Abstract
Medicinal benefits of Allium vegetables, such as garlic, have been noted throughout recorded history, including protection against cancer and cardiovascular disease. We now demonstrate that garlic constituent diallyl trisulfide (DATS) increases longevity of C. elegans by affecting the skn-1 pathway. Treatment of worms with 5-10 μM DATS increased worm mean lifespan even when treatment is started during young adulthood. To explore the mechanisms involved in the DATS-mediated increase in longevity, we treated daf-2, daf-16, and eat-2 mutants and found that DATS increased the lifespan of daf-2 and daf-16 mutants, but not the eat-2 mutants. Microarray experiments demonstrated that a number of genes regulated by oxidative stress and the skn-1 transcription factor were also changed by DATS treatment. Consistently, DATS treatment leads to the induction of the skn-1 target gene gst-4, and this induction was dependent on skn-1. We also found that the effects of DATS on worm lifespan depend on skn-1 activity in both in the intestine and ASI neurons. Together our data suggest that DATS is able to increase worm lifespan by enhancing the function of the pro-longevity transcription factor skn-1.
Keywords: C. elegans, garlic, diallyl trisulfide, gst-4, aging, microarray, skn-1
1. Introduction
Health benefits of Allium vegetables, including garlic, have been reported for centuries (Rivlin, 2001). More recent systematically conducted population-based studies, animal experiments, and in vitro studies provide additional support for the long-established use of garlic and other Allium vegetables for medicinal purposes. For example, epidemiological studies have indicated that a diet rich in Allium vegetables is associated with reduced risk of gastric (You et al., 1989), colorectal (Tanaka et al., 2004), esophageal (Gao et al., 1999), and prostate cancer (Hsing et al., 2002). The cancer preventive effects of Allium vegetables are attributed to its ability to slow cancer cell proliferation, increase activity of detoxifying enzymes, and act as an antioxidant and a free radical scavenger (reviewed in (Shukla and Kalra, 2007) and (Powolny and Singh, 2008)).
Multiple studies have suggested that Allium vegetables may have beneficial effects with regards to cardiovascular disease. Specifically, recent reports indicate that garlic and its components can reduce the blood pressure in hypertensive individuals (Ried et al., 2008). Animal trials also indicate that garlic supplementation is able to reduce total serum cholesterol, LDL, triglycerides and slightly increase HDL (Ali et al., 2000; Slowing et al., 2001), and also lower homocysteine levels (Yeh and Yeh, 2006). Even though human trials have not shown as dramatic effects of garlic as those observed in animals, the overall trends indicate that garlic may be able to lower the risk of cardiovascular disease by affecting those parameters (Bordia et al., 1998; Gardner et al., 2001; Gardner et al., 2007; Zhang et al., 2001). In addition to modifying lipid profiles, garlic and its components were also shown to be an effective anti-oxidant, decrease oxidation of LDL (Lau, 2006), and attenuate peroxidation in the aortic tissue and reduce atherosclerotic plaque deposits (Durak et al., 2002). Besides having beneficial effects with regards to cancer and heart disease, Allium vegetables were also shown to reduce risk factors for other diseases associated with aging such as diabetes and various neurologic diseases (reviewed in (El-Sabban and Abouazra, 2008; Gorinstein et al., 2007; Powolny and Singh, 2008; Ried et al., 2008; Sobenin et al., 2008)). Together these studies indicate that garlic may be a useful dietary supplement for the prevention of chronic diseases.
The reported favorable effects of Allium vegetables are usually attributed to the organosulfur compounds which are released from the vegetables during cutting or chewing (Block, 1985). Allicin, which is the primary compound released from garlic, is very unstable and upon decomposition yields a variety of organosulfur compounds including diallyl sulfide, diallyl disulfide, and diallyl trisulfide (DATS). These compounds are responsible for the characteristic smells and flavors of garlic, onions, and similar Allium vegetables.
Because Allium vegetables seem to have beneficial effects in the prevention of diseases like diabetes and cancer which are strongly linked to aging in terms of prevalence, we raised the question of whether constituents of these vegetables affect aging. In the present study, we explored this possibility using the non-parasitic nematode C. elegans as a model and diallyl trisulfide (DATS) as a prototypical garlic-derived sulfur compound. Here, we report for the first time that DATS treatment using pharmacologically relevant doses increases the lifespan of C. elegans. Via microarrays and treatment of transgenic worms, we find that DATS activates parts of the oxidative stress response and that this activation requires the skn-1 transcription factor. The skn-1 gene encodes the worm homolog of the Nrf2 transcription factor, and in worms skn-1 is involved in responses to oxidative stress and dietary restriction (An and Blackwell, 2003; Bishop and Guarente, 2007). We further find that the effects of DATS on worm longevity require the action of the skn-1 transcription factor in both the intestine and ASI neurons, which are the two sites of skn-1 expression. Together our data suggest that DATS is able to increase worm lifespan by enhancing the function of the pro-longevity transcription factor skn-1. Similar mechanisms may be involved in the effects of DATS in people.
2. Experimental procedures
2.1 Strains
Caenorhabditis Elegans strains TJ1060 (spe-9(hc88); fer-15(b26)) (Fabian and Johnson, 1995), DA1113 (eat-2 (ad1113)) (Raizen et al., 1995), CF1038 (daf-16(mu86)) (Lin et al., 1997), CL2166 (dvIs19[pAF15(gst-4::GFP::NLS)]) (Link and Johnson, 2002), and TJ356 (zIs356) (Henderson and Johnson, 2001) were provided by the Caenorhabditis Genetics Center (Minneapolis, MN) which is supported by NIH funding. ALF105 (eat-2 (ad1113); dvIs19[pAF15(gst-4::GFP::NLS)]) and GL227 (daf-2 (e1371); spe-9(hc88); fer-15(b26)) were generated by standard crosses. LG333 (skn-1(zu135);Is007[skn-1::gfp]), LG335 (skn-1(zu135)/nT1[qIs51]), LG348 (skn-1(zu135)/nT1[qIs51];geIs9[gpa-4p::skn-1b::gfp]), and LG357 (skn-1(zu135)/nT1[qIs51];geIs10[ges-1p::skn-1c::gfp]) strains were a generous gift from Drs. Nicholas A. Bishop and Leonard Guarente (MIT, Cambridge, MA) (Bishop and Guarente, 2007). CL691 (dvIs19[pAF15(gst-4::GFP::NLS); skn-1(zu67) IV/nT1[uncD-?(n754); let-?]) was a generous gift from Dr. Chris Link (U. Colorado, Boulder, CO) (Rea et al., 2007).
2.2 Lifespan analyses
Lifespan analyses were performed at 20°C on duplicate or triplicate NGA plates (Fisher and Lithgow, 2006). Briefly, in order to synchronize the worm population appropriate C. elegans strains were treated with hypochlorite treatment and the resulting eggs were placed on NGA plates spotted with equal amounts of OP50-1. At day #1 of adulthood, 40 worms were transferred to two or three NGA plates containing 40 μM 5-Fluoro-2′deoxyuridine (FUdR) to inhibit the growth of progeny and spotted with OP50-1. Immediately before use these plates were freshly spotted with indicated doses of DATS (2.5 – 20 μM final concentration) dissolved in 50 μL DMSO or a similar amount of DMSO alone, as a control. Animals were scored every 2-3 days for survival by examining for touch provoked movement. Worms which did not respond to repeated touching were scored as dead. Every week worms were transferred onto plates freshly spotted with DATS and DMSO to assure that active compound is present throughout the entire experiment. The experiment was terminated when all worms were scored as dead or censored. At least two trials were performed for all genotypes, and the data shown represent one of two or more replicates with similar effects on longevity. Results from both trials are summarized in Table 1. Data was analyzed using Stata8 (Stata Corp LP, College Station, TX) and Kaplan-Meier survival curves were prepared using Graphpad Prism 5 (Graphpad Software, San Diego, CA).
Table 1.
Lifespan Data
| Genotype | Treatment | Mean | N | p-value |
|---|---|---|---|---|
| spe-9(hc88); fer-15(b26) | DMSO | 23.9 | 80 | - |
| spe-9(hc88); fer-15(b26) | 2.5 μM DATS | 25.4 | 85 | NS |
| spe-9(hc88); fer-15(b26) | 5 μM DATS | 26.7 | 81 | 0.015 |
| spe-9(hc88); fer-15(b26) | 10 μM DATS | 26.9 | 82 | 0.045 |
| spe-9(hc88); fer-15(b26) | 20 μM DATS | 23.2 | 83 | NS |
| spe-9(hc88); fer-15(b26) | DMSO | 20.2 | 120 | - |
| spe-9(hc88); fer-15(b26) | 5 μM DATS | 23.0 | 120 | <0.001 |
| spe-9(hc88); fer-15(b26) | 10 μM DATS | 21.7 | 119 | 0.035 |
| spe-9(hc88); fer-15(b26) | 20 μM DATS | 21.1 | 120 | NS |
| spe-9(hc88); fer-15(b26) | DMSO(Killed OP50) | 32.5 | 120 | - |
| spe-9(hc88); fer-15(b26) | 2.5 μM DATS | 34.5 | 120 | 0.046 |
| spe-9(hc88); fer-15(b26) | 5 μM DATS | 35.4 | 120 | 0.001 |
| spe-9(hc88); fer-15(b26) | 10 μM DATS | 34.6 | 118 | 0.029 |
| spe-9(hc88); fer-15(b26) | DMSO(Killed OP50) | 32.3 | 120 | - |
| spe-9(hc88); fer-15(b26) | 2.5 μM DATS | 31.6 | 117 | NS |
| spe-9(hc88); fer-15(b26) | 5 μM DATS | 34.1 | 121 | NS |
| spe-9(hc88); fer-15(b26) | 10 μM DATS | 35.5 | 123 | 0.009 |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | DMSO | 35.5 | 85 | - |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | 5 μM DATS | 41.9 | 80 | <0.001 |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | 10 μM DATS | 40.4 | 87 | 0.001 |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | 20 μM DATS | 37.3 | 90 | NS |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | DMSO | 35.0 | 120 | - |
| daf-2 (e1371); spe-9(hc88); fer-15(b26) | 10 μM DATS | 38.6 | 120 | 0.003 |
| daf-16(mu86) | DMSO | 14.7 | 88 | - |
| daf-16(mu86) | 10 μM DATS | 16.2 | 86 | 0.008 |
| daf-16(mu86) | DMSO | 13.4 | 120 | - |
| daf-16(mu86) | 10 μM DATS | 14.5 | 120 | 0.001 |
| eat-2 (ad1113) | DMSO | 25.7 | 136 | - |
| eat-2 (ad1113) | 10 μM DATS | 26.7 | 136 | NS |
| eat-2 (ad1113) | DMSO | 21.8 | 84 | - |
| eat-2 (ad1113) | 10 μM DATS | 21.0 | 81 | NS |
| spe-9(hc88); fer-15(b26) | DMSO | 23.9 | 120 | - |
| spe-9(hc88); fer-15(b26) | 10 μM DATS | 25.9 | 120 | <0.001 |
| skn-1(zu135) | DMSO | 14.9 | 122 | - |
| skn-1(zu135) | 10 μM DATS | 15.2 | 120 | NS |
| skn-1(zu135) | DMSO | 14.0 | 118 | - |
| skn-1(zu135) | 10 μM DATS | 14.0 | 124 | NS |
| skn-1(zu135); geIs9[gpa-4p::skn-1b::gfp] | DMSO | 20.3 | 116 | - |
| skn-1(zu135); geIs9[gpa-4p::skn-1b::gfp] | 10 μM DATS | 21.0 | 120 | NS |
| skn-1(zu135); geIs9[gpa-4p::skn-1b::gfp] | DMSO | 15.2 | 100 | - |
| skn-1(zu135); geIs9[gpa-4p::skn-1b::gfp] | 10 μM DATS | 15.1 | 120 | NS |
| skn-1(zu135); geIs10[ges-1p::skn-1c::gfp] | DMSO | 21.5 | 123 | - |
| skn-1(zu135); geIs10[ges-1p::skn-1c::gfp] | 10 μM DATS | 21.2 | 122 | NS |
| skn-1(zu135); geIs10[ges-1p::skn-1c::gfp] | DMSO | 17.3 | 40 | - |
| skn-1(zu135); geIs10[ges-1p::skn-1c::gfp] | 10 μM DATS | 17.9 | 40 | NS |
Lifespan studies using killed OP50-1 were preformed by spotting NGA plates with OP50-1 and UV irradiating the dried plates in a Stratalinker (Stratagene Inc., La Jolla, CA) prior to use as described previously (Sutphin and Kaeberlein, 2009). We also added 80 μL of the bactericidal antibiotic kanamycin from a 10 mM stock shortly before use (Garigan et al., 2002). Worms were grown on these plates from egg hatching and adult worms were transferred to new plates for measurement of adult lifespan. The plates used for adult worms were spotted with DMSO or DATS dissolved in DMSO as described above. The NGA plates used for these experiments did not include FUdR.
DATS acts as a repellent for worms at the concentrations tested which resulted in an increase in worms leaving the plate or burrowing compared to control (not shown and (Bargmann et al., 1990)). This was particularly prevalent at the early time points when worm mobility is greatest, and this effect likely biases against a positive effect on lifespan as the more robust animals are perhaps more able to leave the plate. To minimize the use of censoring and maintain maximal study populations, we used an extra plate of worms prepared in parallel to replace worms lost especially at the early time points as previously described (Viswanathan et al., 2005).
2.3 Pumping Assay
Day 1 adult N2 worms were transferred to NGA plates spotted with DMSO or 10 μM DATS for 24 hours. Individual worms were scored for pumping over 20 seconds via the use of a hand counter. Similar results were obtained in two independent experiments.
2.4 Visualization of GFP fluorescence in daf-16::GFP, skn-1::GFP, and gst-4p::GFP
Day 1 adult worms were transferred to NGA plates freshly spotted with the indicated amount of DATS or DMSO as control. After 24 hrs of exposure changes in the expression or distribution of GFP expression were observed using Olympus BX51 fluorescent microscope. Photographs of all worms from a given experiment were captured at 10X magnification using a digital camera on the same day using the same microscope and camera settings to facilitate comparison. The gst-4p::GFP fluorescence was measured by analyzing digital images with the ImageJ program (NIH, Bethesda, MD) (Abramoff, 2004).
Day 1 skn-1(zu135);Is007[skn-1::gfp] transgenic worms were treated for 24 hours with 100 μM DATS or for 4 days with 10 μM DATS (or DMSO only as a negative control). GFP fluorescence in ASI neurons was measured by digital imaging using 40X magnification followed by analysis with the ImageJ program.
2.5 Microarray gene expression analyses
TJ1060 (spe-9(hc88); fer-15(b26)) worms were synchronized by hypochlorite treatment, and 40 day 1 adult worms were transferred onto NGA plates spotted with equal amount of OP50-1 and 10 μM DATS or DMSO (control). There were 12 plates for each DATS and control group, and animals were treated with DATS or DMSO for 24 hrs at 20°C. Each plate containing 40 worms was prepared as a separate replicate. The total RNA extraction and microarray analyses have been performed as described previously (McColl et al., 2008). Concentration of the RNA and sample quality/integrity was measured using a ND-1000 Spectrophotometer (NanodropTechnologies) and Bioanalyzer Pico Chip (Agilent Inc.). 500 ng of total RNA was amplified using Amino Allyl Message Amplification II Kit (Ambion Inc., Austin, TX). Sample integrity and concentration was again determined using a ND-1000 Spectrophotometer and Bioanalyzer Pico Chip. Amplified RNA was subsequently labeled with CyDye Post Labeling Reactive Dyes (Cy3 and Cy5, GE Healthcare Bio-Sciences Corp., Piscataway, NJ) with reagents and protocol from the Amino Allyl Message Amplification II Kit. Labeled RNA was fragmented to improve hybridization using an RNA Fragmentation kit (Ambion Inc., Austin TX). Hybridization was performed using Lucidea Slide Pro machine (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) following the manufacturer’s protocol. Nuclease-free Water, 20% SDS, 20 X SSC solutions were purchased from Ambion Inc. The arrays were procured from Washington University in St. Louis Genomic Sequencing Center and consisted of 20,333 unique probes that covered 22,490 C. elegans genes. The arrays were scanned using the Scan Array Express 2-Laser Scanner (PerkinElmer Life Sciences, Waltham, MA). In order to generate the GPR files arrayscan images were quantified with the GenePix 5.1 software package (Molecular Devices Corp., Sunnyvale, CA) using GAL file overlays provided by the Washington University in St. Louis Genomic Sequencing Center. Wormbase was used to obtain the gene annotations.
Results of the microarray data were analyzed using the following statistical tests: 2-sample t-test adjusted for multiple testing by the Benjamini-Hochberg correction and clustering analyses of significant genes (HOPACH) (McColl et al., 2008). Normalized data were transferred into an Excel XP spreadsheet.
3. Results
3.1 Effects of DATS on Caenorhabditis elegans lifespan
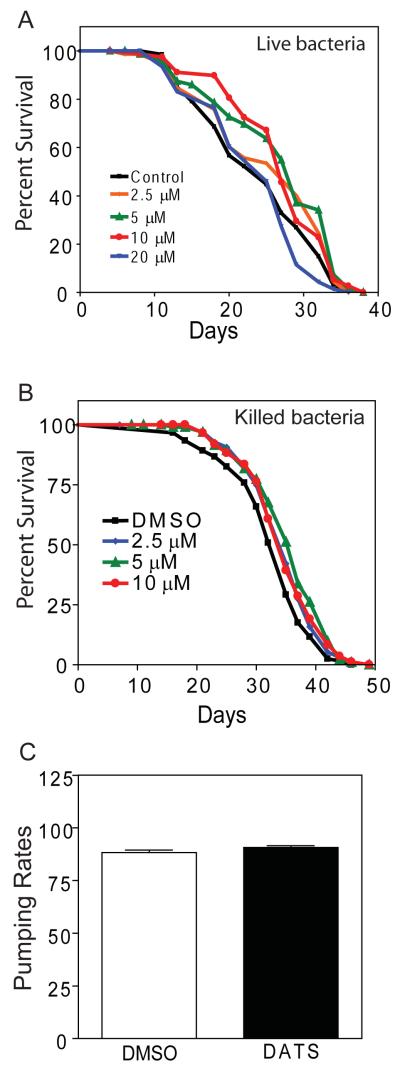
To study the effects of DATS on longevity of C. elegans, we treated the TJ1060 (spe-9(hc88); fer-15(b26)) strain with 2.5-20 μM DATS. When treatment was started at egg hatching, we found that eggs hatched and larvae developed into adults only when exposed to DATS concentrations of less than 10 μM (not shown). As a result, we chose to start treatment during young adulthood to allow treatment with a wider range of doses. In adults, we observed that worms exposed to 5 and 10 μM DATS showed an increased lifespan compared to animals treated with the DMSO vehicle alone or 20 μM DATS (Figure 1A). Exposure to 5 and 10 μM DATS increased the mean lifespan of the worms by 11.7% and 12.6%, respectively (Mean lifespan = 23.9 days for DMSO, 25.4 for 2.5 μM, 26.7 for 5 μM, 26.9 for 10 μM, and 23.2 for 20 μM. p=0.015 for 5 μM and p=0.0452 for 10 μM DATS vs. DMSO by log-rank test. N = 80 for DMSO, 85 for 2.5 μM, 81 for 5 μM, 82 for 10 μM, and 83 for 20 μM). These concentrations of DATS also increased longevity in a second trial (Table 1). Together these results indicated that DATS increased the lifespan of adult worms at a dose between 5-10 μM with a loss of the beneficial effects at a dose of 20 μM. Further, DATS doses above 20 μM appear to be toxic as treatment of worms with 100 μM DATS shortened lifespan relative to control (not shown).
Figure 1.
DATS increases Caenorhabditis elegans lifespan. (A) TJ1060 (spe-9(hc88); fer-15(b26)) worms were synchronized, transferred to NGA plates spotted with the indicated doses of DATS or DMSO as a control starting on day 1 of adulthood, and survival was assessed at 20°C by touch-provoked movement. (B) TJ1060 (spe-9(hc88); fer-15(b26)) worms were synchronized, transferred to NGA plates spotted with UV and kanamycin killed OP50 along with the indicated doses of DATS or DMSO as a control starting on day 1 of adulthood, and survival was assessed at 20°C by touch-provoked movement. (C) Pumping rates counted over 20 seconds for N2 worms treated with either DMSO or 10 μM DATS. Data shown in each panel are representative of two independent experiments.
Organosulfides have been shown to have anti-microbial properties (O’Gara et al., 2000; Tsao and Yin, 2001a; Tsao et al., 2003; Tsao and Yin, 2001b). For C. elegans, their E. coli diet is also pathogenic and limits maximal lifespan, so the effect of DATS on lifespan could represent a direct effect on the bacteria instead of the worms (Garigan et al., 2002). To examine this possibility, we tested whether DATS altered the lifespan of worms grown on killed bacteria. We performed lifespans using OP50 bacteria killed by a combination of UV-light and kanamycin and then spotted with a range of DATS concentrations (Garigan et al., 2002; Sutphin and Kaeberlein, 2009). We found that the use of killed bacteria significantly increased worm lifespan as previously described (Garigan et al., 2002). However, DATS further increased the lifespan of treated worms with a maximum effect observed between 5-10 μM (Figure 1B). Specifically, exposure to 5 and 10 μM DATS increased the mean lifespan of the worms by 9.0% and 6.8%, respectively (Mean lifespan = 32.5 days for DMSO, 34.5 for 2.5 μM, 35.4 for 5 μM, and 34.6 for 10 μM. p=0.001 for 5 μM; p=0.0288 for 10 μM DATS vs. DMSO by log-rank test. N = 120 for DMSO, 120 for 2.5 μM, 120 for 5 μM, and 118 for 10 μM). DATS also increased longevity in a second trial (Table 1). This finding suggests that DATS is able to increase worm lifespan independent of its effects on bacterial survival.
Garlic has been previously shown to be a repellent for worms (Bargmann et al., 1990). As a result, we hypothesized that DATS treatment could lead to changes in pharyngeal pumping rates as means to minimize exposure to the compound. Changes in pharyngeal pumping can lead to dietary restriction-like effects in worms (Lakowski and Hekimi, 1998). To test this possibility, we treated worms with 10 μM DATS for 24 hours and then counted pumping rates for individual worms over 20 seconds. We found that DATS treatment did not decrease pumping rate compared to DMSO treated control animals (Figure 1C) (DMSO mean 88.3+/−1.1 vs. DATS mean 90.8+/−0.8, N = 10 for DMSO and N = 8 for DATS). We also failed to see a decrease in pumping rates for DATS treated animals in a second trial with 10 animals for each treatment (not shown). These findings suggest that DATS does not increase lifespan by reducing food intake.
3.2 DATS-induced increase in lifespan does not require daf-2 or daf-16
Mutations affecting the daf-2-daf-16 signaling pathway have been shown to result in dramatic increases in worm longevity (Kenyon et al., 1993). Additionally, daf-2 has been shown to act during adulthood alone in terms of its effect on longevity (Dillin et al., 2002). Given the ability of DATS treatment starting during adulthood to increase worm lifespan, we examined the effects of DATS on the daf-2 pathway. Reductions in daf-2 signaling cause daf-16 to translocate from the cytoplasm into the nucleus where it activates expression of target genes (Henderson and Johnson, 2001; Lee et al., 2001; Lin et al., 2001; McElwee et al., 2003; McElwee et al., 2004; Murphy et al., 2003; Oh et al., 2006). Hence, we wished to test whether DATS treatment acted to reduce daf-2 signaling in treated worms.
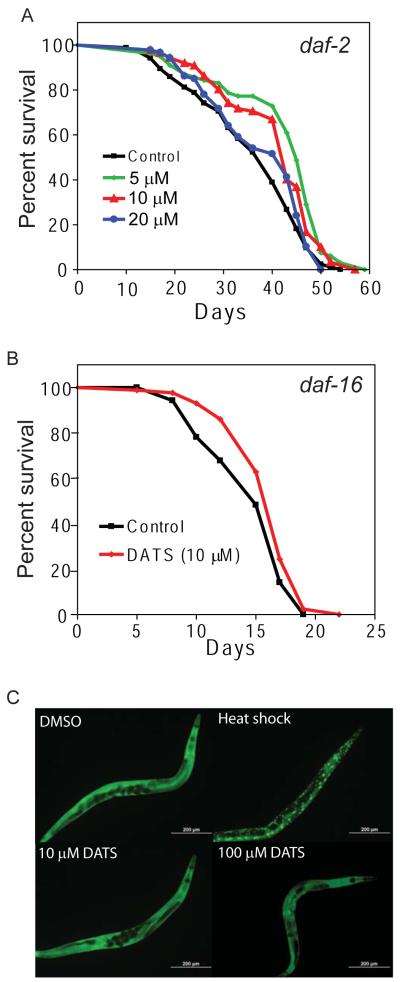
To test the role of the daf-2 signaling pathway in the effects of DATS on worms, we first treated a daf-2 mutant with DATS to examine whether treatment would produce further increases in lifespan. We treated the GL227 strain (daf-2 (e1371) spe-9(hc88); fer-15(b26)) with 5-20 μM DATS and measured the effects on lifespan. We found that DATS treatment further increased the lifespan of daf-2(e1371) relative to DMSO control treated animals (Figure 2A). Exposure to 5 and 10 μM DATS increased the mean lifespan of the worms by 17.8% and 13.8%, respectively (Mean lifespan = 35.5 days for DMSO, 41.9 for 5 μM, 40.4 for 10 μM, and 37.3 for 20 μM. p<0.0001 for 5 μM; p=0.0013 for 10 μM DATS vs. DMSO by log-rank test. N = 85 for DMSO, 80 for 5 μM, 87 for 10 μM and 90 for 20 μM). A second trial also showed an increase in daf-2 longevity (Table 1). We then tested the effects of DATS treatment on a daf-16 mutant as the increased longevity of daf-2 mutants requires daf-16 (Kenyon et al., 1993). We treated the CF1038 strain (daf-16(mu86)) with 10 μM DATS and measured the effect on worm survival. We found that DATS treatment also increased the lifespan of daf-16(mu86) by 9.8% (Figure 2B) (DMSO mean survival 14.7 days vs. 16.2 for DATS, p=0.0083 by Log-rank test, N = 88 for control and 86 for DATS). A second trial also showed an increase in daf-16 longevity (Table 1). Further, we treated worms carrying a transgene encoding a daf-16:GFP fusion protein with either 10 or 100 μM DATS for 24 hours. We found that neither dose of DATS produced daf-16 nuclear translocation while a brief heat shock resulted in nuclear accumulation of daf-16 (Figure 2C). Together these observations indicated that the DATS-mediated increase in worm lifespan was independent of daf-2 signaling.
Figure 2.
Effects of DATS on lifespan are independent of daf-2 and daf-16. (A) DATS increases mean lifespan of daf-2(e1371) worms. GL227 (daf-2 (e1371); spe-9(hc88); fer-15(b26)) worms were transferred at day 1 of adulthood to plates freshly spotted DMSO or 5 μM, 10 μM, or 20 μM DATS and then scored for adult survival by touch provoked movement. Data shown are representative of two separate experiments. (B) DATS increases the lifespan of daf-16(mu86) mutants. CF1038 day 1 adult worms were placed on plates freshly spotted with 10 μM DATS or DMSO (control) and then scored for survival by touch provoked movement. Data shown are representative of two separate experiments. (C) DATS treatment does not induce daf-16 translocation in TJ356 (daf16::GFP) worms. Day 1 adult TJ356 animals were exposed to DMSO (negative control), heat shock (2 hrs, positive control), 10 μM, or 100 μM DATS for 24 hrs. Localization of daf-16:GFP was then assessed by fluorescent microscopy at 10 x magnification.
3.3 DATS fails to augment eat-2 lifespan
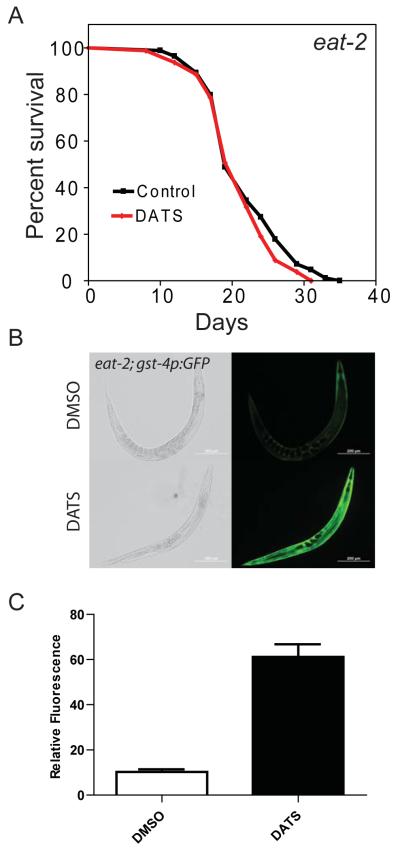
Our results suggest that DATS treatment acts independently of the daf-2 signaling pathway, so we tested additional longevity mutants with DATS. We treated the eat-2(ad1113) mutant, which have enhanced longevity and serve as a worm model of dietary restriction, with 10 μM DATS and examined the effects on lifespan (Lakowski and Hekimi, 1998). Interestingly, the eat-2(ad1113) mutants exposed to DATS did not exhibit changes in mean lifespan when compared to control animals (DMSO control mean 25.7 days vs. DATS 26.7 days, p=0.81 by Log-rank test, N = 136 for both DMSO and DATS) (Figure 3A) (Table 1). One possible explanation for the lack of effect of DATS on the lifespan of the eat-2(ad1113) mutants is a failure of these mutants, which show reduced pharyngeal pumping, to ingest the compound. To address this possibility, we treated the ALF105 strain (eat-2(ad1113);gst-4p::GFP) worm mutant with 10 μM DATS. We observed that the gst-4p::GFP transgene was still induced following DATS treatment (Figure 3B and 3C) (DMSO GFP fluorescence mean 10.3 vs. 61.2 for DATS, p < 0.0001 by t-test, N = 13 for DMSO and 9 for DATS). The induction of GFP by DATS treatment suggests that DATS is still ingested or absorbed by these worms despite the reduced pharyngeal pumping. The failure of DATS to enhance the lifespan of an eat-2 mutant perhaps suggests that downstream pathways associated with dietary restriction could interact or overlap with the pathways activated by DATS.
Figure 3.
DATS fails to increase the lifespan of eat-2 mutants. (A) Day 1 adult DA1113 (eat-2(ad1113)) worms were exposed to DMSO and 10 μM DATS and scored for survival by touch provoked movement. (B) Day 1 adult eat-2(ad1113); gst-4p:GFP worms were treated with 10 μM DATS or DMSO (control) for 24 hours. Expression of gst-4::GFP was imaged by fluorescence microscopy at 10 X magnification. (C) Quantification of GFP fluorescence in images used in (B).
3.4 Microarray analysis of DATS treated worms
To better understand how DATS treatment could produce lifespan extension in worms, we performed microarray analyses using DATS-treated spe-9(hc88); fer-15(b26) worms to explore which genes are influenced by 24 hours of DATS treatment. We used a t-test adjusted for multiple testing to give a false discovery rate of 0.05 to identify 31 genes that were differentially expressed between control and DATS treated worms (Table 2). This group of genes included several genes involved in the response to oxidative stress such as pqm-1 and gst-4 (Leiers et al., 2003; Tawe et al., 1998) (Table 2). Also included were several genes necessary for normal growth, viability, and fatty acid metabolism, including elo-2 and elo-5 (Horikawa et al., 2008; Kniazeva et al., 2004; Kniazeva et al., 2003) (Table 2).
Table 2.
Differentially expressed genes in DATS treated worms
| Gene | Function | Fold Change |
|---|---|---|
| elo-5 | Elongase involved in branched chain FA synthesis | 2.64 |
| gst-4 | GST. Induced by oxidative stress | 2.48 |
| hacd-1 | 3-hydroxyacyl-CoA dehydrogenase. Activated by fasting. | 2.43 |
| F55H12.2 | Unknown | 2.43 |
| vit-1 | Vitellogenin lipoprotein | 2.41 |
| Y38F1A.6 | Mitochondrial hydroxyacid-oxoacid transhydrogenase | 2.40 |
| col-179 | Collagen. Pathogen hypersensitive by RNAi | 2.33 |
| folt-2 | Putative folate transporter | 2.18 |
| C06B3.6 | Unknown | 2.15 |
| F25E5.8 | Unknown | 2.12 |
| cah-4 | Carbonic anhydrase. Induced in alkaline environment | 2.11 |
| ZK228.4 | Unknown | 2.10 |
| F56A4.3 | GST-like | 2.01 |
| clec-265 | C-type lectin | 1.93 |
| F19B2.5 | Helicase-like transcription factor family | 1.91 |
| F18E3.7 | D-aspartate oxidase. | 1.86 |
| elo-2 | Palmitic acid elongase | 1.83 |
| msra-1 | Methionine sulfoxide-S-reductase | 1.82 |
| cyp-32B1 | Cytochrome P450 CYP4/CYP19/CYP26 | 1.81 |
| pqm-1 | Oxidative-stress induced zinc finger gene | 1.75 |
| F49E2.5 | Unknown | 0.62 |
| T19D7.6 | Unknown | 0.52 |
| B0281.3 | Unknown zinc finger gene | 0.52 |
| C29F7.2 | Predicted kinase. Cadmium hypersensitive by RNAi | 0.48 |
| cyp-37A1 | Cytochrome P450 CYP4/CYP19/CYP26 | 0.48 |
| lbp-5 | Intracellular fatty acid binding protein | 0.47 |
| Y40B10A.6 | Predicted O-methyltransferase | 0.43 |
| cyp-34A9 | dod-16; cytochrome P450 CYP2 | 0.42 |
| acl-12 | Predicted ysophosphatidic acid acyltransferase | 0.42 |
| spp-20 | Saposin | 0.38 |
To facilitate more detailed analysis of the effects of DATS treatment on gene expression patterns given the small number of differentially expressed genes identified above, we then analyzed the top 100 genes identified by our microarray analysis as ranked by p-value to include genes which were closer to statistical significance (Supplemental Table 1). We used data from a recent study which used microarrays to study the effects of oxidative stress on gene expression in worms and the role of the skn-1 transcription factor in these responses (Park et al., 2009). Comparison of our data with their data set revealed that 16 genes were up-regulated by both oxidative stress and DATS treatment (representation factor 6.0, p < 5.858e-09 by hypergeometric probability). Up-regulated genes include C32H11.4 which encodes a member of the DUF141 gene family; F18E3.7 which encodes a D-amino acid oxidase; F42D1.2 and T21C12.2 which encode the tatn-1 tyrosine aminotransferase and the hpd-1 4-hydroxyphenylpyruvate dioxygenase and are involved in tyrosine degradation; and gst-4 (Fisher et al., 2008; Katane et al., 2007). Four of these overlapping genes (C06B3.6, C32H11.4, F56A4.3, and gst-4) are also skn-1 dependent (representation factor 6.6, p < 0.003 by hypergeometric probability) (Figure 4). This suggests that DATS activates some genes involved in the oxidative stress response including the known or putative skn-1 target genes C06B3.6, C32H11.4, F56A4.3, and gst-4. DATS also lead to the up-regulation of four collagen genes which are down-regulated by oxidative stress (representation factor 3.7, p < 0.023 by hypergeometric probability) and the down-regulation of eight genes which are usually up-regulated by oxidative stress (representation factor 6.2, p < 3.099e-05 by hypergeometric probability). This latter group includes the dod-16 cytochrome P450 which is up-regulated by daf-2 signaling and contributes to the enhanced longevity of daf-2 mutants (Murphy et al., 2003). Perhaps the opposing effects of DATS treatment on these genes regulated by oxidative stress are consistent with a reduction of overall levels of oxidative stress by DATS.
Figure 4.
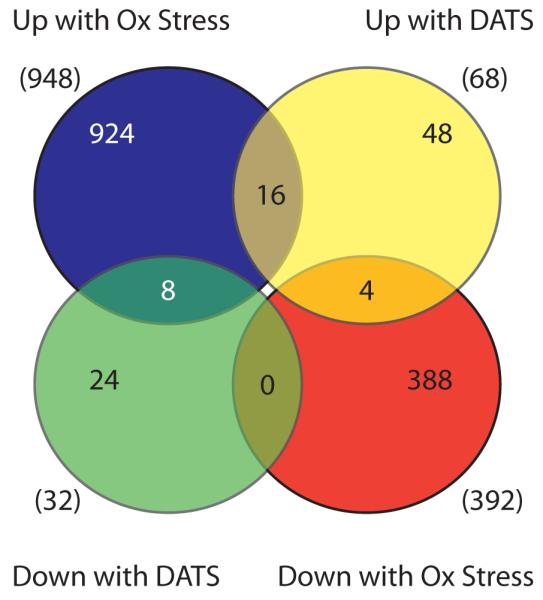
DATS alters the expression of oxidative stress response genes. Venn diagram showing overlap of the top 100 genes regulated by DATS as identified by microarray analysis and the genes identified by Park et. al. as being differentially expressed in worms treated with hyperoxia (Park et al., 2009). Numbers in parentheses represent the total number of genes in each group.
Use of the DAVID program to identify over-represented functional classes among the top 100 genes identified by microarray revealed several groups at a 5% false-discovery rate. Among these were enzymes with oxidoreductase activity with 12 genes having this annotation, cytochrome P450 genes (5 genes), and iron binding proteins (8 genes). Genes involved in lipid metabolism (14 genes) were also over-represented which included three elongases (elo-2, elo-5, and elo-6) which are involved in the synthesis of polyunsaturated fatty acids (Kniazeva et al., 2004; Kniazeva et al., 2003). Also included is the hacd-1 gene which is involved in β-oxidation (Van Gilst et al., 2005).
Hand review of the top 100 genes identified also revealed several genes of interest from an aging perspective. Specifically, DATS treatment up-regulated expression of the daf-36 gene, which is involved in the synthesis of the dafachronic acid ligands which regulate daf-12 function with regards to dauer arrest and longevity (Rottiers et al., 2006). The gfi-1 gene, recently also described as fstr-1, which is required for the retrograde response to impaired mitochondrial function in clk-1 mutants, is also up-regulated by DATS (Cristina et al., 2009). Finally, DATS treatment also up-regulated expression of the F43E2.5 gene, also known as msra-1, which encodes a methionine sulfoxide reductase A that repairs oxidized methionine residues in proteins and is required for the enhanced longevity of daf-2 mutants (Minniti et al., 2009).
Our results, suggest that the gene expression changes produced by DATS include not only aspects of the oxidative stress response but also include other metabolic and hormonal changes.
3.5 DATS induces gst-4::GFP expression via activation of skn-1
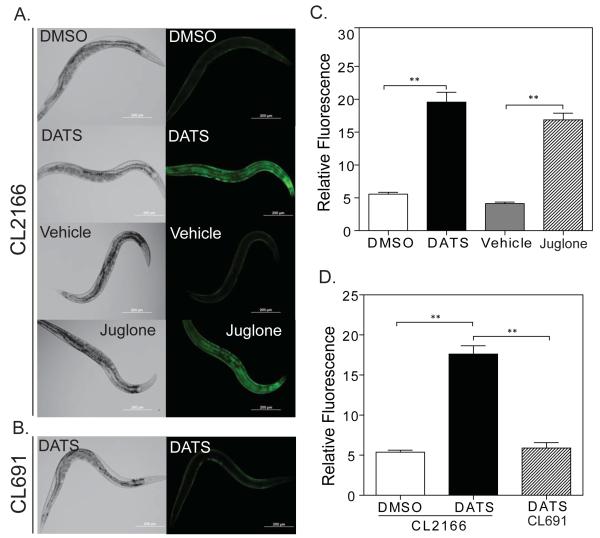
Notably, our microarray data revealed that RNA levels of the gst-4 glutathione S-transferase were almost 2.5 fold higher in DATS-treated worms as compared to controls. In order to verify the findings of microarray analysis, we tested the expression of a gst-4p:GFP transgene in the CL2166 (dvIs19[pAF15(gst-4::GFP::NLS)) transgenic worm strain treated with DATS (Link and Johnson, 2002). We exposed the gst-4p::GFP transgenic worms to 10 μM DATS for 24 hours and observed changes in the expression of GFP indicative of gst-4 induction in the worms. We observed increased fluorescence throughout the body of the worm, particularly in the hypodermis and intestine (Figure 5A and 5C) (DMSO GFP mean fluorescence 5.6 vs. 19.6 for DATS, p <0.0001 by t-test, N = 10 for each). The magnitude of GFP induction following DATS treatment was comparable to the effects of 38 μM juglone, which is a potent source of oxidative stress, on worms treated for 1 hour and then allowed to recover for 6 hours (Figure 5A and 5C) (Vehicle GFP mean fluorescence 4.1 vs. 16.9 for juglone, p<0.0001 by t-test, N = 11 for DMSO and 10 for juglone). Prior work has shown this dose of juglone to produce significantly greater induction of gst-4p:GFP than other sources of oxidative stress, such as hydrogen peroxide and paraquat (Choe et al., 2009). Our observation confirms the activation of gst-4 expression seen in the microarray study and suggests that DATS is able to activate gst-4 expression to similar levels as strong sources of oxidative stress.
Figure 5.
DATS induces expression of the oxidative stress response gene gst-4. (A) Day 1 adult dvIs19[pAF15(gst-4::GFP::NLS worms were treated with DMSO or 10 μM DATS for 24 hrs, or dvIs19[pAF15(gst-4::GFP::NLS transgenic worms were treated with 38 μM juglone dissolved in 100% EtOH or a similar volume of EtOH (vehicle) for 1 hour followed by a 6 hour recovery. The expression of gst-4::GFP was assessed by fluorescence microscopy using 10X magnification. The photographs shown are representative images from one of two separate experiments. Images were captured on the same day with identical camera settings to allow direct comparison. (B) Representative photo of skn-1 mutant dvIs19[pAF15(gst-4::GFP::NLS); skn-1(zu67)/nT1[uncD-?(n754); let-?] worms treated with 10 μM DATS for 24 hours. (C) Graph of GFP fluorescence from images in A. ** = p<0.0001. (D) Graph of GFP fluorescence from images in B and dvIs19[pAF15(gst-4::GFP::NLS)] worms treated in parallel (not shown). ** = p<0.0001.
These data also suggest a role for the skn-1 transcription factor in the effects of DATS on worms because the gst-4 gene is a phase II enzyme which is regulated by the skn-1 transcription factor in response to oxidative stress (An and Blackwell, 2003; Choe et al., 2009; Kell et al., 2007; Tawe et al., 1998). skn-1 is the C. elegans homologue of the mammalian Nuclear Factor Erythroid-derived 2-Related Factor (Nrf2) and coordinates the responses to oxidative stress in worms (An and Blackwell, 2003; Oliveira et al., 2009; Park et al., 2009). skn-1 has also been shown to play an important role in modulating worm longevity (Choe et al., 2009; Tullet et al., 2008).
To test whether the induction of gst-4 expression following treatment of worms with DATS requires skn-1, we treated the CL691 (dvIs19[pAF15(gst-4::GFP::NLS)); skn-1(zu67) IV/nT1[uncD-?(n754); let-?]) strain, which carries a balanced skn-1 mutation and the gst-4p:GFP transgene, with 10 μM DATS. The skn-1 homozygous animals were identified by the absence of the unc mutation carried by the nT1 balancer chromosome. We found that the CL691 strain failed to induce gst-4p:GFP expression in response to DATS treatment whereas CL2166 worms treated in parallel showed robust induction (Figure 5B and 5D) (DMSO control GFP mean fluorescence 5.4 vs. 17.6 for CL2166 DATS vs. 5.9 for CL691 DATS. p<0.0001 by t-test for CL2166 DATS vs. DMSO and CL2166 DATS vs. CL691 DATS. N = 12 for DMSO, 13 for CL2166 DATS, and 11 for CL691 DATS). This suggests that skn-1 is required for the expression of gst-4 following treatment with DATS.
3.6 skn-1 is required for lifespan extension by DATS
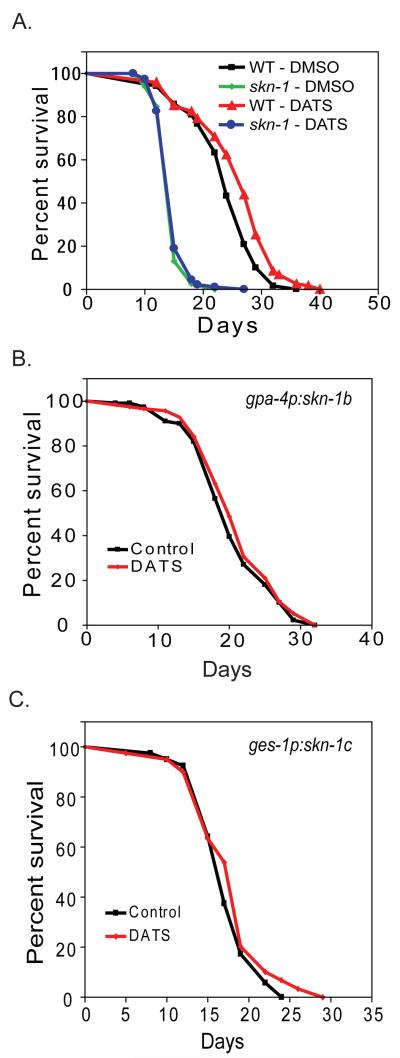
The skn-1 transcription factor has recently been shown to be required for increased worm longevity produced by daf-2 mutations, dietary restriction, and the anti-diabetic medication metformin (Bishop and Guarente, 2007; Onken and Driscoll, 2010; Tullet et al., 2008). Furthermore, the genetic activation of skn-1 via the knock-down of wdr-23 by RNAi has been shown to produce an increase in mean but not maximal lifespan (Choe et al., 2009). Since, we observe an increase in mean but not maximal lifespan, as well as activation of skn-1 following treatment of worms with DATS, we hypothesized that skn-1 could be required for the effects of DATS on worm longevity. To test this hypothesis, we treated the TJ1060 (spe-9(hc88); fer-15(b26)) and LG335 (skn-1(zu135)/nT1[qIs51]) strains with 10 μM DATS. LG335 worms homozygous for skn-1(zu135) were identified by the absence of the visible myo-2:GFP transgene carried on the nT1 balancer chromosome. We found that DATS treatment of TJ1060 increased mean worm lifespan by 8.6% from 23.9 days to 25.9 days (p=0.0003 by Log-rank test, N = 120 for each) whereas DATS treatment of LG335 had a minimal effect on lifespan (14.9 days vs. 15.2 days, 1.9% increase. p=0.573 by Log-rank test, N = 122 for DMSO and 120 for DATS) (Figure 6A). DATS also failed to increase the lifespan of skn-1(zu135) in a second trial (Table 1), and DATS also failed to increase the lifespan of skn-1 RNAi treated worms (not shown). This data indicates that skn-1 is required for DATS treatment to produce an increase in lifespan.
Figure 6.
skn-1 is required for the enhanced longevity of DATS treated worms. (A) TJ1060 (spe-9(hc88); fer-15(b26)) or LG335 (skn-1(zu135)/nT1[qIs51]) worms were grown on NGA plates. Day 1 adult spe-9(hc88); fer-15(b26) and skn-1(zu135) mutant worms were transferred to new plates spotted with DMSO or 10 μM DATS, and the adult lifespan measured by touch provoked movement. (B) LG348 (skn-1(zu135)/nT1[qIs51];geIs9[gpa-4p::skn-1b::gfp]) worms, which express the skn-1b isoform only in ASI neurons, were grown on NGA plates. Day 1 adult skn-1(zu135) mutant worms were transferred to new plates spotted with DMSO or 10 μM DATS, and the adult lifespan measured by touch provoked movement. (C) LG357 (skn-1(zu135)/nT1[qIs51];geIs10[ges-1p::skn-1c::gfp]) worms, which express the skn-1c isoform only in the intestine, were grown on NGA plates. Day 1 adult skn-1(zu135) mutant worms were transferred to new plates spotted with DMSO or 10 μM DATS, and the adult lifespan measured by touch provoked movement.
skn-1 is expressed in both the worm intestine and the ASI neurons. The response to dietary restriction requires skn-1 only in the ASI neurons whereas the increase in worm longevity produced by daf-2 mutations requires skn-1 in the intestine, and the increased longevity produced by metformin treatment requires skn-1 in both tissues (Bishop and Guarente, 2007; Onken and Driscoll, 2010; Tullet et al., 2008). We asked which site(s) of skn-1 expression was the most important for the effects of DATS on worms by using of two transgenic worm strains LG348 (skn-1(zu135)/nT1[qIs51];geIs9[gpa-4p::skn-1b::gfp; rol-6(su1006)]) and LG357 (skn-1(zu135)/nT1[qIs51];geIs10[ges-1p::skn-1c::gfp; rol-6(su1006)]) (Bishop and Guarente, 2007). These strains use an integrated transgene to restore skn-1 function in either the intestine (LG357) or ASI neurons (LG348) in a skn-1 mutant. We used these strains because at the time none of the existing skn-1 mutations disrupted the isoform expressed solely in ASI neurons.
LG348 (skn-1(zu135)/nT1[qIs51];geIs9) worms homozygous for skn-1(zu135) were identified by the absence of the visible myo-2:GFP transgene carried on the nT1 balancer chromosome, and were treated with 10 μM DATS to examine the effects on longevity. LG348 expresses the neuronal skn-1b isoform in ASI neurons via a gpa-4p:skn-1b transgene, and hence allows the role of intestinal skn-1 to be directly tested. We found that skn-1(zu135);geIs9 failed to show an increase in longevity following DATS treatment (DMSO control 20.3 days vs. DATS 21.0 days, 3.5% increase. p=0.322 by Log-rank test. N = 116 for DMSO and 120 for DATS) (Figure 6B). To examine the role of neuronal skn-1 in the response to DATS, we treated LG357 (skn-1(zu135)/nT1[qIs51];geIs10) worms, which only express skn-1c in the intestine via a ges-1p:skn-1c transgene, with DATS and assessed the effects on worm lifespan (Bishop and Guarente, 2007). Similarly to skn-1(zu135)/;geIs9 animals, the skn-1(zu135);geIs10 worms exposed to 10 μM DATS did not show any increase in mean lifespan compared to DMSO-treated control (DMSO mean survival 21.5 days vs. 21.2 for DATS, no increase. p=0.82 by Log-rank test. N = 123 for DMSO and 122 for DATS) (Figure 6C). We also failed to see an increase in longevity with DATS treatment in a second trial for each mutant (Table 1). This suggests that activation of skn-1 in both the intestine and ASI neurons is required to produce DATS-induced lifespan extension. A similar requirement for skn-1 was recently found for metformin to increase worm longevity (Onken and Driscoll, 2010).
3.7 DATS activates skn-1 in ASI neurons
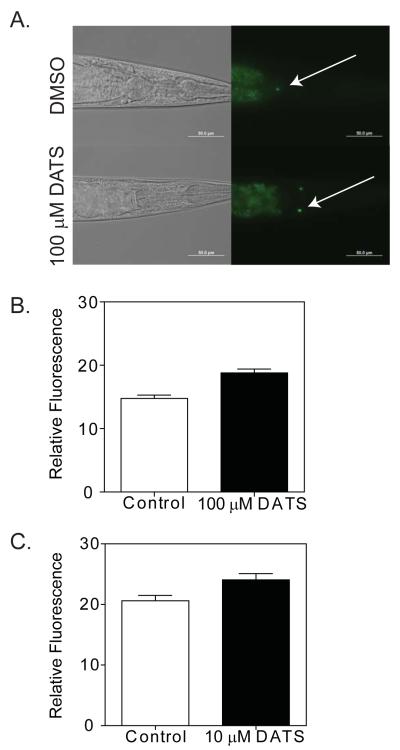
The worm response to dietary restriction has been shown to require skn-1 in the ASI neurons, and subjecting transgenic LG333 (skn-1(zu135);Is007[skn-1::gfp;rol-6dm]) worms to dietary restriction produces an increase in skn-1:GFP levels in the ASI neurons (Bishop and Guarente, 2007). We asked whether DATS treatment had similar effects on worms by treating skn-1(zu135);Is007[skn-1::gfp;rol-6dm] with DATS. In order to maximize the effects on skn-1, we treated worms with 100 μM DATS for 24 hours, and we found that this treatment led to a small but significant increase in GFP expression in the ASI neurons (mean DMSO control GFP intensity 14.76 vs 18.79 for DATS, 27.3% increase, p<0.0001 by t-test. N = 21 for DMSO and 22 for DATS) (Figure 7A and 7B). We also examined skn-1(zu135);Is007[skn-1::gfp;rol-6dm] worms treated with 10 μM DATS, and we found that the induction of GFP expression in the ASI neurons was delayed relative to worms treated with higher DATS doses. However, after 4 days of treatment, we observed a 16.8% increase in GFP expression in the ASI neurons (DMSO control mean 20.6 vs 24.06 for DATS, p=0.014 by t-test, N = 29 for DMSO and 30 for DATS) (Figure 7C). Together these results suggest that DATS influences skn-1 activity in the ASI neurons.
Figure 7.
DATS increases skn-1 expression in ASI neurons. (A) skn-1(zu135);Is007[skn-1::gfp] worms were treated with DMSO or 100 μM DATS for 24 hours before being digitally imaged using 40X magnification. Arrows = ASI neurons. (B) Graph of GFP fluorescence in ASI neurons from images as in A. (C) Graph of GFP fluorescence in ASI neurons in adult worms treated with DMSO or 10 μM DATS for 4 days at 20°C.
4. Discussion
4.1 DATS Increases Longevity in Worms
DATS is one of many organosulfides released from garlic upon chewing, crushing, or cutting the bulbs and is in part responsible for the characteristic taste and smell of garlic flavored foods (Block, 1985). DATS has also been linked to the anti-cancer and pro-cardiovascular benefits of garlic in clinical and animal studies (Breithaupt-Grogler et al., 1997; El-Sabban and Abouazra, 2008; Gao et al., 1999; Gardner et al., 2001; Hsing et al., 2002; Lau, 2006; Powolny and Singh, 2008; Ried et al., 2008; Shukla and Kalra, 2007; Tanaka et al., 2004; Yeh and Yeh, 2006; You et al., 1989). We found that DATS also extends C. elegans longevity in a dose-dependent manner (Figure 1). Interestingly, DATS is able to produce life extension in the worms regardless of whether the treatment is initiated at the first day of adulthood or applied to eggs (Figure 1). We find that the maximal effective dose with regards to longevity is between 5-10 μM, and that higher doses, such as 100 μM, eventually reduce longevity compared to control. The dose-response effects that we observe are consistent with DATS acting on a pharmacologic target instead of a non-specific action. It is also important to note that the effective dose we used is well within the range that can be observed in mammals, because a single intravenous injection of 10 mg of DATS administered to rats achieves a maximal blood concentration of up to 31 μM (Sun et al., 2006). We are unsure of tissue concentration of DATS in treated worms given the difficulties involved in indirectly delivering the compound to worms and the unknown degree to which DATS is ingested or absorbed. However, the concentration in worms is unlikely to significantly exceed the DATS concentration added to the plate.
DATS is not the only organosulfide which may have effects on longevity. There are numerous oil-soluble organosulfides, such as DATS, as well as a number of water-soluble organosulfides. Therefore, even though we conducted our initial studies with DATS, it is not yet clear whether the choice of organosulfide or dosing regiment is optimal with regards to maximizing the effects on longevity. As DATS is intrinsically unstable at the temperatures tested, it is possible that other organosulfide compounds could prove superior to DATS with regards to stability under these conditions (Block, 1985). Similarly, even though we treated animals weekly with DATS, a more frequent dosing regiment could prove to be more effective. We have tried pilot experiments with more frequent dosing and encountered problems with the repulsive effects of DATS on worms (not shown) (Bargmann et al., 1990). It is further possible that other organosulfides may have greater absorption or in vivo potency in worms relative to DATS.
To gain insights into the effects DATS on longevity, we used known genetic mutants to determine whether DATS can extend the lifespan of long-lived daf-2 and eat-2 mutants. From these experiments, we concluded that DATS acts independently of the daf-2 and daf-16 genes, because it was able to extend the lifespan of daf-2 mutants and daf-16 mutants and did not cause nuclear localization of a daf-16:GFP fusion protein in treated worms (Figure 2). The extension of daf-2 longevity by DATS is notable as, similarly to daf-16, skn-1 activity is negatively regulated by daf-2 signaling and skn-1 is required for the increased longevity of specific daf-2 mutants, such as daf-2(e1368) (Tullet et al., 2008). It may be possible that while skn-1 is required for daf-2 longevity, the reductions in daf-2 signaling do not preclude skn-1 from responding to other stimuli and producing further increases in the expression of target genes. This would be consistent with the findings that multiple kinases have the ability to positively and negatively regulate skn-1 activity (An et al., 2005; Inoue et al., 2005; Kell et al., 2007; Tullet et al., 2008).
In contrast, we found that DATS treatment was unable to extend the lifespan of either eat-2 mutants, which represent a worm model of dietary restriction (Lakowski and Hekimi, 1998; Rea et al., 2007). While the definition of dietary restriction in C. elegans is still somewhat vague, the skn-1 gene has been shown to be required for at least some dietary restriction regiments (Bishop and Guarente, 2007; Greer and Brunet, 2009; Mair et al., 2009). The activation of skn-1 we observed in DATS treated animals could in part account for the failure of DATS to further extend eat-2 longevity (Figure 3 and Figure 5).
4.2 DATS activates skn-1 in worms
Our microarray studies revealed an increase in genes activated in worms exposed to oxidative stress in response to DATS treatment (Figure 4). Furthermore, these genes were also enriched in skn-1-dependent genes, such as the gst-4 gene (An and Blackwell, 2003; Leiers et al., 2003; Park et al., 2009; Tawe et al., 1998). Consistent with this finding, treatment of the CL2166 strain, which carries an integrated gst-4p:GFP transgene, with DATS produces a significant increase in GFP expression, especially in the intestine. gst-4 is a well established target gene for the skn-1 transcription factor which coordinates responses to oxidative stress in C. elegans (An and Blackwell, 2003). skn-1 is also an ortholog of the Nrf2 transcription factor which mediates responses to oxidative stress in vertebrates (An and Blackwell, 2003). We also showed that the skn-1 gene is required for the increase in gst-4 expression as induction of the gst-4p:GFP transgene is lost in a skn-1 mutant (Figure 5).
To further elucidate the role of skn-1 in mediating the effects of DATS on C. elegans longevity, we used genetic mutants and transgenic worms. skn-1 has three isoforms which include an A and C isoforms expressed in the intestine and a B isoform expressed in the ASI neurons (An and Blackwell, 2003; Bishop and Guarente, 2007). Loss of all three isoforms blocks the beneficial effects of DATS on worm lifespan (Figure 6). Restoration of skn-1 in either the ASI neurons or intestine with transgenes is unable to restore responsiveness to DATS, which suggests a requirement in both tissues for the biologic effects of DATS (Figure 6). These data tie the lifespan effects of DATS to the activation of skn-1. Interestingly, our findings are supported by similar results observed in worms treated with metformin where increases in longevity depended on skn-1 actions in both tissues (Onken and Driscoll, 2010).
Results of our study and previous reports by others point out that activation of skn-1 leads to increases in mean longevity without consistently increasing maximal longevity in worms (Choe et al., 2009; Onken and Driscoll, 2010). Both pharmacologic activation of skn-1 with DATS or metformin produce these effects on lifespan, as does genetic activation of skn-1 via RNAi mediated inactivation of the wdr-23 gene which mediates the degradation of skn-1, produce these effects on lifespan. It is not clear why skn-1 is not as effective at extending maximal lifespan as other transcription factors, such as daf-16 or daf-12 (Gerisch et al., 2001; Jia et al., 2002; Kenyon et al., 1993). One possibility is the presence of inhibitory phosphorylation sites, such as serine 393 which is phosphorylated by gsk-3, that can act as a brake on increases in skn-1 nuclear localization or transcriptional activity (An et al., 2005). Consistently, over-expression of a skn-1 mutant lacking this phosphorylation site results in increases in mean and maximal lifespan (Tullet et al., 2008). Additionally, there appear to be inhibitory effects of skn-1 on its own activity as skn-1 mutants show greater increases in lifespan and oxidative stress resistance from the over-expression of skn-1 by transgenes than do worms with an intact skn-1 gene (An et al., 2005; Tullet et al., 2008). The mechanisms leading to these observations are unclear but could reflect chromatin modifications or other changes affecting chromosomal skn-1 genes more than skn-1 extrachromosomal arrays. This would be consistent with the greater effects on longevity of skn-1 transgenes in extrachromosomal arrays compared to integrated transgenes (Bishop and Guarente, 2007; Tullet et al., 2008). Finally, skn-1 may regulate genes which both increase and decrease worm survival and result in the observed increase in mean survival. Consistent with this hypothesis, the dod-24 gene, which encodes a secreted protein involved in immune defenses, is up-regulated by skn-1 and yet acts to limit the maximal longevity of worms (Bishop and Guarente, 2007; Murphy et al., 2003). In contrast, the dod-24 gene and several related genes are strongly down-regulated in long-lived daf-12 and daf-16 mutants (Fisher and Lithgow, 2006; McElwee et al., 2004; Murphy et al., 2003).
4.3 Implications for understanding organosulfide actions in vertebrates
Organosulfides are well known to activate the skn-1 homolog Nrf2 in higher animals, and the anti-cancer actions of organosulfides have been linked to Nrf2, as well as the antioxidant and xenobiotic detoxification genes induced by this transcription factor (Chen et al., 2004; Kalayarasan et al., 2008; Kwak et al., 2004; Patel and Maru, 2008). Our work poses the question of whether the health benefits of garlic, other than cancer prevention, are also due the activation of Nrf2.
Recent work with Nrf2 −/− animals has demonstrated roles for Nrf2 in multiple biologic processes which are related to the effects of organosulfides. For example, Nrf2 is required to minimize lipid accumulation in the livers of mice fed a high-fat diet (Tanaka et al., 2008). Importantly, part of this response is the suppression of mRNA levels for genes involved in cholesterol and lipid synthesis, such as 3-hydroxy-3-methylglutaryl coenzyme A reductase and fatty acid synthase (Tanaka et al., 2008). Furthermore, accelerated atherosclerosis seen in middle-aged LDLR −/− mice is associated with a failure to induce oxidative-stress response genes in the blood vessel wall. Treatment of these mice with the anti-diabetic drug rosiglitazone activated Nrf2, induced antioxidant genes, reduced oxidative stress, and attenuated the development of atherosclerosis (Collins et al., 2009). Perhaps DATS and/or other organosulfides could alter lipid levels or influence cardiovascular disease via activating Nrf2 which could act via either mechanism. Future work will address important questions such as how Nrf2 −/− animals respond to garlic-derived organosulfides and whether organosulfides might extend the lifespan of vertebrates.
Supplementary Material
Top 100 differentially expressed genes in DATS treated animals following 24 hour treatment of worms with DATS.
Acknowledgments
This work was supported by a pilot project grant from the University of Pittsburgh Cancer and Aging program project grant (CA103730). A.F. was supported by a grant from the NIA (K08 AG028977). S.V.S was supported by a grant from the NCI (CA113363).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131:951S–4S. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- You WC, Blot WJ, Chang YS, Ershow A, Yang ZT, An Q, Henderson BE, Fraumeni JF, Jr., Wang TG. Allium vegetables and reduced risk of stomach cancer. J Natl Cancer Inst. 1989;81:162–4. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Haruma K, Kunihiro M, Nagata S, Kitadai Y, Manabe N, Sumii M, Yoshihara M, Kajiyama G, Chayama K. Effects of aged garlic extract (AGE) on colorectal adenomas: a double-blinded study. Hiroshima J Med Sci. 2004;53:39–45. [PubMed] [Google Scholar]
- Gao CM, Takezaki T, Ding JH, Li MS, Tajima K. Protective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer Res. 1999;90:614–21. doi: 10.1111/j.1349-7006.1999.tb00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G, Fraumeni JF., Jr. Allium vegetables and risk of prostate cancer: a population-based study. J Natl Cancer Inst. 2002;94:1648–51. doi: 10.1093/jnci/94.21.1648. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Kalra N. Cancer chemoprevention with garlic and its constituents. Cancer Lett. 2007;247:167–81. doi: 10.1016/j.canlet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305–14. doi: 10.1016/j.canlet.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13. doi: 10.1186/1471-2261-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Al-Qattan KK, Al-Enezi F, Khanafer RM, Mustafa T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot Essent Fatty Acids. 2000;62:253–9. doi: 10.1054/plef.2000.0152. [DOI] [PubMed] [Google Scholar]
- Slowing K, Ganado P, Sanz M, Ruiz E, Tejerina T. Study of garlic extracts and fractions on cholesterol plasma levels and vascular reactivity in cholesterol-fed rats. J Nutr. 2001;131:994S–9S. doi: 10.1093/jn/131.3.994S. [DOI] [PubMed] [Google Scholar]
- Yeh YY, Yeh SM. Homocysteine-lowering action is another potential cardiovascular protective factor of aged garlic extract. J Nutr. 2006;136:745S–749S. doi: 10.1093/jn/136.3.745S. [DOI] [PubMed] [Google Scholar]
- Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998;58:257–63. doi: 10.1016/s0952-3278(98)90034-5. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Chatterjee LM, Carlson JJ. The effect of a garlic preparation on plasma lipid levels in moderately hypercholesterolemic adults. Atherosclerosis. 2001;154:213–20. doi: 10.1016/s0021-9150(00)00466-4. [DOI] [PubMed] [Google Scholar]
- Gardner CD, Lawson LD, Block E, Chatterjee LM, Kiazand A, Balise RR, Kraemer HC. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med. 2007;167:346–53. doi: 10.1001/archinte.167.4.346. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Lowe D, Giles P, Fell S, Board AR, Baughan JA, Connock MJ, Maslin DJ. A randomized trial of the effects of garlic oil upon coronary heart disease risk factors in trained male runners. Blood Coagul Fibrinolysis. 2001;12:67–74. doi: 10.1097/00001721-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Lau BH. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. J Nutr. 2006;136:765S–768S. doi: 10.1093/jn/136.3.765S. [DOI] [PubMed] [Google Scholar]
- Durak I, Ozturk HS, Olcay E, Can B, Kavutcu M. Effects of garlic extract on oxidant/antioxidant status and atherosclerotic plaque formation in rabbit aorta. Nutr Metab Cardiovasc Dis. 2002;12:141–7. [PubMed] [Google Scholar]
- El-Sabban F, Abouazra H. Effect of garlic on atherosclerosis and its factors. East Mediterr Health J. 2008;14:195–205. [PubMed] [Google Scholar]
- Gorinstein S, Jastrzebski Z, Namiesnik J, Leontowicz H, Leontowicz M, Trakhtenberg S. The atherosclerotic heart disease and protecting properties of garlic: contemporary data. Mol Nutr Food Res. 2007;51:1365–81. doi: 10.1002/mnfr.200700064. [DOI] [PubMed] [Google Scholar]
- Sobenin IA, Nedosugova LV, Filatova LV, Balabolkin MI, Gorchakova TV, Orekhov AN. Metabolic effects of time-released garlic powder tablets in type 2 diabetes mellitus: the results of double-blinded placebo-controlled study. Acta Diabetol. 2008;45:1–6. doi: 10.1007/s00592-007-0011-x. [DOI] [PubMed] [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–9. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–93. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–9. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Fabian TJ, Johnson TE. Identification genes that are differentially expressed during aging in Caenorhabditis elegans. J.Gerontol.A Biol.Sci.Med.Sci. 1995;50:B245–B253. doi: 10.1093/gerona/50a.5.b245. [DOI] [PubMed] [Google Scholar]
- Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–82. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Link CD, Johnson CJ. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 2002;353:497–505. doi: 10.1016/s0076-6879(02)53072-x. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr.Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AL, Lithgow GJ. The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging Cell. 2006;5:127–138. doi: 10.1111/j.1474-9726.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Sutphin GL, Kaeberlein M. Measuring Caenorhabditis elegans life span on solid media. J Vis Exp. 2009 doi: 10.3791/1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Thomas JH, Horvitz HR. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol. 1990;55:529–38. doi: 10.1101/sqb.1990.055.01.051. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–15. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Abramoff MD, M. PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J Biol Chem. 2008;283:350–7. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66:2269–73. doi: 10.1128/aem.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S, Yin M. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J Antimicrob Chemother. 2001a;47:665–70. doi: 10.1093/jac/47.5.665. [DOI] [PubMed] [Google Scholar]
- Tsao SM, Hsu CC, Yin MC. Garlic extract and two diallyl sulphides inhibit methicillin-resistant Staphylococcus aureus infection in BALB/cA mice. J Antimicrob Chemother. 2003;52:974–80. doi: 10.1093/jac/dkg476. [DOI] [PubMed] [Google Scholar]
- Tsao SM, Yin MC. In-vitro antimicrobial activity of four diallyl sulphides occurring naturally in garlic and Chinese leek oils. J Med Microbiol. 2001b;50:646–9. doi: 10.1099/0022-1317-50-7-646. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc.Natl.Acad.Sci.U.S.A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr.Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–45. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- McElwee J, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2:111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–43. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat.Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- Leiers B, Kampkotter A, Grevelding CG, Link CD, Johnson TE, Henkle-Duhrsen K. A stress-responsive glutathione S-transferase confers resistance to oxidative stress in Caenorhabditis elegans. Free Radic Biol Med. 2003;34:1405–15. doi: 10.1016/s0891-5849(03)00102-3. [DOI] [PubMed] [Google Scholar]
- Tawe WN, Eschbach ML, Walter RD, Henkle-Duhrsen K. Identification of stress-responsive genes in Caenorhabditis elegans using RT-PCR differential display. Nucleic Acids Res. 1998;26:1621–7. doi: 10.1093/nar/26.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa M, Nomura T, Hashimoto T, Sakamoto K. Elongation and desaturation of fatty acids are critical in growth, lipid metabolism and ontogeny of Caenorhabditis elegans. J Biochem. 2008;144:149–58. doi: 10.1093/jb/mvn055. [DOI] [PubMed] [Google Scholar]
- Kniazeva M, Crawford QT, Seiber M, Wang CY, Han M. Monomethyl branched-chain fatty acids play an essential role in Caenorhabditis elegans development. PLoS Biol. 2004;2:E257. doi: 10.1371/journal.pbio.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva M, Sieber M, McCauley S, Zhang K, Watts JL, Han M. Suppression of the ELO-2 FA elongation activity results in alterations of the fatty acid composition and multiple physiological defects, including abnormal ultradian rhythms, in Caenorhabditis elegans. Genetics. 2003;163:159–69. doi: 10.1093/genetics/163.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Tedesco PM, Johnson TE. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–69. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AL, Page KE, Lithgow GJ, Nash L. The Caenorhabditis elegans K10C2.4 Gene Encodes a Member of the Fumarylacetoacetate Hydrolase Family: A CAENORHABDITIS ELEGANS MODEL OF TYPE I TYROSINEMIA. J Biol.Chem. 2008;283:9127–9135. doi: 10.1074/jbc.M708341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katane M, Seida Y, Sekine M, Furuchi T, Homma H. Caenorhabditis elegans has two genes encoding functional d-aspartate oxidases. FEBS J. 2007;274:137–49. doi: 10.1111/j.1742-4658.2006.05571.x. [DOI] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Yamamoto KR. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A. 2005;102:13496–501. doi: 10.1073/pnas.0506234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Hormonal control of C. elegans dauer formation and life span by a Rieske-like oxygenase. Dev.Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000450. doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minniti AN, Cataldo R, Trigo C, Vasquez L, Mujica P, Leighton F, Inestrosa NC, Aldunate R. Methionine sulfoxide reductase A expression is regulated by the DAF-16/FOXO pathway in Caenorhabditis elegans. Aging Cell. 2009;8:690–705. doi: 10.1111/j.1474-9726.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–15. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell A, Ventura N, Kahn N, Johnson TE. Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic Biol Med. 2007;43:1560–6. doi: 10.1016/j.freeradbiomed.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RP, Porter Abate J, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–41. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–38. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breithaupt-Grogler K, Ling M, Boudoulas H, Belz GG. Protective effect of chronic garlic intake on elastic properties of aorta in the elderly. Circulation. 1997;96:2649–55. doi: 10.1161/01.cir.96.8.2649. [DOI] [PubMed] [Google Scholar]
- Sun X, Guo T, He J, Zhao M, Yan M, Cui F, Deng Y. Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi. 2006;126:521–7. doi: 10.1248/yakushi.126.521. [DOI] [PubMed] [Google Scholar]
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A. 2005;102:16275–80. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–83. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–27. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Panowski SH, Shaw RJ, Dillin A. Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PLoS ONE. 2009;4:e4535. doi: 10.1371/journal.pone.0004535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1:841–51. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development. 2002;129:221–31. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- Chen C, Pung D, Leong V, Hebbar V, Shen G, Nair S, Li W, Kong AN. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–90. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Kalayarasan S, Sriram N, Sureshkumar A, Sudhandiran G. Chromium (VI)-induced oxidative stress and apoptosis is reduced by garlic and its derivative S-allylcysteine through the activation of Nrf2 in the hepatocytes of Wistar rats. J Appl Toxicol. 2008;28:908–19. doi: 10.1002/jat.1355. [DOI] [PubMed] [Google Scholar]
- Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133–48. doi: 10.1016/j.mrfmmm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Patel R, Maru G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic Biol Med. 2008;44:1897–911. doi: 10.1016/j.freeradbiomed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–64. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Top 100 differentially expressed genes in DATS treated animals following 24 hour treatment of worms with DATS.