Abstract
Although cancer and neurodegenerative disease are two distinct pathological disorders, emerging evidence indicates that these two types of disease share common mechanisms of genetic and molecular abnormalities. Recent studies show that individual microRNAs (miRNAs) could be involved in the pathology of both diseases, indicating that the mechanisms of these two seemingly dichotomous diseases converge in the dysregulation of gene expression at the post-transcriptional level. Given the increasing evidence showing that miRNA-based therapeutic strategies that modulate the activity of one or more miRNAs are potentially effective for a wide range of pathological conditions, the involvement of miRNAs in the common pathways of leading both diseases suggests a bright future for developing common therapeutic approaches for both diseases. Moreover, the miRNAs that are dysregulated in both diseases may hold promise as uniquely informative diagnostic markers. Here, we review recent studies on the miRNAs that have been implicated in both cancer and neurodegenerative diseases.
Keywords: microRNA, cancer, neurodegenerative disease
Introduction
Two of the most clinically problematic classes of disease impacting the world's aging populations are cancer and neurodegenerative disorders. Although there are stark differences between cancer cells and neurons, with the former dividing rapidly and the latter relatively quiescent and non-replicating, a growing body of evidence supports common genetic mechanisms involved in dysregulated cancer cell growth and the progression of neurodegenerative disease. Mutations in a variety of genes involved in regulation of the cell cycle, DNA repair pathways, protein turnover, oxidative stress, and autophagy have been implicated in both of these otherwise dichotomous diseases (Morris et al., 2010). More recently, changes in microRNA (miRNA)-based regulation have also emerged as potential regulators of both cancer and neurodegenerative pathologies (Cooper et al., 2009). In particular, individual miRNAs have been implicated in the initiation and progression of both malignant neoplasias and neurodegenerative conditions by either regulating common pathways associated with both diseases or by targeting genes specific to each disease.
miRNAs are a class of short, non-coding RNAs that are endogenously expressed to regulate the expression of many genes involved in various biological functions. These regulatory molecules bind to sequences in the 3′ untranslated regions of expressed mRNAs, resulting primarily in degradation of the mRNA transcript or repression of protein translation (Fabian et al., 2010). The specificity of miRNA targets is determined primarily by a short, 7-nucleotide ‘seed sequence’ allowing for both a wide range of targets for particular miRNAs and multiple miRNAs targeting a single mRNA transcript. Studies over the past decade have demonstrated that dysregulation of miRNA expression plays important roles in the pathogenesis of various diseases. miRNAs have been intensively investigated as potential diagnostic markers and therapeutic targets and show great promise as the next generation of diagnostic and therapeutic tools. The convergence of the pathogenesis of cancer and neurodegenerative diseases at the level of miRNA regulation therefore suggests a potential common therapeutic and diagnostic strategy for the two diseases. Here, we review recent studies on the miRNAs that have been implicated in both cancer and neurodegenerative diseases.
miRNAs and common pathways in cancer and neurodegenerative disease
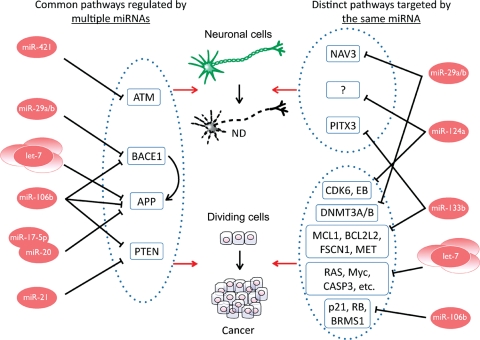
Genes representing distinct pathways in modulating cellular processes, including amyloid precursor protein (APP), ataxia-telangiectasia mutated (ATM), phosphatase and tensin homolog (PTEN), parkinson protein 2 E3 ubiquitin protein ligase (PARK2), protein tyrosine phosphatase delta, β-secretase (BACE1), and mammalian target of rapamycin (mTOR), are involved in the pathogenesis of both cancer and neurodegenerative diseases (Morris et al., 2010). Some of these genes have been demonstrated to be targets of miRNA regulation, which in turn suggests that these miRNAs are also involved in the pathogenesis of both diseases (Figure 1).
Figure 1.
Summary of the common pathways underlying neurodegenerative diseases and cancer that are mediated by miRNAs. An individual miRNA can be involved in the development of both diseases by two mechanisms. A miRNA can target a gene that represents a common cellular pathway that leads to neurodegeneration and uncontrolled tumor cell growth. An individual miRNA can play roles in controlling both neurodegeneration and tumorigenesis by targeting disparate pathways that are specific for each pathogenic process. ‘?’ indicates that direct targets have not been validated.
miRNAs have been implicated as potential regulators of APP, which is related to both Alzheimer's disease (AD) and malignant growth. APP is an integral membrane protein, increased expression of which has been associated with AD in several studies (Podlisny et al., 1987; Rovelet-Lecrux et al., 2006; Theuns et al., 2006; LaFerla et al., 2007), with evidence showing that APP is concentrated at neuronal synapses and is the primary component of AD-associated amyloid plaques following proteolysis. In the context of somatic cells, APP has been shown to increase epithelial cell proliferation and migration, although the mechanism has not been fully defined (Schmitz et al., 2002). In addition, APP has been reported to be over-expressed in various cancers, including oral cavity, esophageal, pancreatic, neuroendocrine, thyroid, and colorectal cancers (Hansel et al., 2003; Ko et al., 2004; Arvidsson et al., 2008; Krause et al., 2008). These results suggest the potential role of APP in cancer pathogenesis. Several miRNAs, including miR-17-5p, miR-20a, and miR-106b, were shown to regulate APP in vitro through reduction of endogenous APP expression following transient transfection in HeLa cells (Hebert et al., 2009), and the same study reported a significant decrease in miR-106b expression in brain tissue of AD patients compared with controls. The let-7 family miRNAs have also been shown to repress expression of the APP ortholog in Caenorhabditis elegans, APL-1, providing in vivo evidence of APP regulation by let-7 (Niwa et al., 2008). In addition to the above, miR-29a/b-1 is believed to be involved in indirectly regulating APP function by directly targeting BACE1/β-secretase. BACE1 is an aspartic acid protease potentially important to the pathophysiology of AD (Cole and Vassar, 2007), with evidence suggesting that elevated expression of BACE1 can result in inappropriate cleavage of APP and an increased load of amyloid β-peptides in patients with sporadic AD (Li et al., 2004). A recent study showed that the miR-29a/b-1 cluster down-regulates BACE1 expression in vitro, that delivery of miR-29a and miR-29b-1 in cell culture reduces the formation of amyloid β-peptides in a reversible manner, and that levels of these miRNAs are significantly reduced in AD patients with dementia who express abnormally high levels of BACE1 (Hebert et al., 2008). The above data therefore suggest that aberrant expression of these miRNAs could play an important role in the development of both cancer and AD by modulating expression of APP.
Ataxia-telangiectasia (AT) is a neurodegenerative disorder caused by mutations of either ATM or ataxia-telangiectasia and Rad3 related (ATR) protein kinases. ATM and ATR together act as critical regulators of double-strand break DNA repair by phosphorylating crucial protein substrates involved in downstream signaling to large DNA-repair networks (Matsuoka et al., 2007). In somatic cells, ATM and ATR act as tumor suppressor genes, with activation of ATM and ATR inducing apoptosis and cell-cycle arrest (Morris et al., 2010). Mutations in ATM and ATR have been identified in several types of tumor, with about 40% of patients homozygous for ATM mutations developing cancer (Morris et al., 2010). These data suggest that ATM and ATR play a critical role in tumorigenesis as well. Recent studies show that the ATM protein is a regulatory target of miR-421 (Hu et al., 2010b), and miR-421 over-expression has been demonstrated in both gastric cancer tissues and a diffuse large B-cell lymphoma cell line (Lawrie et al., 2008; Jiang et al., 2010). The regulatory effect of miR-421 on ATM expression suggests that this miRNA may be involved in the pathogenesis of both AT and cancer.
The tumor suppressor PTEN inhibits activation of the PI3K/Akt/mTOR signaling pathway which otherwise leads to enhanced cell survival and growth in a number of human cancers (Wong et al., 2010). PTEN signaling has also been implicated in the development of Parkinson's disease (PD) through activation of two genes involved in neural protection from oxidative stress, PTEN-induced putative kinase (PINK1), and parkinson disease 7 (PARK7, also known as DJ-1) (Morris et al., 2010). And PTEN is a regulatory target of miRNAs—specifically, miRNA-mediated down-regulation of PTEN through the activity of miR-21 is well established in the literature, and significant increases in miR-21 levels have been observed in a number of human tumors (Meng et al., 2007; Zhang et al., 2010). Another study has demonstrated a causal relationship between miR-106b and down-regulation of PTEN (Poliseno et al., 2010). These data suggest that aberrant expression of these two miRNAs could be involved in both neurodegeneration and cancer pathologies through down-regulating expression of PTEN.
The above studies indicate that some of the common pathways involved in both cancer and neurodegenerative diseases are targets of miRNAs. Other common pathways, such as the PARK2 cell-cycle regulatory pathway, which is known to play an important role in the development of autosomal recessive early onset PD and involves many genes including cyclin E, c-Jun, and β-catenin, have not been investigated as regulatory targets of miRNAs. A preliminary examination based on miRmate (Du et al., 2009) and other publicly available target prediction methods shows that many of these genes, including PARK2, CDK2, and E2F1, that are involved in both diseases are potential targets of multiple miRNAs. The role of miRNAs in regulating these genes and in the initiation and progression of both diseases certainly warrants further investigation.
miRNA regulation of pathways unique to cancer or neurodegenerative disease
While the above data show an individual miRNA can be involved in the development of both cancer and neurodegenerative diseases by targeting genes that represent common pathways for both diseases, other studies have shown that a single miRNA can also be involved in both cancer and neurodegenerative disorders by targeting disparate pathways, each associated with one pathology or the other (Figure 1). For example, a study by Kim et al. (2007) has suggested that miR-133b may play a role in PD by suppressing the expression of the PITX3 transcription factor. They showed that miR-133b functions in a negative feedback loop by suppressing the expression of PITX3, which in turn increases expression of miR-133b to regulate the expression of PITX3 in midbrain dopaminergic neurons (DNs). Additional data show that miR-133b expression is significantly decreased in midbrain tissue from PD patients relative to normal controls, further supporting the relevance of miR-133b in the pathogenesis of PD (Kim et al., 2007; Parent and Parent, 2010). In the context of tumorigenesis, miR-133b has been shown to be down-regulated in a number of tumor types, including esophageal, lung, and colon cancers (Bandres et al., 2006; Crawford et al., 2009; Hu et al., 2010a; Kano et al., 2010). Reported targets of miR-133b in cancer vary, and include genes involved in pro-survival signals such as myeloid cell leukemia sequence 1 (MCL1, also known as BCL2L3) and BCL2-like 2 (BCL2L2) (Crawford et al., 2009), oncogenic actin-binding factors like fascin homolog 1 (FSCN1) (Kano et al., 2010), and the met proto-oncogene receptor tyrosine kinase (MET) (Hu et al., 2010a). These data suggest that miR-133b may be involved in the pathology of both neurodegeneration and cancer by targeting distinct pathways that are specific for each disease. Another miRNA, miR-124a has been related to tumorigenesis through its modulation of the expression of the cyclin-dependent kinase 6 (CDK6) oncogene and the retinoblastoma (RB) tumor suppressor gene (Lujambio et al., 2007). In HeLa cells, over-expression of miR-124a was also shown to induce a neuron-specific expression profile (Lim et al., 2005), and nearly one-fifth of the down-regulated genes identified were also deregulated in DNs isolated from affected areas of the brains of patients with idiopathic PD (Simunovic et al., 2009). Another study identified 27 validated targets of miR-124a, eight of which were deregulated in PD neurons (Karginov et al., 2007; Simunovic et al., 2009). Results from these studies have been reported to correlate well with results from the MIRECORDS database, which shows that 24% of validated miR-124 targets are alternatively regulated in PD (http://mirecords.umn.edu) (Sonntag, 2010). Although the role of each of the miR-124/target interactions in the development of PD needs further investigation, these data highlight the varied roles that miR-124a may play in the pathologies of both cancer and neurodegenerative disorders.
In addition to the miRNAs that target the distinct pathways specific for each disease, the fact that one miRNA has many targets likely places some miRNAs in more complicated biological networks capable of regulating the pathogenic process of both diseases (Figure 1). For example, in addition to targeting BACE1, a gene that plays roles in both diseases, studies in lung cancer and acute myelogenous leukemia have shown that miR-29 family members directly target DNA methyltransferases DNMT3A and DNMT3B (Fabbri et al., 2007; Garzon et al., 2009), which are known to play an important role in cancer development by controlling telomere length and recombination in mammalian cells. Increasing miR-29 levels restored both normal methylation patterns and tumor suppressor gene expression in lung cancer cell lines, and inhibited tumorigenicity both in vitro and in a mouse model (Fabbri et al., 2007). Another study identified neuron navigator 3 (NAV3) as the principal target of miR-29a in the context of AD (Shioya et al., 2010). Members of the miR-29 family of miRNAs may therefore target multiple proteins involved in both neurodegenerative diseases and cancers by both targeting common pathways for these two diseases and regulating additional pathways that are specific for each disease. Both the let-7 and miR-106b families of miRNAs have been identified as regulators of APP; these miRNAs have also been indicated to target additional genes involved in cancer development. The let-7 family of miRNAs is widely considered to act as tumor suppressors in many types of cancer through their regulatory effects on a variety of target proteins (Johnson et al., 2005; Lee and Dutta, 2007; Sampson et al., 2007; Boyerinas et al., 2010). The miR-106b family has been shown to promote cell-cycle progression and overcome chemotherapy-induced G1-arrest through negative regulation of the cyclin-dependent kinase inhibitor 1A (p21/CDKN1A) (Ivanovska et al., 2008). More recent evidence shows that miR-106b may also be involved in promoting an invasive metastatic phenotype in breast cancer cells by targeting breast cancer metastasis suppressor 1 (BRMS1) and RB1 (Pan et al., 2009). The likelihood that each miRNA has multiple targets suggests that one that is involved in both diseases will affect multiple physiological pathways in cells. The effect of dysregulation of major pathways by aberrant expression of miRNAs relevant to both diseases certainly warrants further investigation—in order to comprehensively elucidate the mechanisms by which a single miRNA contributes to the two diseases and to evaluate the therapeutic potential of targeting these miRNAs in both contexts.
Overall, the above studies indicate that many miRNAs are involved in the pathogenesis of both neurodegenerative disease and cancer, suggesting that there is potential value in targeting the same miRNAs in the treatment of both diseases.
Diagnostic potential of miRNAs in neurodegenerative disease and cancer
While the miRNAs involved in both neurodegenerative disease and cancer have potential as therapeutic targets or agents in the treatment of both diseases, miRNAs that are differentially expressed in the two diseases relative to normal tissues may serve as valuable diagnostic markers. Many studies have investigated the potential role of miRNAs as biomarkers in distinguishing cancers from normal tissues, in distinguishing subtypes of tumors, and in predicting the prognosis of cancer patients (Yu et al., 2008; Lebanony et al., 2009; Ng et al., 2009; Du et al., 2010). Moreover, recent studies have shown that miRNAs can be released from cells into peripheral circulation and can be detected in serum, and that miRNA levels in serum change in response to the incidence of cancer (Chen et al., 2008; Mitchell et al., 2008). Studies have also investigated the potential of miRNAs as biomarkers of neurodegenerative disease. For example, Cogswell et al. (2008) identified miRNA dysregulation in the brains of AD patients, and also found AD-specific miRNA changes in cerebrospinal fluid (CSF), suggesting a non-invasive diagnostic approach based on the miRNA profile in CSF. The above studies indicate that miRNAs have significant potential as diagnostic markers for both cancer and neurodegenerative disease. The involvement of the same miRNAs in both cancer and neurodegenerative disease suggests the possibility of developing common diagnostic tools based on the miRNA expression. As an example, miR-133b is indicated to be down-regulated in midbrain tissues from PD patients (Kim et al., 2007; Parent and Parent, 2010) as well as in a number of cancer types (Bandres et al., 2006; Crawford et al., 2009; Hu et al., 2010a; Kano et al., 2010). Given that miRNAs are readily detectable in blood and other biofluids, their value as non-invasive biomarkers for both diseases certainly warrants further investigation.
Summary
Results from studies such as those discussed above highlight the multiple roles that individual miRNAs may play in the regulation of both the disparate and common pathways involved in the pathogenesis of neurodegenerative disease and malignant growth. Further investigation is clearly needed to fully understand the consequences of aberrant expression of these miRNAs and to fully characterize their roles in the initiation and progression of both diseases. The convergence of the regulatory mechanisms of these two diseases certainly suggests that such investigations will not only illuminate the relationships between the two diseases, but will also provide an experimental basis for the development of common miRNA-based therapeutic and diagnostic strategies.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (R01 CA129632).
Conflict of interest: none declared.
Acknowledgements
The authors gratefully acknowledge the technical assistance of Paul Card (Department of Internal Medicine, UT Southwestern) and thank Michael Peyton (Hamon Center for Therapeutic Oncology Research, UT Southwestern) for thoughtful insights and discussions.
References
- Arvidsson Y., Andersson E., Bergstrom A., et al. Amyloid precursor-like protein 1 is differentially upregulated in neuroendocrine tumours of the gastrointestinal tract. Endocr. Relat. Cancer. 2008;15:569–581. doi: 10.1677/ERC-07-0145. doi:10.1677/ERC-07-0145. [DOI] [PubMed] [Google Scholar]
- Bandres E., Cubedo E., Agirre X., et al. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. doi:10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyerinas B., Park S.M., Hau A., et al. The role of let-7 in cell differentiation and cancer. Endocr. Relat. Cancer. 2010;17:F19–F36. doi: 10.1677/ERC-09-0184. doi:10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- Chen X., Ba Y., Ma L., et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. doi:10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- Cogswell J.P., Ward J., Taylor I.A., et al. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J. Alzheimers Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- Cole S. L., Vassar R. The Basic Biology of BACE1: a key therapeutic target for Alzheimer's disease. Curr. Genomics. 2007;8:509–530. doi: 10.2174/138920207783769512. doi:10.2174/138920207783769512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T.A., Wan L., Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. doi:10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M., Batte K., Yu L., et al. MicroRNA 133B targets pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem. Biophys. Res. Commun. 2009;388:483–489. doi: 10.1016/j.bbrc.2009.07.143. doi:10.1016/j.bbrc.2009.07.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Schageman J.J., Subauste M.C., et al. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol. Cancer Res. 2009;7:1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. doi:10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Schageman J.J., Irnov Girard L., et al. MicroRNA expression distinguishes SCLC from NSCLC lung tumor cells and suggests a possible pathological relationship between SCLCs and NSCLCs. J. Exp. Clin. Cancer Res. 2010;29:75–80. doi: 10.1186/1756-9966-29-75. doi:10.1186/1756-9966-29-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M., Garzon R., Cimmino A., et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. doi:10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., Sonenberg N., Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. doi:10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- Garzon R., Liu S., Fabbri M., et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood. 2009;113:6411–6418. doi: 10.1182/blood-2008-07-170589. doi:10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel D.E., Rahman A., Wehner S., et al. Increased expression and processing of the Alzheimer amyloid precursor protein in pancreatic cancer may influence cellular proliferation. Cancer Res. 2003;63:7032–7037. [PubMed] [Google Scholar]
- Hebert S.S., Horre K., Nicolai L., et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. USA. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. doi:10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S.S., Horre K., Nicolai L., et al. MicroRNA regulation of Alzheimer's amyloid precursor protein expression. Neurobiol. Dis. 2009;33:422–428. doi: 10.1016/j.nbd.2008.11.009. doi:10.1016/j.nbd.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Hu G., Chen D., Li X., et al. miR-133b regulates the MET proto-oncogene and inhibits the growth of colorectal cancer cells in vitro and in vivo. Cancer Biol. Ther. 2010a;10:190–197. doi: 10.4161/cbt.10.2.12186. doi:10.4161/cbt.10.2.12186. [DOI] [PubMed] [Google Scholar]
- Hu H., Du L., Nagabayashi G., et al. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc. Natl. Acad. Sci. USA. 2010b;107:1506–1511. doi: 10.1073/pnas.0907763107. doi:10.1073/pnas.0907763107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I., Ball A.S., Diaz R.L., et al. MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol. Cell. Biol. 2008;28:2167–2174. doi: 10.1128/MCB.01977-07. doi:10.1128/MCB.01977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Guo J., Xiao B., et al. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J. Gastroenterol. 2010;45:17–23. doi: 10.1007/s00535-009-0135-6. doi:10.1007/s00535-009-0135-6. [DOI] [PubMed] [Google Scholar]
- Johnson S.M., Grosshans H., Shingara J., et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. doi:10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kano M., Seki N., Kikkawa N., et al. miR-145, miR-133a and miR-133b: tumor suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int. J. Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. doi:10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- Karginov F.V., Conaco C., Xuan Z., et al. A biochemical approach to identifying microRNA targets. Proc. Natl. Acad. Sci. USA. 2007;104:19291–19296. doi: 10.1073/pnas.0709971104. doi:10.1073/pnas.0709971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Inoue K., Ishii J., et al. A microRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. doi:10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S.Y., Lin S.C., Chang K.W., et al. Increased expression of amyloid precursor protein in oral squamous cell carcinoma. Int. J. Cancer. 2004;111:727–732. doi: 10.1002/ijc.20328. doi:10.1002/ijc.20328. [DOI] [PubMed] [Google Scholar]
- Krause K., Karger S., Sheu S.Y., et al. Evidence for a role of the amyloid precursor protein in thyroid carcinogenesis. J. Endocrinol. 2008;198:291–299. doi: 10.1677/JOE-08-0005. doi:10.1677/JOE-08-0005. [DOI] [PubMed] [Google Scholar]
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. doi:10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lawrie C.H., Saunders N.J., Soneji S., et al. MicroRNA expression in lymphocyte development and malignancy. Leukemia. 2008;22:1440–1446. doi: 10.1038/sj.leu.2405083. doi:10.1038/sj.leu.2405083. [DOI] [PubMed] [Google Scholar]
- Lebanony D., Benjamin H., Gilad S., et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J. Clin. Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. doi:10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. doi:10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Lindholm K., Yang L.B., et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc. Natl. Acad. Sci. USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. doi:10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L.P., Lau N.C., Garrett-Engele P., et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. doi:10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lujambio A., Ropero S., Ballestar E., et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. doi:10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- Matsuoka S., Ballif B.A., Smogorzewska A., et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. doi:10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- Meng F., Henson R., Wehbe-Janek H., et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. doi:10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P.S., Parkin R.K., Kroh E.M., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. doi:10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L.G., Veeriah S., Chan T.A. Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene. 2010;29:3453–3464. doi: 10.1038/onc.2010.127. doi:10.1038/onc.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E.K., Chong W.W., Jin H., et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. doi:10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- Niwa R., Zhou F., Li C., et al. The expression of the Alzheimer's amyloid precursor protein-like gene is regulated by developmental timing microRNAs and their targets in Caenorhabditis elegans. Dev. Biol. 2008;315:418–425. doi: 10.1016/j.ydbio.2007.12.044. doi:10.1016/j.ydbio.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S., Yu F., Gong C., et al. Tumor Invasion, and metastasis initiated by miR-106b in breast cancer by targeting BRMS1 and RB. Cancer Res. 2009;69 (Meeting Abstract Supplement), Abstract 6157. [Google Scholar]
- Parent M., Parent A. Substantia nigra and Parkinson's disease: a brief history of their long and intimate relationship. Can. J. Neurol. Sci. 2010;37:313–319. doi: 10.1017/s0317167100010209. [DOI] [PubMed] [Google Scholar]
- Podlisny M.B., Lee G., Selkoe D.J. Gene dosage of the amyloid beta precursor protein in Alzheimer's disease. Science. 1987;238:669–671. doi: 10.1126/science.2960019. doi:10.1126/science.2960019. [DOI] [PubMed] [Google Scholar]
- Poliseno L., Salmena L., Riccardi L., et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci. Signal. 2010;3:ra29. doi: 10.1126/scisignal.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovelet-Lecrux A., Hannequin D., Raux G., et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006;38:24–26. doi: 10.1038/ng1718. doi:10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Sampson V.B., Rong N.H., Han J., et al. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. doi:10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- Schmitz A., Tikkanen R., Kirfel G., et al. The biological role of the Alzheimer amyloid precursor protein in epithelial cells. Histochem. Cell Biol. 2002;117:171–180. doi: 10.1007/s00418-001-0351-5. doi:10.1007/s00418-001-0351-5. [DOI] [PubMed] [Google Scholar]
- Shioya M., Obayashi S., Tabunoki H., et al. Aberrant microRNA expression in the brains of neurodegenerative diseases: miR-29a decreased in Alzheimer disease brains targets neurone navigator 3. Neuropathol. Appl. Neurobiol. 2010;36:320–330. doi: 10.1111/j.1365-2990.2010.01076.x. doi:10.1111/j.1365-2990.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- Simunovic F., Yi M., Wang Y., et al. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson's disease pathology. Brain. 2009;132:1795–1809. doi: 10.1093/brain/awn323. doi:10.1093/brain/awn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag K.C. MicroRNAs and deregulated gene expression networks in neurodegeneration. Brain Res. 2010;1338:48–57. doi: 10.1016/j.brainres.2010.03.106. doi:10.1016/j.brainres.2010.03.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuns J., Brouwers N., Engelborghs S., et al. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am. J. Hum. Genet. 2006;78:936–946. doi: 10.1086/504044. doi:10.1086/504044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K.K., Engelman J.A., Cantley L.C. Targeting the PI3K signaling pathway in cancer. Curr. Opin. Genet. Dev. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. doi:10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.L., Chen H.Y., Chang G.C., et al. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. doi:10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Zhang J.G., Wang J.J., Zhao F., et al. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin. Chim. Acta. 2010;411:846–852. doi: 10.1016/j.cca.2010.02.074. doi:10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]