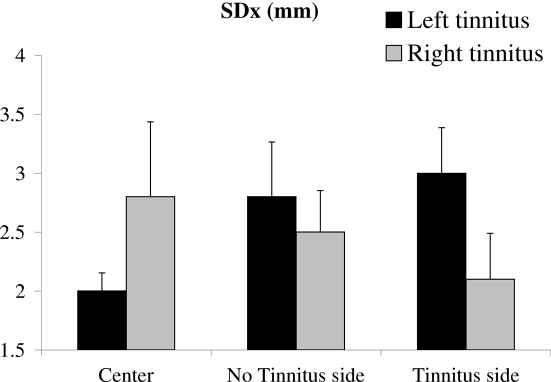
No main gaze position effect was evident. The gaze position factor interacted with the type of tinnitus results shown in Figure 2).
There was a significant interaction between the group and the gaze position on the SDx (F(2,38) = 3.36; p < 0.045). For subjects with left tinnitus (bilateral or unilateral), SDx was significantly higher under lateral gaze conditions (tinnitus or non-tinnitus side) as compared with the center gaze condition. In contrast, no significant difference between the three conditions was observed for subjects with right tinnitus. The results are illustrated in Figure 2.
Figure 2.
Significant interaction between gaze position and subgroup of tinnitus subjects: for subjects with left tinnitus only (as determined in text) medio-lateral body sway is optimal for the straight ahead gaze position but deteriorated significantly when looking away, on either side. For right tinnitus gaze position modulation is not significant.
Discussion
Perceived tinnitus
In this paper we study subjects with somatic tinnitus who spontaneously noticed a modulation of their subjective tinnitus perception in pitch or intensity after any kind of movement or muscular exercise or muscular pressure. Tinnitus is sometimes perceived only in very quiet situations (Tucker et al., 2005; Knobel and Sanchez, 2008) or when attention is focused on tinnitus. As our posture experiments have been carried out in a relatively noisy setting (e.g., computers, investigators…) not all subjects were able to hear their tinnitus during our testing. Yet, it should be emphasized that all patients had a chronic history of tinnitus, with a duration of more than 1 year, the mean duration being 5.6 years. Those individuals (10/23) who constantly heard their tinnitus were asked to evaluate the intensity of tinnitus during our tasks. We observed that the intensity of tinnitus was much greater when the eyes were closed than when the eyes were left open. Some modulation of perceived tinnitus intensity was observed with gaze direction even though it was not statistically significant. It therefore follows that differences in the perceived intensity of tinnitus might possibly be subtended by attention mechanisms.
Posturography
Instability with eyes closed (Romberg test)
For both groups of subjects almost all postural parameters were significantly lower for the eyes open condition as opposed to the eyes closed condition. Such results confirm that vision and related oculomotor signals provide better stability in tinnitus subjects, as is the case for controls (Kapoula and Le, 2006).
A significant interaction between the group and the vision (eyes open, eyes closed) was observed for the FFTx only. The values for this parameter were particularly high for the eyes closed condition in the tinnitus subjects. Note that the interaction was significant for the power spectrum of high frequencies (>2 Hz) which are believed to be related to reflexive loop, e.g., spinal cord involvement (Paillard et al., 2002).
Increased lateral body sway in tinnitus (regardless of vision conditions)
The key finding here is that tinnitus patients show a specific deficit in lateral body sway only. Before we discuss this we should highlight the absence of overall postural deficits in such patients affecting other parameters, particularly the surface. This indicates appropriate recruitment of patients excluding cases with acute vestibular syndrome. Importantly, we found significant difference between tinnitus and controls for most of the parameters concerning the medio-lateral sway data (SDx, FFTx, and the Px). One could argue that the results are biased by the two patients with acoustic neuroma who underwent surgery 13 and 6 years ago (patients 9, 18; see Table 1). Statistical analysis without these two patients provides identical results. Therefore, the effect on lateral body sway concerns the group of patients. The specificity of the effect on the medio-lateral sway argues against the simple causal role of attention, fatigue or other confounding factors. Moreover, for the open eyes condition, the power spectrum for all frequency bands (low, medium, high) was positively correlated with the duration of the tinnitus; the Pearson correlation coefficient was 0.48, 0.51, and 0.48, respectively, all significant at p < 0.05.
The specificity of deficit along the medio-lateral axis could be related to the tinnitus itself. Our results are congruent with studies reporting an effect of sound stimulation on postural control and particularly on medio-lateral sway (Tanaka et al., 2001; Priplata et al., 2002; Deviterne et al., 2005; Alessandrini et al., 2006; Dozza et al., 2007). According to Alessadrini et al. (2006), sound stimulation could activate the vestibular system leading to lower limb reflex and body shift. We hypothesize that tinnitus may act similarly. As tinnitus was not always perceived at the time of our laboratory testing we imply that the hypothetical action of tinnitus as a source of sound stimulation constitutes a chronic stimulation of the neural system, i.e., an auditory distractor. A comparison of the postural performance of the subgroup of patients who heard their tinnitus with those who did not showed no significant difference; this is in line with the idea of chronic stimulation. Alternatively, poor lateral stability in tinnitus subjects could be related either to infra-clinic peripheral abnormality, e.g., vestibular receptor (Borel et al., 2002; Mbongo et al., 2005), or to a subtle central dysfunction including the cerebellar loop integrating vestibular somatosensory and auditory signals.
Gaze specific – posture instability in left tinnitus only
First one should note that the majority of patients were right handed and consequently we did not have a sufficient number of left handed patients in order to make a comparison. Eye dominance was determined by asking the subject to fixate a target via a hole, initially binocularly and then closing each eye alternatively; the eye for which the target was seen was the dominant eye. About half of the patients were right eye dominant (12/23). There was no difference, however, between the two groups in terms of their postural stability (all p > 0.05). Sixty-seven percent of the patients with left or left dominant tinnitus were right eye dominant, while among the patients with right or right dominant tinnitus only 50% were right eye dominant. Further studies with larger groups of patients are necessary in order to test how eye dominance and the tinnitus side might possibly be related if at all. Eye dominance is itself a complex concept, and the distinction between sensory–visual and motor dominance remains poorly understood (Mapp et al., 2003; Matheron et al., 2008). In what follows we will discuss gaze specificity in posture according to the laterality of tinnitus.
Lateral gaze (either to the tinnitus side or to the non-tinnitus side) deteriorated postural stability comparing with the straight ahead gaze position but only in subjects with left or left dominant tinnitus. In contrast, in subjects with right tinnitus, posturographic values did not significantly vary between the three gaze conditions. The results suggest that the modulation of both tinnitus intensity and postural stability by gaze position exists particularly in subjects with left tinnitus. The question is why such gaze position effects are present for left tinnitus only. Neuroanatomical differences and/or differences in terms of neural projections between the left and the right auditory cortex and oculomotor areas controlling eye position might explain our results. A behavioral study of healthy subjects from Lewald and Ehrenstein (2001) supports this hypothesis: the authors found a significant difference in sound localization error between the left gaze condition (error of 1.84°) and the right gaze condition (error of 0.61°). They explained this difference in terms of the relation between the spatial sound localization and the right posterior parietal cortex (rPPC); also patients with lesions of the rPPC showed a systematic shift in sound localization to the left side.
The primary straight ahead gaze position is known to be a privileged position even for controls, for instance, saccades to such position are more accurate (Kapoula and Robinson, 1986). As shown in Figure 2 postural control is rather optimal for such central gaze position. Gaze dependent postural instability in left tinnitus could reflect abnormal integrating processes in eye positions, vestibular, somatosensory, and vestibular visual cues when gazing away from the center.
Conclusion
All patients studied here had history of chronic tinnitus modulated by movements. Such patients present abnormal medio-lateral body sway in quiet stance. Moreover, in patients with left tinnitus only, posture stability deteriorates when patients fixate away from the center. The straight ahead gaze position might be the landmark for posture control, any deviation from this could adversely affect posture as a consequence perhaps of abnormal connections between auditory and oculomotor areas in the right cortex. The influence of tinnitus on postural control might be mediated via activation of the vestibular system as suggested for the case of external sound stimulation by Alessandrini et al. (2006). Future studies are needed to identify the respective role of peripheral versus central system dysfunction on the postural control in tinnitus. Also, supra-postural double tasks combining posture and attention such as those employed by Riley et al. (1999) and McNevin and Wulf (2002) would be of interest.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Gabi Lipede for improving the English.
References
- Alessandrini M., Lanciani R., Bruno E., Napolitano B., Di Girolamo S. (2006). Posturography frequency analysis of sound-evoked body sway in normal subjects. Eur. Arch. Otorhinolaryngol. 263, 248–252 10.1007/s00405-005-0965-7 [DOI] [PubMed] [Google Scholar]
- Amiridis I. G., Hatzitaki V., Arabatzi F. (2003). Age-induced modifications of static postural control in humans. Neurosci. Lett. 350, 137–140 [DOI] [PubMed] [Google Scholar]
- Andersson G., Westin V. (2008). Understanding tinnitus distress: introducing the concepts of moderators and mediators. Int. J. Audiol. 47(Suppl. 2), S106–S111 [DOI] [PubMed] [Google Scholar]
- Bernard-Demanze L., Dumitrescu M., Jimeno P., Borel L., Lacour M. (2009). Age-related changes in posture control are differentially affected by postural and cognitive task complexity. Curr. Aging Sci. 2, 139–149 [PubMed] [Google Scholar]
- Borel L., Harlay F., Magnan J., Chays A., Lacour M. (2002). Deficits and recovery of head and trunk orientation and stabilization after unilateral vestibular loss. Brain 125, 880–894 10.1093/brain/awf085 [DOI] [PubMed] [Google Scholar]
- Brandt T. (1999). Vertigo: Its Multisensory Syndromes. London: Springer [Google Scholar]
- Day B. L., Steiger M. J., Thompson P. D., Marsden C. D. (1993). Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J. Physiol. (Lond.) 469, 479–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Flicio C. M., Melchior Mde O., Ferreira C. L., Da Silva M. A. (2008). Otologic symptoms of temporomandibular disorder and effect of orofacial myofunctional therapy. Cranio 26, 118–125 [DOI] [PubMed] [Google Scholar]
- Deviterne D., Gauchard G. C., Jamet M., Vancon G., Perrin P. P. (2005). Added cognitive load through rotary auditory stimulation can improve the quality of postural control in the elderly. Brain Res. Bull. 64, 487–492 [DOI] [PubMed] [Google Scholar]
- Dozza M., Horak F. B., Chiari L. (2007). Auditory biofeedback substitutes for loss of sensory information in maintaining stance. Exp. Brain Res. 178, 37–48 10.1007/s00221-006-0709-y [DOI] [PubMed] [Google Scholar]
- Dumitrescu M., Lacour M. (2006). “Nouveau critèresquantitatifs d'analyse du contrôle postural,” in Efficience et déficiences du contrôle postural, eds Pérennou D., Lacour M. (Marseille: Solal; ), 65–75 [Google Scholar]
- Eggermont J. J., Roberts L. E. (2004). The neuroscience of tinnitus. Trends Neurosci. 27, 676–682 10.1016/j.tins.2004.08.010 [DOI] [PubMed] [Google Scholar]
- Gagey P.M., Weber B. (2005). “Stabilométrie,” in Posturologic: régulation et dérèglements de la station debout, ed. Masson E. (Issy-les-Moulineaux: Elsevier Masson; ), 45–59 [Google Scholar]
- Gatev P., Thomas S., Kepple T., Hallett M. (1999). Feedforward ankle strategy of balance during quiet stance in adults. J. Physiol. (Lond.) 514(Pt 3), 915–928 10.1111/j.1469-7793.1999.915ad.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomer E., Dupui P., Bessou P. (1994). Spectral frequency analysis of dynamic balance in healthy and injured athletes. Arch. Int. Physiol. Biochim. Biophys. 102, 225–229 [DOI] [PubMed] [Google Scholar]
- Halford J. B., Anderson S. D. (1991). Tinnitus severity measured by a subjective scale, audiometry and clinical judgement. J. Laryngol. Otol. 105, 89–93 [DOI] [PubMed] [Google Scholar]
- Heinecke K., Weise C., Schwarz K., Rief W. (2008). Physiological and psychological stress reactivity in chronic tinnitus. J. Behav. Med. 31, 179–188 [DOI] [PubMed] [Google Scholar]
- Hesser H., Andersson G. (2009). The role of anxiety sensitivity and behavioral avoidance in tinnitus disability. Int. J. Audiol. 48, 295–299 10.1080/14992020802635325 [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J. (1990). Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res. 8, 221–254 [DOI] [PubMed] [Google Scholar]
- Jonsson E., Seiger A., Hirschfeld H. (2005). Postural steadiness and weight distribution during tandem stance in healthy young and elderly adults. Clin. Biomech. (Bristol, Avon) 20, 202–208 10.1016/j.clinbiomech.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Kapoula Z., Le T. T. (2006). Effects of distance and gaze position on postural stability in young and old subjects. Exp. Brain Res. 173, 438–445 10.1007/s00221-006-0382-1 [DOI] [PubMed] [Google Scholar]
- Kapoula Z., Robinson D. A. (1986). Saccadic undershoot is not inevitable: saccades can be accurate. Vision Res. 26, 735–743 10.1016/0042-6989(86)90087-8 [DOI] [PubMed] [Google Scholar]
- Knobel K. A., Sanchez T. G. (2008). Influence of silence and attention on tinnitus perception. Otolaryngol. Head Neck Surg. 138, 18–22 10.1016/j.otohns.2007.09.023 [DOI] [PubMed] [Google Scholar]
- Kohen-Raz R., Himmelfarb M., Tzur S., Kohen-Raz A., Shub Y. (1996). An initial evaluation of work fatigue and circadian changes as assessed by multiplate posturography. Percept. Mot. Skills 82, 547–557 [DOI] [PubMed] [Google Scholar]
- Lacour M., Bernard-Demanze L., Dumitrescu M. (2008). Posture control, aging, and attention resources: models and posture-analysis methods. Neurophysiol. Clin. 38, 411–421 [DOI] [PubMed] [Google Scholar]
- Le T. T., Kapoula Z. (2006). Distance impairs postural stability only under binocular viewing. Vision Res. 46, 3586–3593 [DOI] [PubMed] [Google Scholar]
- Le T. T., Kapoula Z. (2008). Role of ocular convergence in the Romberg quotient. Gait Posture 27, 493–500 10.1016/j.gaitpost.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Levine R. A., Abel M., Cheng H. (2003). CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp. Brain Res. 153, 643–648 10.1007/s00221-003-1747-3 [DOI] [PubMed] [Google Scholar]
- Levine R. A., Nam E. C., Oron Y., Melcher J. R. (2007). Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Prog. Brain Res. 166, 195–207 10.1016/S0079-6123(07)66017-8 [DOI] [PubMed] [Google Scholar]
- Lewald J., Ehrenstein W. H. (2001). Effect of gaze direction on sound localization in rear space. Neurosci. Res. 39, 253–257 [DOI] [PubMed] [Google Scholar]
- Mapp A. P., Ono H., Barbeito R. (2003). What does the dominant eye dominate? A brief and somewhat contentious review. Percept. Psychophys. 65, 310–317 [DOI] [PubMed] [Google Scholar]
- Matheron E., Yang Q., Le T. T., Kapoula Z. (2008). Effects of ocular dominance on the vertical vergence induced by a 2-diopter vertical prism during standing. Neurosci. Lett. 444, 176–180 [DOI] [PubMed] [Google Scholar]
- Mbongo F., Patko T., Vidal P. P., Vibert N., Tran Ba Huy P., de Waele C. (2005). Postural control in patients with unilateral vestibular lesions is more impaired in the roll than in the pitch plane: a static and dynamic posturography study. Audiol. Neurootol. 10, 291–302 10.1159/000086081 [DOI] [PubMed] [Google Scholar]
- McNevin N. H., Wulf G. (2002). Attentional focus on supra-postural tasks affects postural control. Hum. Mov. Sci. 21, 187–202 [DOI] [PubMed] [Google Scholar]
- Meric E., Chery-Croze S. (1996). Traduction et validation de l'échelle subjective de mesure de la sévérité de l'acouphène (Subjective Tinnitus Severity Scale, JBS Halford et al. 1991). J. F. ORL 45, 409–412 [Google Scholar]
- Meric E., Chery-Croze S. (1997). Translation and validation of the questionnaire “Tinnitus Handicap Questionnaire, 1990”. J. Otolaryngol. 26, 167–170 [PubMed] [Google Scholar]
- Miller M. A., Rahe R. H. (1997). Life changes scaling for the 1990s. J. Psychosom. Res. 43, 279–292 [DOI] [PubMed] [Google Scholar]
- Nashner L. M. (1979). Organization and programming of motor activity during posture control. Prog. Brain Res. 50, 177–184 10.1016/S0079-6123(08)60818-3 [DOI] [PubMed] [Google Scholar]
- Nicolas-Puel C., Faulconbridge R. L., Guitton M., Puel J. L., Mondain M., Uziel A. (2002). Characteristics of tinnitus and etiology of associated hearing loss: a study of 123 patients. Int. Tinnitus J. 8, 37–44 [PubMed] [Google Scholar]
- Paillard T., Costes-Salon C., Lafont C., Dupui P. (2002). Are there differences in postural regulation according to the level of competition in judoists? Br. J. Sports Med. 36, 304–305 10.1136/bjsm.36.4.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priplata A., Niemi J., Salen M., Harry J., Lipsitz L. A., Collins J. J. (2002). Noise-enhanced human balance control. Phys. Rev. Lett. 89, 238101. [DOI] [PubMed] [Google Scholar]
- Riley M. A., Stoffregen T. A., Grocki M. J., Turvey M. T. (1999). Postural stabilization for the control of touching. Hum. Mov. Sci. 18, 795–817 [Google Scholar]
- Shulman A. (1991). “Classification of tinnitus,” in Tinnitus, Diagnosis and Treatment, eds Shulman A., Aran A., Tonndof J., Feldman H., Vernon J. A. (Philadelphia: Lea and Febiger Publications; ), 248–252 [Google Scholar]
- Tanaka T., Kojima S., Takeda H., Ino S., Ifukube T. (2001). The influence of moving auditory stimuli on standing balance in healthy young adults and the elderly. Ergonomics 44, 1403–1412 10.1080/00140130110110601 [DOI] [PubMed] [Google Scholar]
- Tranter R. M., Graham J. R. (2009). A review of the otological aspects of whiplash injury. J. Forensic Leg. Med. 16, 53–55 10.4197/Med.16-1.5 [DOI] [PubMed] [Google Scholar]
- Tucker D. A., Phillips S. L., Ruth R. A., Clayton W. A., Royster E., Todd A. D. (2005). The effect of silence on tinnitus perception. Otolaryngol. Head Neck Surg. 132, 20–24 10.1016/j.otohns.2005.08.016 [DOI] [PubMed] [Google Scholar]
- Vio M. M., Holme R. H. (2005). Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov. Today 10, 1263–1265 [DOI] [PubMed] [Google Scholar]
- Wilson P. H., Henry J., Bowen M., Haralambous G. (1991). Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. J. Speech Hear. Res. 34, 197–201 [PubMed] [Google Scholar]
- Winter D. A., Patla A. E., Ishac M., Gage W. H. (2003). Motor mechanisms of balance during quiet standing. J. Electromyogr. Kinesiol. 13, 49–56 10.1016/S1050-6411(02)00085-8 [DOI] [PubMed] [Google Scholar]
- Winter D. A., Prince F., Frank J. S., Powell C., Zabjek K. F. (1996). Unified theory regarding A/P and M/L balance in quiet stance. J. Neurophysiol. 75, 2334–2343 [DOI] [PubMed] [Google Scholar]
- Zigmond A. S., Snaith R. P. (1983). The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 [DOI] [PubMed] [Google Scholar]