Abstract
Recent climate change has caused the distributions of many species to shift poleward, yet few empirical studies have addressed which species will likely be vulnerable to longer-term climate changes. To investigate past consequences of climate change, we calculated the population extinction rates of 35 reptile species from 87 Greek land-bridge islands in the Mediterranean that occurred over the last 16,000 years. Population extinction rates were higher for those species that today have more northern distributions. We further found that northern species requiring cool, mesic habitats had less available suitable habitat among islands, implicating loss of suitable habitat in their elevated extinction rates. These extinctions occurred in the context of increasing fragmentation, with islands shrinking and separating as sea levels rose. Thus, the circumstances faced by reptiles on the islands are similar to challenges for numerous species today that must cope with a changing climate while living in an increasingly human-fragmented landscape. Our island-biogeographical approach to investigating historical population extinctions gives insight into the long-term patterns of species responses to climate changes.
Keywords: habitat fragmentation, island biogeography, conservation, faunal relaxation, herpetology, Aegean Sea
Introduction
There is now evidence that many species from a wide variety of taxonomic groups have experienced poleward range shifts over the last several decades of climate change (Walther et al. 2002, Parmesan and Yohe 2003, Root et al. 2003, Parmesan 2006). Despite this fingerprint of global climate change on biological systems, inference about climate impacts on populations over the next century is limited on several fronts. First, studies documenting recent range shifts are generally restricted to species for which extensive time-series data are available (Parmesan et al. 1999) or which have experienced rapid shifts (Shoo et al. 2006). Therefore, documented cases of range shifts are dominated by species that are generally well-studied and vagile, such as birds and butterflies (Thomas and Lennon 1999, Wilson et al. 2005, Chamaille-Jammes et al. 2006, but see Hickling et al. 2006, LaSorte and Thompson 2007). Second, the majority of studies on range shifts has focused on the leading (poleward) edge of the range and hence range expansion (Hampe and Petit 2005, Thomas et al. 2006). Movement of the trailing edge of the range, however, may be more important for species conservation, because this drives the contraction of a species’ range; rapid range retraction from the trailing edge coupled with a slow expansion at the leading edge could place species in jeopardy of substantial population reduction or extinction (Huntley 1999, Franco et al. 2006). Finally, current climate change is occurring simultaneously with habitat fragmentation as humans are changing landscapes (Warren et al. 2001, Travis 2003). Fragmentation will inhibit range shifts not only because it limits poleward range expansion (Honnay et al. 2002), but also because it can increase population extinction rates at the trailing edge (Koprowski et al. 2005). At present it is unknown which species in habitat fragments (e.g., national parks, relict habitats) will be most susceptible to climate change.
Although studies on the effects of recent climate change on species have understandably focused on recent changes in the distributions and abundances of species, additional insight can be gained by investigating longer-term patterns of population extinctions and species distributional changes (Davis et al. 2000, Barnosky et al. 2003). Since the height of the last glaciation, the world’s climate has changed dramatically; mean temperatures have increased and precipitation patterns have changed regionally (Issar 2003). By examining patterns of species distributional changes during these past climatic changes, we can identify characteristics of species that may make them vulnerable to current and future climate changes.
We investigated the natural population extinctions of 35 reptile species from 87 land-bridge islands in the northeast Mediterranean Sea that separated from the mainland starting 16,000 years ago at the end of the last ice age. These islands today harbor only impoverished species communities compared to mainland areas of equal sizes, indicating that population extinctions have been common (see Results). The environmental changes faced by these 35 species were similar to those that will be experienced by large numbers of species over the next century of climate change, including both habitat change and fragmentation. In the eastern Mediterranean the early Holocene (11,000-7,000 BP) was a period of wet, mild conditions during which there were extensive forested areas (Caner and Algan 2002, Magny et al. 2002, Mudie et al. 2002, Eastwood et al. 2007), while the period after 6,000 BP saw increasing aridity (Eastwood et al. 2007) and reduction in forest cover over most (Rossignol-Strick 1999b, Roberts et al. 2001, Geraga et al. 2005), but not all (Bottema and Sarpaki 2003), of the region. Thus, the 35 study species experienced climate change and a concomitant vegetation change. Second, increasing global temperatures led to a 120-m rise in sea level that resulted in the sequential fragmentation of a continuous coastal landscape into land-bridge islands. Hence, the study species experienced a progressive fracturing of continuous populations into ever-smaller island groups, mirroring the situation faced by many species today that live in an increasingly human-fragmented landscape.
Here, we first calculate the natural extinction rates of island populations of 35 reptile species using the current presence/absence distribution of each species. Second, we analyze the relationship between these extinction rates and the distributional patterns of the species on the mainland. In particular, we ask whether species that have more northerly ranges on the mainland are more likely to have higher population extinction rates on the study islands. We would expect this to be the case if more northern species have lower tolerance for the hotter, drier conditions and habitats at the equatorial boundary of their ranges. Thus, we are using the latitudinal range of species on the mainland as a surrogate for a number of traits that might be associated with climatic preferences or tolerances of species. Finally, we ask how the amount of suitable habitat available for each species influenced extinction rates. This question addresses the importance of climatic effects on the biotic environment for the vulnerability of species to extinction.
Methods
Species selection
We analyzed data from 87 Greek islands. Present-day reptile distributions were collected from the literature (Chondropoulos 1986, 1989) and were supplemented by field surveys by JF. The present reptile fauna of all Aegean islands consist of 49 species. Of these, we included the 35 species that occurred on at least one of our 87 study islands and for which we could obtain data on population densities and habitat specialization. The 35 species analyzed in the present study include 28 species from an earlier study (Foufopoulos and Ives 1999b) and 7 additional species with newly collected distributional information. A listing of the distribution and ecological characteristics of all species is available in the Online Appendix.
Calculation of extinction rates
We calculated population extinction rates for each species from the current presence/absence of species on islands that were formerly connected to a larger landmass during the last ice age (Perissoratis and Conispoliatis 2003). To infer the past distribution of reptile species among islands, we divided islands into 5 archipelagos that were either connected to the mainland (Eastern Aegean, Argosaronic, Sporades and Ionian clusters) or formed a large continuous landmass (Cyclades) during the last glacial maximum. We inferred the sequence and timing of island formation from bathymetric charts and the known rate of sea-level rise since the height of the Wisconsin-Würm glaciation (Pirazzoli 1991, Perissoratis and Conispoliatis 2003). This resulted in 5 island cladograms whose roots represent the ancestral landmasses and tips the present-day islands (Fig. 1., see also (Foufopoulos and Ives 1999a).
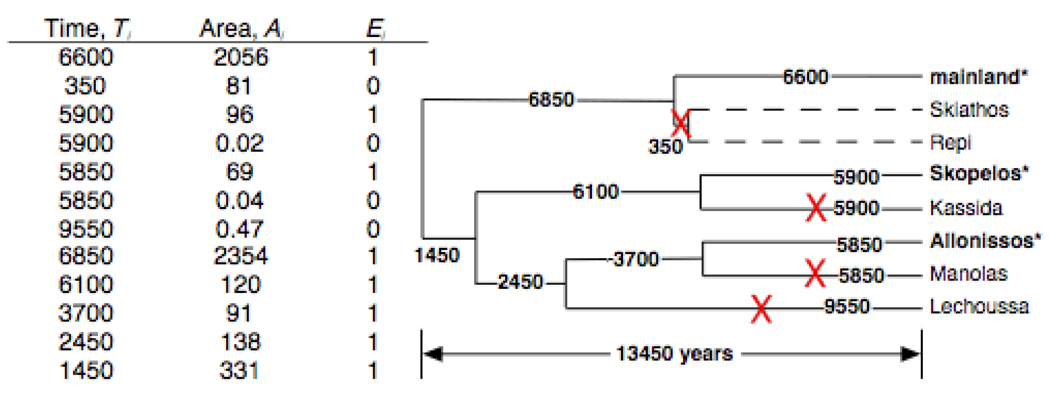
Fig. 1.
Illustration of the calculation of extinction rates for an example species, the Turkish Gecko Hemidactylus turcicus, on the Sporades Archipelago. This species survives today on two islands and on the mainland adjacent to the archipelago (bold text). The cladogram gives the sequence of island separations over the last 13,450 years as obtained from bathymetric maps, and the times of separation were estimated using the known progression of sea level rise. Extinction events (X’s) were assigned using a parsimony assumption that minimized the number of extinctions needed to give the observed presence/absence distribution among islands. Extant populations could either persist or go extinct during the period before the next separation event (or present day), and these events Ei (extinction = 0, persistence = 1) are given in the table along with the time periods over which these events occurred. The table includes not only information on the present islands, but also on the ancestral islands. These values were used in equation 1 to give extinction rates r. Note that data for the separate islands of Skiathos and Repi (dashed lines) are not included, because H. turcicus was assumed to have gone extinct on the ancestral landmass. Thus, the species never occurred on these islands.
We then mapped the patterns of species presence/absence onto these island cladograms under the parsimony assumption that a species occurred on an ancestral island if it occurred on any of its daughter islands that formed as the island fragmented (Fig. 1). The assumption that current presence indicates past occurrence and not immigration is based on a wealth of evidence demonstrating that the chances of over-water colonizations – whether natural or as the result of anthropogenic activities – are very low (Foufopoulos and Ives 1999b). First, in contrast to tropical island regions like the Caribbean or Melanesia that have warm waters and abundant vegetation forming rafts that enable dispersal (Calsbeek and Smith 2003), in the Aegean the relatively cold waters, general absence of floating vegetation, and substantial inter-island distances make over-water travel very difficult. Second, if substantial overwater colonization had occurred, one would expect islands closer to either the mainland or other larger islands to harbor more species. However, analyses revealed that there is no such relationship; instead, the number of species on islands was negatively associated with the time at which islands were isolated during the island fragmentation process (Foufopoulos and Ives 1999b). Third, lack of over-water colonization is reflected in some seemingly idiosyncratic distributions where species have failed to disperse across narrow but deep straits separating islands. Taxonomic analyses based on morphological and molecular criteria resulted in the description of 5 allopatric Podarcis lizard species, more than 25 subspecies of Podarcis erhardii, and at least 13 subspecies of Cyrtopodion kotschyi in the Aegean Sea alone (Mayer and Tiedemann 1980, Beutler 1981, Mayer and Tiedemann 1981, Gruber 1986, Kasapidis et al. 2005, Poulakakis et al. 2005a, Poulakakis et al. 2005b, Lymberakis et al. 2008). The distribution patterns of these groups mirror the time of separation of islands rather than geographic proximity, showing lack of substantial gene flow across water straits. Finally, detailed population genetic analyses of the lizard P. erhardii demonstrated lack of an isolation-by-distance effect in the genetic structure of the examined populations and a progressive loss of genetic diversity over time. Both of these relationships indicate the absence of significant gene flow even between populations separated by narrow water straights (Poulakakis et al. 2005c, Hurston et al. in review).
To calculate extinction rates, we assume that the instantaneous rate of extinction of a species is constant between the time of island formation and fragmentation (ancestral islands) or since the time of formation (extant islands). We also assume that the extinction rate depends on the size of the island. Specifically, for island i we assume the instantaneous extinction rate is r − a(Ai − A̅) where r is the overall (average) extinction rate and a measures the sensitivity of the extinction rate to island area, Ai. For extant islands Ai is the current area of the island, and for ancestral islands Ai is the size of the island immediately before it separated into daughter islands, giving the minimum size of the ancestral island; this assumes that the minimum island area sets the extinction risk. The average area of islands (both past and present) from the archipelagos on which the species occurred, A̅, is subtracted from Ai so that r gives the average extinction rate over all islands, since the average of (Ai − A) over all islands i is zero. With these assumptions, the probability that a species goes extinct on island i is given by the logistic regression model
| (1) |
where Ei is the fate (0 = extinction, 1 = persistence) of the species on the present or ancestral island i, and Ti is the time between when the island was formed and either when it divided or today. This model produces estimates of extinction rates, rather than extinction probabilities, because time Ti is incorporated; preliminary analyses (not presented) showed that time was a significant predictor of population extinctions. In summary, the logistic regression given by equation 1 estimates for each species the mean extinction rate r and the parameter a that represents the sensitivity of extinction rates to island area, with larger values of a corresponding to increased susceptibility to extinction on smaller islands. Figure 1 illustrates the information used in the calculations.
In fitting equation 1, rather than use the logit link function normally used in logistic regression (McCullagh and Nelder 1989), instead we used the log link function, because this easily accommodates the exponential form of equation 1. Logistic regression is known to give biased estimates of the coefficients, and we therefore modified the method of Firth (1993) that incorporates a penalty into the likelihood proportional to the determinant of the corresponding information matrix. To obtain confidence intervals for the estimates of extinction rates r and area sensitivity coefficient a, we performed bootstrapping. This not only revealed the precision of the estimates but also any residual bias that was not corrected by the modified Firth method. Extinction rates and area sensitivities for all species are presented in the Online Appendix along with bootstrapped confidence intervals.
Our approach involving the reconstruction of the sequence of island separation and species loss gives a more-refined picture of extinction rates than would be obtained from the current presence/absence patterns of species among islands alone. For example, if a species is absent from an entire archipelago despite being present on a nearby mainland, we scored these multiple absences as only a single, ancient population extinction event. This guards, at least in part, against incorrectly inflated estimates of extinction rates for species that currently have sparse distributions among islands.
Our estimation of extinction rates assumes that species were originally uniformly distributed across the ancestral landmass, since we assume that absences of species from islands indicate extinctions. The (sub)fossil record from the islands, although far from complete, supports the existence of rich pre-fragmentation species communities with several documented cases of persistence (fossils from islands where the species still survive at present) and also demonstrates extinctions (fossils on islands of species that went subsequently extinct) (Bachmayer et al. 1975, Schneider 1975, Szyndlar 1991). Nonetheless, to assess the sensitivity of our results to the two underlying assumptions of the estimation of extinction rates – uniform ancestral distributions of species and no colonization between islands – we performed two simulation studies (Appendix A). In the first, we violated the assumption that species were distributed uniformly throughout the ancestral landmass from which extinctions were calculated by instead supposing that the ancestral species distributions were patchy. Second, we violated the assumption that there was no immigration between islands by incorporating colonization. Although both of these model assumptions are supported by considerable evidence, these simulation studies nonetheless make our results more secure. Even large violations of both assumptions do not change our overall conclusions (Appendix A).
Extinction rates and species characteristics
We hypothesized that if the current latitudinal distribution of species on the mainland reflect their sensitivity to climatic conditions, then there should be a relationship between the latitudinal range of the 35 species and their extinction rates on Aegean islands. To determine the latitudinal ranges of species, we identified the northern and southern-most points of each species’ present range from published distributional information (Böhme 1981) and museum records. The northern and southern-most range points were averaged to give the latitudinal midpoint (LM) of the species’ range. Although there are multiple ways of measuring the latitudinal range of species, our approach is simple and incorporates the full range of latitudes at which species are found. We explore alternative measures of species ranges, specifically the northern and southern range limits, and the latitudinal span (= northern − southern limit) in Appendix B.
We regressed extinction rates on the latitudinal midpoints of ranges. Because the species are phylogenetically related, such regressions run the risk of type I errors, identifying a statistically significant pattern when in fact there is none (e.g., Martins and Garland 1991). Therefore, we analyzed the data with a regression method that incorporates the species phylogeny. Standard phylogenetic comparative methods assume that the residual variation from the regression can be described by a Brownian motion evolutionary process; this is the assumption of both independent contrasts (Felsenstein 1985, Garland et al. 1992) and Generalized Least Squares approaches (Martins and Hansen 1997, Garland and Ives 2000). Rather than make this strict assumption, instead we use the program “Regressionv2.m” from Lavin et al. (2008) in which the strength of phylogenetic signal exhibited by the residuals of the analysis is estimated simultaneously with the regression coefficients. The strength of the phylogenetic signal is given by a parameter d that varies from 0 when residuals are independent, to 1 when the phylogenetic correlations among residuals are consistent with Brownian motion evolution. Thus, the parameter d statistically adjudicates between non-phylogenetic regression and regression under a Brownian motion model of evolution. To correct for heteroscedasticity in the residuals, we square-root transformed the extinction rates prior to analyses.
In addition to the latitudinal midpoint of species ranges, we considered two other species traits that were previously shown to be related to extinction rates of reptile populations on Aegean islands, maximum population density and the breadth of habitats used by the species (Foufopoulos and Ives 1999b). To measure population density, species were categorized into four groups according to the maximum density (number per unit area) they achieve on the study islands. We use these maximal estimates as species-specific traits, rather than as an attribute of any one population. Because we are using these estimates as traits that affect extinction rates, we are assuming that maximum densities have not changed appreciably over the course of the Holocene. We used 4 broad categories of maximum densities because uncertainty in these species traits prevents any finer-scale designations. To obtain a metric of the breadth of habitats used by species, we identified 10 habitat categories that summarize the types of habitats on the islands (Watson 1964). Our metric of breadth of habitat use is the fraction of these 10 categories used by each species. We performed multiple regression as above, using Regressionv2.m to account for possible phylogenetic signal in the residuals (Lavin et al. 2008).
Extinction rates and suitable habitats
We also performed analyses to determine whether availability of suitable habitat could explain differences in extinction rates. To obtain measures of the amount of suitable habitat remaining on each island for each species, we used ArcGIS to analyze a CORINE 1:100,000 land cover database (EEA 2000) that included 71 of the 87 islands in our study. The 44 land-use/land-cover classes in CORINE were collapsed into the 10 habitat categories used previously to estimate breadth of habitat use. Various reptile groups differ broadly in the minimum area of suitable habitat they require to persist. To scale the amount of suitable habitat by the area requirements of a species, we determined the minimum area of suitable habitat on any island that was supporting a species in each order (turtles: 6.25 ha) or sub-order (snakes: 2.88 ha, lizards: 0.5 ha). The total area of suitable habitat for each species among all islands was then divided by the minimum area each species required according to their order or suborder. Finally, we scaled these values to range between 0 and 1 by dividing this value for each species by the maximum value obtained among all species, thereby giving a metric of the availability of suitable habitat for each species, SH. Because the resulting values were not normally distributed, they were arcsine-transformed SH for subsequent analyses. Note that SH is a measure of island characteristics but scaled according to the habitat requirements for the different groups of species. In contrast, the maximum population density of species and the breadth of habitat use are treated as species traits in the analyses of the last section (Extinction rates and species characteristics).
To determine whether the loss of suitable habitat could explain the higher extinction rates of more-northern species, we regressed SH against the latitudinal midpoint of species ranges, LM, and we then regressed the extinction rates r against SH. Thus, we addressed whether more northern species have less suitable habitat currently available on the study islands, and then whether a lack of availability of suitable habitat could explain higher extinction rates. These analyses were performed using Regressionv2.m (Lavin et al. 2008) to incorporate the possibility of phylogenetic signal.
Potentially confounding factors
We analyzed data to test two general assumptions we used to calculate extinction rates: (i) humans have played an at most minor role in the extinction or redistribution of species among islands, and (ii) species were broadly distributed among ancestral islands, so that the current absence of a species from an island implies population extinction.
(i) Human impact on the distribution of species
It is possible that humans have played a role in the colonization and extinction of species from islands, and therefore that the present distributions reflect human activity rather than natural long-term patterns of climate change and population extinctions. To assess the effect of human habitation, we regressed the number of species per island on whether or not islands were inhabited by humans, including island area in the regression because island area is known to affect the number of species per island. Because we are particularly interested in the extinction rates of northern vs. southern reptile species, we also divided the species into a northern and a southern group, with the northern group having latitudinal midpoints above the mid-latitude of the islands, 37.6°N (N = 22 species), and the southern group below (N = 13). If humans caused the positive relationship between extinction rates and species latitudinal midpoints, then we would expect these regressions to show a larger decrease in the occurrence of species caused by humans for northern species than for southern species. Note that for these analyses the dependent variable is the number of species on islands. Therefore, to determine whether the effect of human habitation on the distribution of species depends on the latitudinal range of species, we have to categorize species as northern or southern and perform separate analyses for both groups.
(ii) Homogeneous pre-fragmentation distribution of species
Our calculations of extinction rates (equation 1) assume that species were distributed uniformly across the ancestral landmasses of the archipelagos on which they currently occur. If species were initially patchily distributed, then the sequential fragmentation of islands during the Holocene might not have led to extinctions. Instead, species that do not occur on an island today might not have existed in the same location prior to fragmentation. This concern is addressed in Appendix A using a simulation study. Here we address this issue empirically.
We constructed a species-area curve for the islands and compared this to a species-area curve for the mainland areas adjacent to the islands with species area data from Chondropoulos (1986, 1989) and field data from one of the authors (JF). The areas on the mainland occupy low-lying regions near the coast, and are topographically and climatically similar to the islands. If this analysis shows that islands have fewer species than mainland areas of the same size, then this would imply a strong role of extinctions in determining the pattern of species occurrences on islands (MacArthur and Wilson 1967). If instead the distribution of species on current islands were determined solely by their former, patchy distribution across the ancestral landmass, then there is no reason to expect that the number of species on islands would be lower than comparably sized areas on the mainland.
Results
Extinction rates, r, which give the instantaneous probability that a population goes extinct from an island of average size, ranged from 0.0095 to 0.47 per 1,000 years (Online Appendix). For the 35 species, there were only 3 whose southern boundary on the mainland was further north than the southern-most island on which it was scored as being absent in the calculation of extinction rates (Online Appendix). Therefore, the extinction rates do not simply reflect the northward movement of the southern range limits of species. The area sensitivity parameter a ranged from −0.034 to 0.070 per 1,000 years per km2. All but two species had positive estimates of a, indicating that extinction rates increased on smaller islands (Online Appendix); of the two species with negative estimates, one was non-significant and the other weakly significant (0.05 > p > 0.02).
Extinction rates and species characteristics
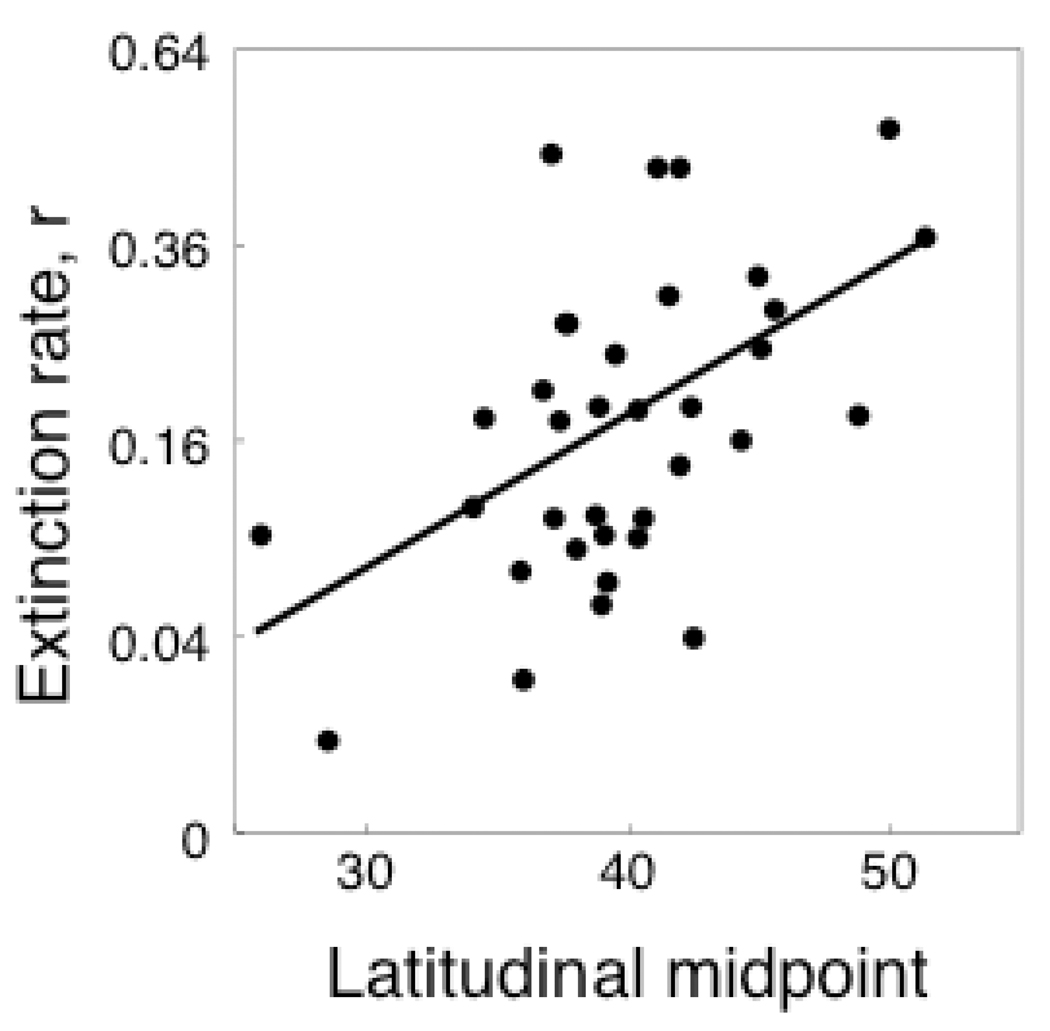
Extinction rates r were positively correlated with the latitudinal midpoint of species ranges on the mainland, LM (Fig. 2, Table 1A). In the regression the estimate of the strength of phylogenetic signal (parameter d) was zero. This indicates that closely related species were not more likely to have similar extinction rates after the effect of LM was removed.
Fig. 2.
Mean population extinction rates r (1000 yr−1, square root transformed) versus the latitudinal midpoint (degrees N) of the range of 35 reptile species, LM. The relationship is statistically significant (p < 0.002, Table 1A).
Table 1.
| A. Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on the latitudinal midpoint of species ranges. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.27. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | −0.205 | −1.14 | |
| Latitudinal midpoint, LM | 0.0158 | 3.52 | < 0.002 |
| B. Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on maximal species densities, the breadth habitats used by species, and LM. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.62. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 0.201 | 1.31 | |
| Maximal density | −0.0486 | −2.52 | < 0.02 |
| Breadth of habitat use | −0.0294 | −2.94 | < 0.01 |
| Latitudinal midpoint, LM | 0.0130 | 3.81 | < 0.001 |
We also performed multiple regression of extinction rates r against LM and two additional variables known to affect extinction rates, the maximum density achieved by species and their breadth of habitat use (Table 1B). Both of these additional variables were statistically significant, as was LM, and there was no evidence of phylogenetic signal in the residual variation (d = 0). The regression coefficient for LM, 0.013, was only slightly lower than in the regression with LM alone (0.016, Table 1A), indicating that the additional two variables do not explain the observed relationship between extinction rates and LM.
Extinction rates and suitable habitat
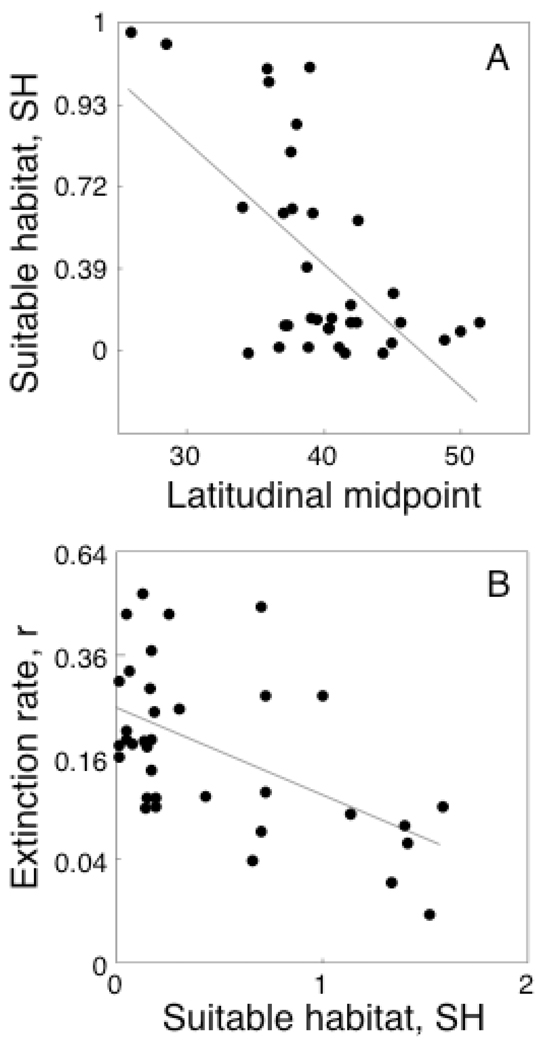
For each species, we calculated a metric SH for the relative amount of suitable habitat available across the study islands. SH was negatively related to the latitudinal midpoint of species ranges, LM, indicating that more northern species have less suitable habitat available (Fig. 3A, Table 2A). Furthermore, extinction rates r were negatively related to SH, indicating that species with more available habitat had lower extinction rates (Fig. 3B, Table 2B). This provides one potential explanation for the relationship between extinction rates r and LM (Fig. 2): northern species had less available suitable habitat, and as a consequence had higher extinction rates.
Fig. 3.
(A) The relative availability of suitable habitat, SH (arcsine transformed) versus the latitudinal midpoint of species ranges, LM. The relationship is statistically significant (p < 0.001, Table 2A). (B) Mean population extinction rates r (1000 yr−1, square root transformed) versus SH (arcsine transformed). The relationship is statistically significant (p < 0.001, Table 2B).
Table 2.
| A. Phylogenetic regression of the arcsine-transformed metric of suitable habitat available on islands, SH, and the latitudinal midpoint of species ranges. The estimate of phylogenetic signal in the residuals is d = 0.13, with N = 35 and R2 = 0.32. At this small value of d, the estimates for the coefficients and t-values are within 1% of those obtained under standard (non-phylogenetic) regression. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 2.38 | 4.85 | |
| Latitudinal midpoint, LM | −0.0479 | −3.90 | < 0.001 |
| B. Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on arcsine-transformed SH. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.30. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 0.498 | 16.5 | |
| Suitable habitat, SH | −0.171 | −3.78 | < 0.001 |
| C. Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on arcsine-transformed SH and LM. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.36. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 0.118 | 0.505 | |
| Suitable habitat, SH | −0.141 | −2.03 | < 0.05 |
| Latitudinal midpoint, LM | 0.0089 | 1.64 | < 0.2 |
A multiple regression of r against both LM and SH provides support for this explanation. Even with the inclusion of LM, the coefficient for SH on extinction rates remains statistically significant. However, the coefficient for LM is reduced from 0.016 (Table 1A) to 0.0089 (Table 2C), and statistical significance of this relationship is lost. This indicates that, while both LM and SH are related to extinction rates, at least part of the explanation for northern species having higher extinction rates is that they have less available habitat.
Human impact on the distribution of species
The number of reptile species on an island was not statistically related to the presence of humans in a regression analysis that accounted for the effects of island area (humans, t = −0.175, p = 0.861; area, t = 10.427, p < 0.001; total model R2 = 0.809, N = 87). These results suggest it is unlikely that the presence of humans has impacted extinction or colonization by reptile species. We performed a similar analysis on the subsets of 22 northern (LM above the mid-latitude of the islands) and 13 southern species (northern species: t = −0.317, p = 0.752; southern species, t = −0.106, p = 0.916). Because neither the subsets of northern nor southern species showed an effect of human presence, it is unlikely that human presence was responsible for the higher extinction rates of northern species (Fig. 2).
Species-area relationships on islands and mainland
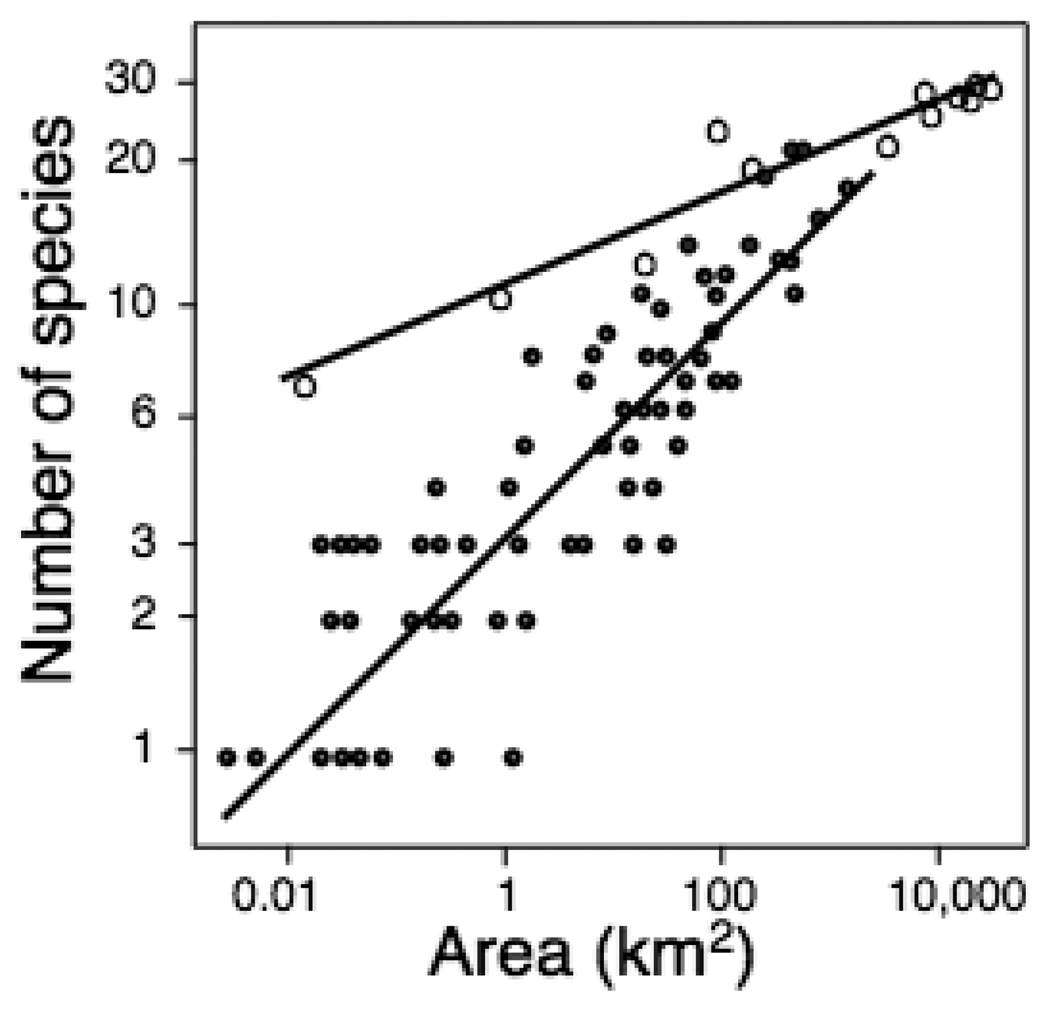
Species-area curves for the islands and the mainland showed lower numbers of species on islands than on comparably sized areas on the mainland (Fig. 4). This suggests that, similar to other land-bridge islands or habitat fragments (Wilcox 1978, Newmark 1987, 1991, Burkey 1995), the reptile communities in the Aegean underwent relaxation following isolation from the mainland. As the steepness of the island slope indicates, the population extinctions underlying this relaxation process were most pronounced on smaller islands. This is consistent with our calculations of area sensitivity, a, in the extinction rates, for which the preponderance of negative species-specific values indicates higher extinction rates on smaller islands. The apparently strong effect of extinction in the island species-area curve relative to that from the mainland indicates that the pattern of species occurrences on islands that we used to calculate extinction rates cannot be attributed to an initially patchy distribution of species across the ancestral landmass.
Fig. 4.
Species-area curves giving the number of species on 87 islands (solid circles) and areas on the mainland (open circles). Islands, in particular small ones, have experienced substantial loss of species in comparison to the mainland. On the mainland, larger sites are prefectures and smaller sites are locations of detailed field surveys. See Valakos et al. (2008).
Discussion
We found that island populations of more-northern reptile species had higher extinction rates r (Fig. 2, Table 1A). This is consistent with the expectation that species from northern climates were impacted more severely by climate change on the study islands during the Holocene (Geraga et al. 2005, Eastwood et al. 2007, Fuchs 2007). While investigating possible explanations for this pattern, we also accounted for two species characteristics known to affect extinction rates: the maximum population density of a species (Pimm et al. 1988, Purvis et al. 2000) and the breadth of habitat types used by the species (Rossignol-Strick 1999a, Owens and Bennett 2000). Our goal was to determine whether these two species characteristics underlie the observed relationship between extinction rates and the midpoint of species’ latitudinal range. For example, more northern species might be species that naturally occur at lower population densities, and the resulting smaller populations might be more prone to extinction. However, including these two species traits did not reduce the strength of the relationship between latitudinal range midpoints and mean extinction rates (Table 1B).
Higher population extinction rates of northern species could be due to direct effects of climate (e.g., temperature) or indirect effects through changes in the biotic environment such as shifts in plant communities. Although it is not possible to completely disentangle direct and indirect effects, circumstantial evidence suggests that higher extinction rates of northern species resulted at least in part from the disproportionate loss of more cool, mesic habitats (e.g., deciduous and coniferous forest). The amount of suitable habitat for each species, SH, was negatively related to the latitudinal midpoint of species ranges, LM. Furthermore, extinction rates r were negatively related to SH. Reduced area of suitable habitat could lead to extinction by making some islands unsuitable, or by restricting the suitable area of islands that thereby reduces population sizes and makes them more prone to extinction. Whereas conditions were cool and moist at the beginning of the Holocene when most of the islands became separated, the region has since experienced increasing aridity (Caner and Algan 2002, Magny et al. 2002, Mudie et al. 2002, Eastwood et al. 2007) and a general decrease in forest cover (Roberts et al. 2001, Rossignol-Strick, 1999, but see Bottema and Sarpaki 2003, Geraga et al. 2005). Consequently, the paleoclimatic record, though incomplete, suggests that in the northeast Mediterranean Basin conditions have become increasingly unsuitable for taxa with cool or mesic habitat preferences.
Human land use could also influence reptile persistence on the islands, and humans caused significant changes in vegetation cover after 6,000 BP (Fuchs 2007) on 40 (46%) of the study islands that have been inhabited. Nevertheless, in field observations (JF, unpublished data) we found that human presence had surprisingly little effect on reptile communities, with all resident species widely distributed throughout anthropogenically modified habitats and no clear differences between areas with and without human habitation. Indeed, in our statistical analyses, after correcting for island area, the presence of humans had no effect on the number of reptile species on islands, either for all species combined or for subsets of northern and southern species. Thus, the absence of a difference in human impacts on the northern and southern subsets of species implies that humans are unlikely to have been responsible for the higher extinction rates of northern species from islands. Our data indicate that while human activities have had effects on the vegetation cover of the islands in the region (Grove and Rackham 2003), this did not translate into observable changes in the local reptile communities, possibly because the gradual expansion of non-intensive agricultural practices allowed local taxa to adapt to an anthropogenically modified landscape.
Our calculations of population extinction rates presuppose that all species were distributed throughout the proto-archipelagos of the Aegean Sea; we assumed that absence from an island indicated extinction either from that island or from its island progenitors. If instead species were patchily distributed across the pre-fragmentation landscape, the current patchwork distribution among islands could reflect the former patchy distributions rather than post-fragmentation population extinctions. However, simulation studies of our procedure for calculating extinction rates showed that our conclusions are robust to even highly patchy initial distribution of species across the ancestral landmass (Appendix A). Furthermore, comparing the species-area curve of the islands to that of the mainland provides empirical evidence that supports the simulation studies. For a given area, islands have fewer species than the mainland; for example, small islands had 1–3 species, whereas areas of the same size, elevation, and habitat heterogeneity had 6–10 species on the mainland (Fig. 4). Furthermore, the slope of the species-area curve for the islands was steeper than for the mainland, which is consistent with the well known pattern of high extinction rates among small populations (MacArthur and Wilson 1967, Groom et al. 2006). If the present distribution of species among islands were caused solely by patchy ancestral distributions, we would expect no difference between island and mainland species-area curves. Indirect evidence for the widespread distribution of reptiles across the pre-fragmentation landscapes also comes from the palynological record. It indicates that during the earliest Holocene while conditions were cool and relatively dry, climatic conditions were similar to what is typical of present-day northern latitudes were these species are widespread today (Digerfeldt et al. 2007). Therefore, in the past northern species likely had broad geographical distributions within this island region. Finally, all of the northern species currently occur on islands spread throughout the broader geographic area (Valakos 2008), often close to sea level, again pointing towards a widespread distribution on the pre-fragmentation landscapes.
In summary, our results suggest that species from cooler, mesic regions were more susceptible to extinction from our study islands, and their elevated susceptibility was caused at least in part by the loss of suitable habitats. This loss was likely driven in large part by climate change that resulted in higher temperatures and increasing aridity during the Holocene. These patterns of extinction occurred within the context of a landscape that was becoming increasingly fragmented during the Holocene as sea levels rose. Not only are islands today depauperate compared to similar areas on the mainland (Fig. 4), but 33 of 35 species had higher extinction rates on smaller islands (area sensitivity parameter a > 0, OA). Thus, fragmentation that led to smaller island areas exacerbated extinctions.
What lessons do these results hold for current climate change? Because the southern range limits of almost all our study species on the mainland lay south of the southern-most island from which they went extinct, the extinctions from islands do not simply represent a retraction of the southern range limit that matches the nearby mainland. Instead, island extinctions occurred north of the present southern range limit on the mainland. Thus, range retraction in the fragmented, island landscape is a sporadic, non-uniform process, with extinctions occurring especially on small islands as a mosaic within the equatorial side of a species range. This represents an interaction of the climate change and fragmentation processes, with more extensive fragmentation (smaller islands) leading to higher extinction rates that are further exacerbated for species that are sensitive to climatic changes. From this example in the Aegean, we would expect to see the effects of climate change on species in a fragmented landscape as inflated extinction rates on the equatorial side of species’ ranges before there is range retraction. These extinctions could give a warning sign of full-blown range contraction.
Extinctions of reptiles on islands in the Aegean over the last 10,000 years share many similarities with anthropogenic climate change and habitat fragmentation as it occurs today. Human activities tend to first destroy flat, lowland habitats that are amenable to agriculture and development. As a result, many natural habitats survive today only as small habitat islands confined to mountainous regions that are surrounded by a ‘sea’ of low domesticated land. Such upland areas tend to be topographically diverse, as are the islands in the Aegean. Similarly, the matrix surrounding habitat islands in many human-dominated landscapes is hostile to slowly dispersing terrestrial organisms like reptiles, presenting obstacles that might be almost as impenetrable as the cold waters of the Aegean Sea. This fragmentation presents a threat in its own right, and also a major challenge for species whose distributions are shifting poleward as the climate warms.
The scenario of population extinctions suggested by our analyses allows integration of several independently observed patterns. As the climate warms, species ranges are expected to contract from their equatorial boundary (Huntley 1999, Franco et al. 2006). Loss of suitable habitat is the primary cause of extinction risk for species throughout the world (Groombridge 1992) and for Mediterranean herptiles in particular (Cox et al. 2006). And fragmentation is well-known to increase population extinction rates, as fragmentation creates small populations and isolates them from the possibility of re-colonization (Newmark 1987). Together these components represent a complex of processes that will likely play out over centuries, rather than decades. While there have been numerous documented cases of rapid, contemporary changes in species ranges (Walther et al. 2002, Parmesan and Yohe 2003, Root et al. 2003, Parmesan 2006), these may represent only that subset of species susceptible to rapid and direct effects of climate change. In contrast, the extinction process of reptiles from Aegean islands has progressed through millennia, and the indirect effect of climate change through habitat loss appears to play a major role. Our results suggest that in the coming centuries, fragmentation will exacerbate the effects of a warming climate leading to differential extinctions of those species that are habitat specialists and/or that naturally occur at low densities. Unfortunately, documenting slow or delayed effects of contemporary climate change on species ranges and population extinction will be difficult. Therefore, patterns of past population extinctions, while not mimicking precisely the patterns we expect over the coming centuries, may nonetheless give insights into the types of processes that will drive changes in species populations in the future.
Acknowledgments
Funding was provided through the University of Wisconsin Department of Zoology, University of Michigan, Princeton Environmental Institute, the Cleveland Dodge Foundation, and National Science Foundation grant DEB-0816613 (ARI) and EF-0914866 (AMK) and National Institutes of Health grant 1R01AI090159-01 (A.M.K.).
Appendix A
Robustness of extinction rate estimates
In our estimation of extinction rates, we assume that species are initially uniformly distributed within the 5 landmasses that eventually divided into the 5 archipelagos we used in our study, and that colonizations did not occur. There is good circumstantial evidence for these two assumptions. Nonetheless, here we investigate the consequences of violations in these two assumptions.
Initial uniform distribution among landmasses
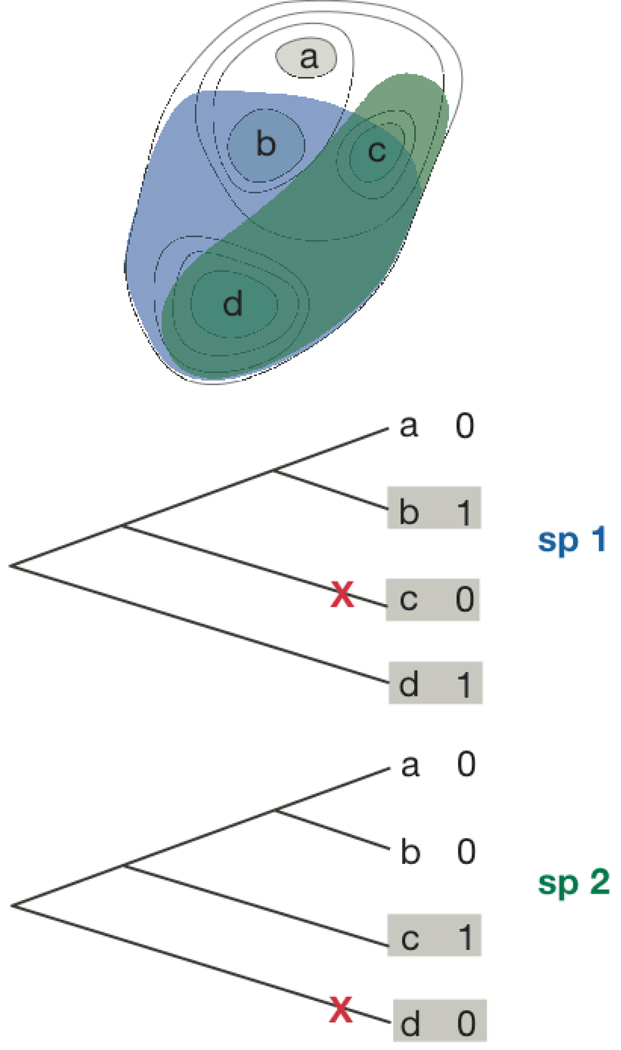
To estimate extinction rates, we assumed that absence of a species indicates extinction either from that island or from an ancestral island. If a species were not uniformly distributed within the initial landmass before island separations started, then we would incorrectly score the absence of that species from islands that had never previously occupied that location as an extinction. This is illustrated in figure A1 that shows the initial distribution of two species, sp 1 and sp 2, and island cladograms with their current presence/absence. Sp 1 (blue) occupied the land area encompassing islands b, c, and d, but not a. The absence of sp 1 on island a today does not represent an extinction. However, absence from island c does represent an extinction. Therefore, for calculating extinction rates, only information from islands b, c, and d (shaded) should be used.
Fig. A1.
Hypothetical initial distributions of two species, sp 1 (blue) and sp 2 (green), among four islands that separated sequentially through time. The contour lines in the top panel give the progressive island boundaries. The island cladograms show the current pattern of presence/absence of sp 1 and sp 2 on islands, with × (red) marking true extinction events. Islands backed by gray shading give the subset of islands from which extinction rates should be calculated.
Sp 2 was initially confined to the area that later formed islands c and d. Therefore, absence from islands a and b do not represent extinctions, and only information from islands c and d should be used to calculate extinction rates. Note that in this case the method we use to calculate extinction rates would count the absence of sp 2 from islands a and b as arising from only a single extinction, on the ancestral island to islands a and b, that is, proto-island a–b. Therefore, even though there are two absences, they are scored as a single extinction. This property of our estimation procedure should reduce its sensitivity to initially non-uniform distributions of species, because recently separated islands are likely to be in close spatial proximity and therefore initial absence of species from that region will be scored as only a single extinction.
Initially non-uniform species distributions will lead to the mis-scoring of absences as extinctions. To simulate the consequences of these pseudo-extinctions, for each species we computed extinction rates after randomly removing extinction events with probability 0.5. For example, for sp 1 we calculate extinction rates assuming an extinction from island c but persistence on islands b and d, and proto-island b–c. For sp 2, we assume an extinction from island d and not from island c. This random procedure does not explicitly account for the biologically expected pattern that geographically neighboring islands are likely to have had the same initial presence/absence of species; for example, islands a and b are nearby and therefore are more likely to be within the initial range of a species (although this is not the case for either sp 1 or sp 2 in our hypothetical example). Nonetheless, because the extinction events can occur on ancestral islands as well as extant islands, removal of these ancestral extinction events is equivalent to the absence of a species from an entire region of the initial landmass. Another simplifying assumption is that we do not consider the size of an island (either extant or ancestral) in randomly removing extinction events, even though larger islands are more likely initially to have had a species. Nonetheless, our simulation to examine violation of the uniform distribution assumption is severe, removing on average 50% of the extinction events from the data set. Therefore, our procedure provides a reasonable test of the robustness of our extinction calculations.
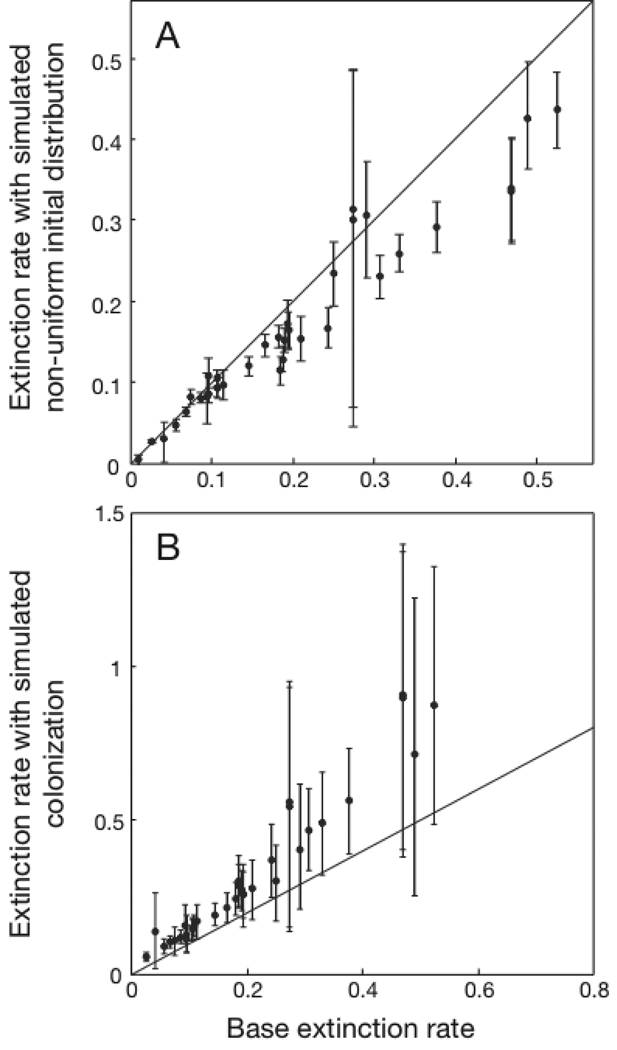
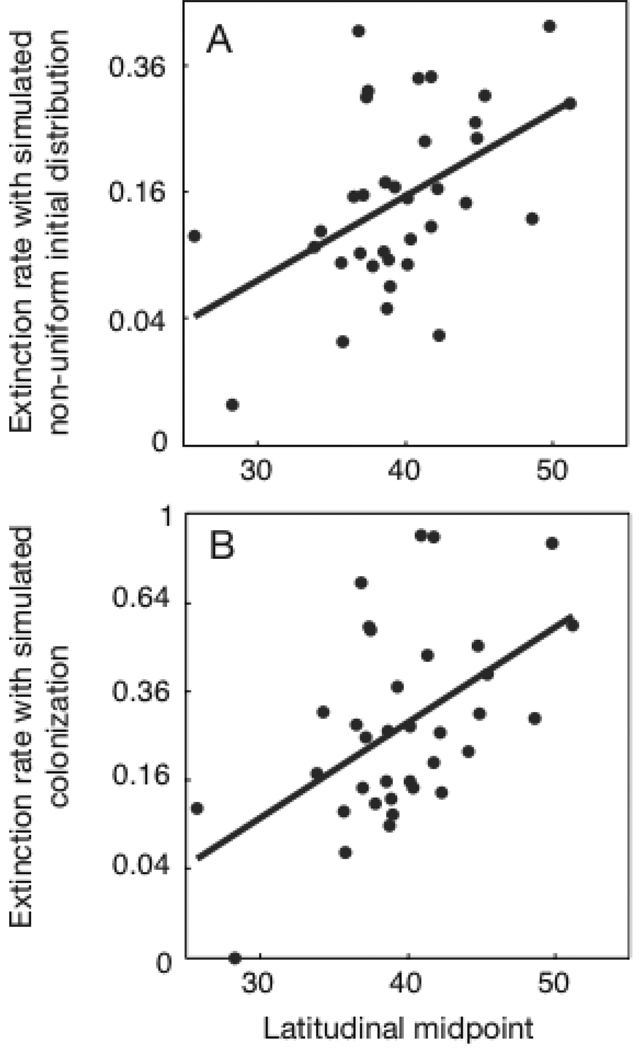
For each species we simulated 1000 data sets by randomly removing extinction events with probability 0.5. Comparing the simulated extinction rate estimates with the base estimates (Eq. 1) obtained assuming a uniform initial distribution of species (Fig. A2A) shows that the random removal of extinction events leads to lower estimates of extinction rates. Nonetheless, there is a strong correlation between the extinction rates simulating non-uniform initial distributions and our base estimates of extinction rates. Furthermore, replacing the base estimates of extinction rates with the simulated extinction rate estimates shows a statistically significant effect of the latitudinal midpoint of species ranges (LM) on extinction rates (Table A1A), only slightly weaker than obtained with the base extinction estimates (Table 2B). The graph of extinction rates vs. LM remains similar (compare Fig. A3A with Fig. 2 in the main text).
Fig. A2.
Extinction rates computed using simulations to mimic (A) non-uniform initial distributions of species among landmasses, and (B) colonization, plotted against the base extinction rates estimated from the data assuming uniform initial distributions and no colonization. Extinction rates for all 35 species are shown. The error bars give the 75% quantiles of the estimated extinction rates from 1000 simulations. For (A) extinction events were removed from the data set with probability 0.5, and for (B) both extinction and persistence events were removed with probability 0.5, and persistence events were converted to extinction events with probability 0.25.
Fig. A3.
Extinction rates computed using simulations to mimic (A) non-uniform initial distributions of species among landmasses, and (B) colonization, plotted against the latitudinal midpoint of species ranges (LM). For (A) extinction events were removed from the data set with probability 0.5, and for (B) both extinction and persistence events were removed with probability 0.5, and persistence events were converted to extinction events with probability 0.25.
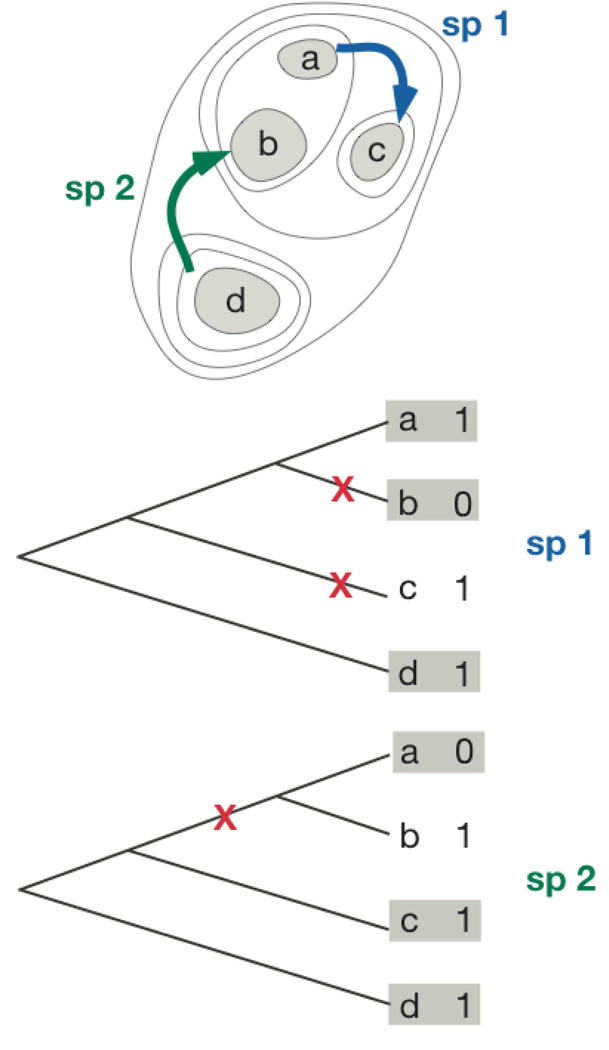
Colonization
To estimate extinction rates, we assume that the presence of a species on an island implies that the species has persisted since the landmass started to divide. If species could colonize islands, then presence on islands could be due to colonization rather than long-term persistence. This is illustrated in figure A4 that shows the colonization of islands by two hypothetical species and island cladograms with their current presence/absence. Sp 1 (blue) colonized island c and sp 2 colonized island b. For sp 1, this obscured an extinction event on island c; even though sp 1 went extinct after the separation of islands c and proto-island a–b, it is currently present on island c. This shows that colonization can hide extinction events.
Fig. A4.
Island colonization and the current hypothetical distributions of two species, sp 1 (blue) and sp 2 (green), among four islands that separated sequentially through time. The contour lines in the top panel give the progressive island boundaries. The island cladograms show the current pattern of presence/absence of sp 1 and sp 2 on islands, with × (red) marking true extinction events. Islands backed by gray shading give the subset of islands from which extinction rates should be calculated.
The effects of colonization on the inferred pattern of extinctions can be more complicated. Because sp 2 colonized island b, it covered the extinction on proto-island a–b. In addition, applying the assumptions that we used to estimate extinction rates, colonization also made it appear that sp 2 persisted on island b. Furthermore, because sp 2 is present on island b and not island a, our method would infer that an extinction had occurred on island a. Thus, there are three changes that must be simulated to estimate extinction rates when colonization is possible: (i) persistence events must be converted to extinction events (e.g., the presence of sp 1 on island c), (ii) persistence events must be removed from the data set (e.g., the persistence of sp 2 on island b, because extinction actually occurred on proto-island a–b), and (iii) extinction events must be removed from the data set (e.g., the extinction of sp 2 from island a, because extinction actually occurred on proto-island a–b). These patterns become more complex when considering colonizations that take place on ancestral proto-islands.
As a simple procedure to mimic the effects of colonization, we simulated data sets by randomly converting persistence events into extinction events with probability 0.25, and randomly removing events (either persistence or extinction events) with probability 0.5. This procedure does not correspond to a specific set of detailed assumptions about how the colonization process occurs, such as whether colonization depends on the distance to nearby islands or island area. Nonetheless, by making the magnitude of the change large (with the assumption that 25% of extinctions are “recovered” through colonization), it provides a reasonable test for the robustness of our conclusions to our base assumption of no colonization.
The estimates of extinction rates were higher when we simulated the effects of colonization than for the base assumptions (Fig. A2B), although the estimates were highly correlated. As a result, the relationship between extinction rates and the latitudinal midpoint of species ranges (LM) remained the same (Table A1B, Fig. A3B).
These two simulation studies of the two main assumptions under which we estimated extinction rates (the uniform initial distribution of species and no colonization) demonstrate that the relative extinction rates among the different species are broadly insensitive to even large violations of the assumptions (Fig. A2), and the conclusion that more northerly species have higher extinction rates remains intact (Table A1, Fig. A3).
Table A1.
| A. The consequences of non-uniform initial distribution of species. Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on species densities, the diversity of habitats used by species, and LM. The extinction rates were calculated as the mean from 1000 simulated data sets in which extinction events were removed with probability 0.5. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.62. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 0.200 | 1.28 | |
| Density | −0.0448 | −2.29 | < 0.04 |
| Habitat diversity, HD | −0.0216 | −2.13 | < 0.05 |
| Latitudinal midpoint, LM | 0.0108 | 3.46 | < 0.002 |
| B. Consequences of simulated colonizations. Phylogenetic regression of square- root transformed extinction rates r (per 1,000 years) on species densities, the diversity of habitats used by species, and LM. The extinction rates were calculated as the mean from 1000 simulated data sets in which extinction and survival (non-extinction) events were removed with probability 0.5, and survival events were converted to extinction events with probability 0.25. The estimate of phylogenetic signal in the residuals is d = 0, with N = 35 and R2 = 0.62. Analyses were performed using Regressionv2.m from Lavin et al. (2008). | |||
|---|---|---|---|
| Effect | Coefficient | t-value | p-value |
| Intercept | 0.122 | 0.49 | |
| Density | −0.0563 | −1.79 | >0.08 |
| Habitat diversity, HD | −0.0442 | −2.55 | < 0.02 |
| Latitudinal midpoint, LM | 0.0200 | 3.56 | < 0.002 |
Appendix B
Alternative measures of species ranges
In the analyses we use the midpoint of species latitudinal ranges to measure the “northerliness” of species. Here we consider three other measures of species ranges: latitudinal span (northern range limit – southern range limit), southern range boundary, and northern range boundary (Table B1). Extinction rates were negatively and marginally significantly related to latitudinal span, positively related to the southern range boundary, and positively but non-significantly related to the northern boundary. These tests are not independent, because the latitudinal midpoints and span depend on the southern and northern boundaries. Nonetheless, they are instructive in showing the overall pattern of extinction rates and the importance of species’ southern boundaries.
Table B1.
Phylogenetic regression of square-root transformed extinction rates r (per 1,000 years) on maximal species densities, the breadth of habitats used by species, and one of either LM, the latitudinal span of species ranges, the latitude of the southern boundary, or the latitude of the northern boundary. Analyses were performed using Regressionv2.m from Lavin et al. (2008).
| Effect | Coefficient | t-value | p-value |
|---|---|---|---|
| Intercept | 0.201 | 1.31 | |
| Maximal density | −0.0486 | −2.52 | < 0.02 |
| Breadth of habitat use | −0.0294 | −2.94 | < 0.01 |
| Latitudinal midpoint, LM | 0.0130 | 3.81 | < 0.001 |
| Intercept | 0.85 | 1.011 | |
| Maximal density | −0.072 | −3.22 | < 0.01 |
| Breadth of habitat use | −0.030 | −2.63 | < 0.02 |
| Latitudinal span | −0.0048 | −1.97 | < 0.06 |
| Intercept | 0.34 | 3.70 | |
| Maximal density | −0.064 | −3.81 | < 0.001 |
| Breadth of habitat use | −0.030 | −3.39 | < 0.002 |
| Southern boundary | 0.013 | 5.24 | < 0.001 |
| Intercept | 0.55 | 3.17 | |
| Maximal density | −0.053 | −2.26 | < 0.04 |
| Breadth of habitat use | −0.029 | −2.47 | < 0.02 |
| Northern boundary | 0.0038 | 1.26 | < 0.22 |
Footnotes
Expanded online edition: Online Appendix: Distributions, ecological data and extinction rates of species in this study.
Contributor Information
Johannes Foufopoulos, Email: jfoufop@umich.edu.
A. Marm Kilpatrick, Email: marm@biology.ucsc.edu.
Anthony R. Ives, Email: arives@wisc.edu.
References
- Bachmayer F, Brinkerink JH, Symeonidis N. Pleistozaene Schildkroeten aus Hoehlen der Insel Kreta. Annales Geologiques des Pays Helleniques. 1975;27:110–122. [Google Scholar]
- Barnosky AD, Hadly EA, Bell CJ. Mammalian response to global warming on varied temporal scales. Journal of Mammalogy. 2003;84:354–368. [Google Scholar]
- Beutler A. Cyrtodactylus kotschyi (Steindachner, 1870)—Aegaeischer Bogenfingergecko. In: Boehme W, editor. Handbuch der Reptilien und Amphibien Europas. Wiesbaden, Germany: AULA Verlag; 1981. pp. 53–74. [Google Scholar]
- Böhme W, editor. Handbuch der Amphibien und Reptilien Europas. Vol. 1–6. Wiesbaden, Germany: Akademische Verlagsgesellschaft; 1981. [Google Scholar]
- Bottema S, Sarpaki A. Environmental change in Crete: a 9000-year record of Holocene vegetation history and the effect of the Santorini eruption. Holocene. 2003;13:733–749. [Google Scholar]
- Burkey TV. Extinction Rates in Archipelagoes - Implications for Populations in Fragmented Habitats. Conservation Biology. 1995;9:527–541. [Google Scholar]
- Calsbeek R, Smith TB. Ocean currents mediate evolution in island lizards. Nature. 2003;426:552–555. doi: 10.1038/nature02143. [DOI] [PubMed] [Google Scholar]
- Caner H, Algan O. Palynology of sapropelic layers from the Marmara Sea. Marine Geology. 2002;190:35–46. [Google Scholar]
- Chamaille-Jammes S, Massot M, Aragon P, Clobert J. Global warming and positive fitness response in mountain populations of common lizards Lacerta vivipara. Global Change Biology. 2006;12:392–402. [Google Scholar]
- Chondropoulos BP. A checklist of the Greek reptiles. I. The lizards. Amphibia-Reptilia. 1986;7:217–235. [Google Scholar]
- Chondropoulos BP. A checklist of Greek reptiles. II. The snakes. Herpetozoa. 1989;2:3–36. [Google Scholar]
- Davis M, Douglas C, Calcote R, Cole KL, Winkler MG, Flakne R. Holocene climate in the western Great Lakes national parks and lakeshores: Implications for future climate change. Conservation Biology. 2000;14:968–983. [Google Scholar]
- Digerfeldt G, P S, Olsson S. Reconstruction of Holocene lake-level changes in Lake Xinias, central Greece. The Holocene. 2007;17:361–367. [Google Scholar]
- Eastwood WJ, Leng MJ, Roberts N, Davis B. Holocene climate change in the eastern Mediterranean region: a comparison of stable isotope and pollen data from Lake Golhisar, southwest Turkey. Journal of Quaternary Science. 2007;22:327–341. [Google Scholar]
- EEA. CORINE land cover database (Version 6/1999) European Environmental Agency NATLAN; 2000. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Foufopoulos J, Ives AR. Reptile distributions and island dendrograms for the islands of the Aegean and Ionion Seas. 1999a [Google Scholar]
- Foufopoulos J, Ives AR. Reptile extinctions on land-bridge islands: Life-history attributes and vulnerability to extinction. American Naturalist. 1999b;153:1–25. doi: 10.1086/303149. [DOI] [PubMed] [Google Scholar]
- Franco AMA, Hill JK, Kitschke C, Collingham YC, Roy DB, Fox R, Huntley B, Thomas CD. Impacts of climate warming and habitat loss on extinctions at species' low-latitude range boundaries. Global Change Biology. 2006;12:1545–1553. [Google Scholar]
- Fuchs M. An assessment of human versus climatic impacts on Holocene soil erosion in NE Peloponnese, Greece. Quaternary Research. 2007;67:349–356. [Google Scholar]
- Garland T, Jr, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Systematic Biology. 1992;41:18–32. [Google Scholar]
- Garland T, Jr, Ives AR. Using the past to predict the present: Confidence intervals for regression equations in phylogenetic comparative methods. American Naturalist. 2000;155:346–364. doi: 10.1086/303327. [DOI] [PubMed] [Google Scholar]
- Geraga M, Tsaila-Monopolis S, Ioakim C, Papatheodorou G, Ferentinos G. Short-term climate changes in the southern Aegean Sea over the last 48,000 years. Palaeogeography Palaeoclimatology Palaeoecology. 2005;220:311–332. [Google Scholar]
- Groom MJ, Meffe GK, Carroll CR. Principles of Conservation Biology. 3d Ed. Sunderland, MA, USA: Sinauer Associates; 2006. [Google Scholar]
- Grove AT, Rackham O. An Ecological History. New Haven, USA: Yale University Press; 2003. The Nature of Mediterranean Europe. [Google Scholar]
- Gruber U. Podarcis erhardii (Bedriaga, 1876)—Aegaeische Mauereidechse. In: Boehme W, editor. Handbuch der Reptilien und Amphibien Europas. Wiesbaden, Germany: AULA Verlag; 1986. pp. 25–49. [Google Scholar]
- Hampe A, Petit RJ. Conserving biodiversity under climate change: the rear edge matters. Ecology Letters. 2005;8:461–467. doi: 10.1111/j.1461-0248.2005.00739.x. [DOI] [PubMed] [Google Scholar]
- Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology. 2006;12:450–455. [Google Scholar]
- Honnay O, Verheyen K, Butaye J, Jacquemyn H, Bossuyt B, Hermy M. Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecology Letters. 2002;5:525–530. [Google Scholar]
- Huntley B. The dynamic response of plants to environmental change and the resulting risks of extinction. In: Mace GM, Balmford A, Ginsberg JR, editors. Conservation in a changing world. Cambridge: Cambridge University Press; 1999. pp. 69–85. [Google Scholar]
- Hurston H, Foufopoulos J, Voith L, Bonanno J, Pafilis P, Valakos E, Anthony N. Effects of fragmentation on genetic diversity in island populations of the Aegean wall lizard Podarcis erhardii (Lacertidae, Reptilia) Molecular Ecology. doi: 10.1016/j.ympev.2009.03.028. in review. [DOI] [PubMed] [Google Scholar]
- Issar AS. Climate changes during Holocene and their impact on hydrological systems. Cambridge, U.K.: Cambridge University Press; 2003. [Google Scholar]
- Kasapidis P, Magoulas A, Mylonas M, Zouros E. The phylogeography of the gecko Cyrtopodion kotschyi (Reptilia : Gekkonidae) in the Aegean archipelago. Molecular Phylogenetics and Evolution. 2005;35:612–623. doi: 10.1016/j.ympev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Koprowski JL, Alanen MI, Lynch AM. Nowhere to run and nowhere to hide: Response of endemic Mt. Graham red squirrels to catastrophic forest damage. Biological Conservation. 2005;126:491–498. [Google Scholar]
- LaSorte FA, Thompson FR. Poleward shifts in winter ranges of North American birds. Ecology. 2007;88:1803–1812. doi: 10.1890/06-1072.1. [DOI] [PubMed] [Google Scholar]
- Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T., Jr Morphometrics of the avian small intestine, compared with non-flying mammals: a phylogenetic approach. Physiological and Biochemical Zoology. 2008;81:526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- Lymberakis P, Poulakakis N, Kaliontzopoulou A, Valakos E, Mylonas M. Two new species of Podarcis (Squamata; Lacertidae) from Greece. Systematics and Biodiversity. 2008;6:307–318. [Google Scholar]
- MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton University Press; 1967. [Google Scholar]
- Magny M, Miramont C, Sivan O. Assessment of the impact of climate and anthropogenic factors on Holocene Mediterranean vegetation in Europe on the basis of palaeohydrological records. Palaeogeography Palaeoclimatology Palaeoecology. 2002;186:47–59. [Google Scholar]
- Martins EP, Garland T., Jr Phylogenetic analyses of the correlated evolution of continuous characters: a simulation study. Evolution. 1991;45:534–557. doi: 10.1111/j.1558-5646.1991.tb04328.x. [DOI] [PubMed] [Google Scholar]
- Martins EP, Hansen TF. Phylogenies and the comparative method: A general approach to incorporating phylogenetic information into the analysis of interspecific data. American Naturalist. 1997;149:646–667. Erratum 153:448. [Google Scholar]
- Mayer W, Tiedemann F. Elektrophoretische Untersuchungen an europaeischen Arten der Gattungen Lacerta und Podarcis. I. Die Podarcis-Formen der griechischen Inseln Milos und Skiros. Sonderdruck der Zeitschrift fuer zoologische Systematik und Evolutionsforschung. 1980;18:147–152. [Google Scholar]
- Mayer W, Tiedemann F. Electrophoretic investigations on the European species of the genus Lacerta and Podarcis. II. To the systematic status of the lizards of the island Piperi (Northern Sporades, Greece) Zoologischer Anzeiger (Jena) 1981;207:143–150. [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 2 edition. London: Chapman and Hall; 1989. [Google Scholar]
- Mudie PJ, Rochon A, Aksu AE. Pollen stratigraphy of Late Quaternary cores from Marmara Sea: land-sea correlation and paleoclimatic history. Marine Geology. 2002;190:233–260. [Google Scholar]
- Newmark WD. A Land-Bridge Island Perspective on Mammalian Extinctions in Western North-American Parks. Nature. 1987;325:430–432. doi: 10.1038/325430a0. [DOI] [PubMed] [Google Scholar]
- Newmark WD. Tropical Forest Fragmentation and the Local Extinction of Understory Birds in the Eastern Usambara Mountains, Tanzania. Conservation Biology. 1991;5:67–78. [Google Scholar]
- Owens IPF, Bennett PM. Ecological basis of extinction risk in birds: Habitat loss versus human persecution and introduced predators. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology Evolution and Systematics. 2006;37:637–669. [Google Scholar]
- Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennent WJ, Thomas JA, Warren M. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Perissoratis C, Conispoliatis N. The impacts of sea-level changes during latest Pleistocene and Holocene times on the morphology of the Ionian and Aegean seas (SE Alpine Europe) Marine Geology. 2003;196:145–156. [Google Scholar]
- Pimm SL, Jones HL, Diamond J. On the Risk of Extinction. American Naturalist. 1988;132:757–785. [Google Scholar]
- Pirazzoli PA. World atlas of Holocene sea-level changes. Amsterdam: Elsevier Science; 1991. [Google Scholar]
- Poulakakis N, Goulielmos G, Antoniou A, Zouros E, Mylonas M. Isolation and characterization of polymorphic microsatellite markers in the wall lizard Podarcis erhardii (Squamata : Lacertidae) Molecular Ecology Notes. 2005a;5:549–551. [Google Scholar]
- Poulakakis N, Lymberakis P, Valakos E, Pafilis P, Zouros E, Mylonas M. Phylogeography of Balkan wall lizard (Podarcis taurica) and its relatives inferred from mitochondrial DNA sequences. Molecular Ecology. 2005b;14:2433–2443. doi: 10.1111/j.1365-294X.2005.02588.x. [DOI] [PubMed] [Google Scholar]
- Poulakakis N, Lymberakis P, Valakos E, Zouros E, Mylonas M. Phylogenetic relationships and biogeography of Podarcis species from the Balkan Peninsula, by baysian and maximum likelihood analyses of mitochondrial DNA sequences. Molecular Phylogenetics and Evolution. 2005c;37:845–857. doi: 10.1016/j.ympev.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N, Reed JM, Leng MJ, Kuzucuoglu C, Fontugne M, Bertaux J, Woldring H, Bottema S, Black S, Hunt E, Karabiyikoglu M. The tempo of Holocene climatic change in the eastern Mediterranean region: new high-resolution crater-lake sediment data from central Turkey. Holocene. 2001;11:721–736. [Google Scholar]
- Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Rossignol-Strick M. The Holocene climatic optimum and pollen records of sapropel 1 in the eastern Mediterranean, 9000-6000 BP. Quarternary Science Reviews. 1999a;18:515–530. [Google Scholar]
- Rossignol-Strick M. The Holocene climatic optimum and pollen records of sapropel 1 in the eastern Mediterranean, 9000-6000 BP. Quaternary Science Reviews. 1999b;18:515–530. [Google Scholar]
- Schneider B. Eine mittelpleistozaene Herpetofauna von der Insel Chios, Aegaeis. Senckenbergiana Biologica. 1975;56:191–198. [Google Scholar]
- Shoo LP, Williams SE, Hero JM. Detecting climate change induced range shifts: Where and how should we be looking? Austral Ecology. 2006;31:22–29. [Google Scholar]
- Szyndlar Z. A review of Neogene and Quaternary snakes of central and eastern Europe. I. Scolecophidia, Boidae, Colubrinae. Estudios Geologicos (Madrid) 1991;47:103–126. [Google Scholar]
- Thomas CD, Franco AMA, Hill JK. Range retractions and extinction in the face of climate warming. Trends in Ecology & Evolution. 2006;21:415–416. doi: 10.1016/j.tree.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Lennon JJ. Birds extend their ranges northwards. Nature. 1999;399:213–213. [Google Scholar]
- Travis JMJ. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proceedings of the Royal Society of London Series B-Biological Sciences. 2003;270:467–473. doi: 10.1098/rspb.2002.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valakos E, Pafilis P, Maragou M, Sotiropoulos K, J F. The amphibians and reptiles of Greece. Frankfurt, Germany: Chimaera Verlag; 2008. [Google Scholar]
- Valakos PE, Maragou M, Sotiropoulos K, Foufopoulos J. The amphibians and reptiles of Greece. Frankfurt, Germany: Chimaera Verlag; 2008. [Google Scholar]
- Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Warren MS, Hill JK, Thomas JA, Asher J, Fox R, Huntley B, Roy DB, Telfer MG, Jeffcoate S, Harding P, Jeffcoate G, Willis SG, Greatorex-Davies JN, Moss D, Thomas CD. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. [DOI] [PubMed] [Google Scholar]
- Watson GE. PhD. New Haven, CT, USA: Yale University; 1964. Ecology and evolution of passerine birds in the islands of the Aegean Sea. [Google Scholar]
- Wilcox BA. Super-Saturated Island Faunas - Species-Age Relationship for Lizards on Post-Pleistocene Land-Bridge Islands. Science. 1978;199:996–998. doi: 10.1126/science.199.4332.996. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Gutierrez D, Gutierrez J, Martinez D, Agudo R, Monserrat VJ. Changes to the elevational limits and extent of species ranges associated with climate change. Ecology Letters. 2005;8:1138–1146. doi: 10.1111/j.1461-0248.2005.00824.x. [DOI] [PubMed] [Google Scholar]