Abstract
Huntington’s disease (HD) is a late-onset, neurodegenerative disease for which there are currently no cures nor disease-modifying treatments. Here we report the identification of several potential anti-inflammatory targets for HD using an ex vivo model of HD that involves the acute transfection of human mutant huntingtin-based constructs into rat brain slices. This model recapitulates key components of the human disease, including the formation of intracellular huntingtin protein (HTT)-containing inclusions and the progressive neurodegeneration of striatal neurons—both occurring within the native tissue context of these neurons. Using this "high-throughput biology" screening platform, we conducted a hypothesis-neutral screen of a collection of drug-like compounds which identified several anti-inflammatory targets that provided neuroprotection against HTT fragment-induced neurodegeneration. The nature of these targets provide further support for non-cell autonomous mechanisms mediating significant aspects of neuropathogenesis induced by mutant HTT fragment proteins.
Keywords: Adenosine, A2A, JNK, IKK, CXCR3, microglia, neurodegeneration, neuroprotection, biolistics, brain slice, drug discovery, inflammation
Introduction
HD arises from the inheritance of dominant, mutant alleles of the huntingtin gene (HD) containing expansions in the polyglutamine domain of HTT that exceed ~35 glutamines (Gusella and Macdonald, 2006). HD affects approximately 5–7 individuals per 100,000 in Western countries, with some 35,000 people suffering from HD in the United States alone (Walker, 2007). Despite the landmark identification and cloning of the HD gene in 1993 (Huntington's Disease Collaborative Research Group, 1993), there are still no clinically validated, disease-modifying drug targets for HD and only palliative treatments are currently available. Indeed, the normal functions of HTT remain uncertain, and while disease mechanism(s) presumably involve gains-of-function from the polyglutamine expansion, they may also involve loss of normal function of the HTT protein as well as interference with the function of the normal allele (Borrell-Pages et al., 2006; Cattaneo et al., 2005; Imarisio et al., 2008).
The lack of clinically validated targets for this fatal disease places an urgent need on the development of biologically relevant and clinically predictive models to support the discovery and development of new targets and drug candidates. One powerful discovery path in the pharmaceutical industry is to screen large compound libraries (often containing 1 million+ compounds) using in vitro assays based on an identified/hypothesized molecular target, ideally one that has previously been validated in clinical usage. This is often followed by cell-based secondary screens, and eventually by demonstration of safety and efficacy in animal models. Although numerous cell-based HD assays are available (Fecke et al., 2009; Varma et al., 2008), and a variety of transgenic and knock-in models of HD have been developed in recent years (Menalled et al., 2009), such an approach depends critically on the hypothetical framework of the original target selection being directly translatable into efficacy in cells, animal models, and eventually humans.
An alternative strategy to a target-based drug discovery approach is phenotypic screening using disease relevant in vitro models. While some disease processes can be recapitulated adequately in dissociated cell culture, recent evidence underscores the complex nature of HD pathogenesis involving the interplay of multiple cell types and brain regions (Gu et al., 2007; Gu et al., 2005; Ilieva et al., 2009). Thus, here we have established a tissue contextual phenotypic model of HD based on the acute transfection of rat corticostriatal brain slices with DNA constructs derived from the human HD gene. This model provides region-specific and cell type-specific neuronal deficits, recapitulating the main features of HD cellular pathology and, importantly, is not restricted to cell autonomous processes, allowing resident interactions among multiple cell types to affect outcome. We show that this assay platform can be implemented at elevated throughput levels for primary screening of focused compound libraries, as well as of specific compound series, for direct evaluation of functional neuroprotection against HD-like degeneration in individual neurons within living brain tissue explants. In an hypothesis-neutral screen of drug-like compounds implicated in neuroprotection, we identified several compound/classes with presumptive anti-inflammatory mechanisms of action, emphasizing the importance of tissue-based screening platforms in capturing non-cell autonomous processes involved in disease pathogenesis.
Materials & Methods
Plasmids
Huntingtin clones were kind gifts from Dr. Chris Ross (Johns Hopkins) and from the Hereditary Disease Foundation, based upon which N-terminal truncations, polyglutamine expansions, and C-terminal fusions with CFP were made and subcloned into the GWiz expression plasmid under the control of the CMV promoter (Genlantis, San Diego, CA). The CFP and YFP expression constructs were made by transferring corresponding sequences from pCFP-N1 and pYFP-N1 (Clontech, Mountain View, CA) into the Gwiz backbone. The MAP2C–YFP construct was a generous gift of Drs. Stepanie Kaech and Gary Banker (Oregon Health & Science University), and the histone 2B–mCherry construct a generous gift of Dr. Rusty Lansford (California Institute of Technology). DNAs for transfections were prepared in large, single lots by contract with Aldeveron (Fargo, ND) to ensure consistency in DNA quality and concentration over multiple screening runs.
Compounds
Small molecule compounds were purchased from Sigma Aldrich (St. Louis, MO), Tocris (Ellisville, MO), BioMol (Plymouth Meeting, PA), CalBioChem (Gibbstown, NJ), Research Diagnostics (Acton, MA), Fluka (St. Louis, MO), TCI America (Portland, OR), and MP Biomedicals (Solon, OH). BDNF was purchased from Peprotech (Rocky Hill, NJ). SAHA was purchased from Alexis (Plymouth Meeting, PA) or synthesized by Evotec (courtesy of CHDI Foundation, Inc.). Compound C, T487, WAY-638, WAY-717, and WAY-781 were synthesized by Wyeth Pharmaceuticals. Unless otherwise noted, compounds were prepared as 1000x stock solutions in DMSO and diluted into brain slice culture medium to a final DMSO concentration of 0.1%. Control brain slices were always treated with the same vehicle (typically 0.1% DMSO) that was used to deliver the compounds.
Preparation of brain slice explants
CD Sprague-Dawley rat pups were obtained from Charles River (Wilmington, MA) and used at postnatal day 10. Brains were cut into 250 µm coronal slices on Vibratomes (Vibratome Co., St. Louis, MO) in chilled medium baths. Approximately 6 such slices could be cut from each rat brain; each was divided into "hemi-coronal" slices which were placed in individual wells in multi-well plates to generate 12 brain slice assays per rat brain. Slices were explanted in interface configuration using culture medium containing 15% heat-inactivated horse serum, 10 mM KCl, 10 mM HEPES, 100 U/ml penicillin/streptomycin, 1 mM MEM sodium pyruvate, and 1 mM L-glutamine in Neurobasal A (Invitrogen, Carlsbad, CA); medium was filtered sterilized at 0.22 µm before use. For routine screening, slices were plated into 12-well plates in which culture support was provided by culture medium set in a low concentration of agarose (0.5%; J.T. Baker, Phillipsburg, NJ). For large molecules and proteins, brain slices were plated into 12-well plate transwell inserts (BD Biosciences, San Jose, CA). Slice cultures were maintained at 32 deg. C in humidified incubators under 5% CO2.
Biolistic transfection
Particle-mediated gene transfer into brain slice explants was done using a Helios Gene Gun (Bio-Rad, Hercules, CA) which was adapted for higher throughput use by replacing its internal controller with custom electronic circuitry driven by a modified Master-8 multi-channel stimulator (AMPI, Jerusalem, Israel). The device was mounted at a vertical angle centered on a cross-hair target for improved accuracy of shooting. For the screening runs described here, "DNA bullets" were made en masse using1.6 µm gold particles as the DNA carrier (Bio-Rad, Hercules, CA) with a total DNA load of 4 µg DNA/ mg Au. The Tefzel tubing used for the manufacture of DNA bullets was immobilized using custom static tubing stretchers rather than the rotating tube-loading device provided by Bio-Rad which allowed a >10x increase in throughput. The unit was set to fire at 95–105 psi upon activation of a foot switch, at an aperture distance from the brain slices of ~2.5 cm.
Antibody staining
The mEM48 (MAB5374) and MAB 5492 mouse monoclonal antibodies and rabbit anti-DARPP32 antibody (AB1656) were purchased from Chemicon (Danvers, MA). Brain slices were fixed for 20 minutes in 4% paraformaldehyde and 4% sucrose in PBS. Slices were transferred to a 24-well plate with 30% sucrose and allowed to sink overnight at 4 degrees followed by one freeze/thaw cycle. Sucrose solution was removed and slices were blocked in 10% normal goat serum/0.1% Tween-20/PBS for 1 hour. Slices were incubated with primary antibodies in 5% normal goat serum/0.1% Tween-20/PBS for 24 h, washed 3 × 10 min in PBS, incubated with secondary antibodies (Invitrogen, Carlsbad, CA) for at least 4 hours, washed again and mounted onto coated slides in Vectashield (Vector Laboratories, Burlingame, CA).
Assessment of medium spiny neuron (MSN) health
YFP-expressing MSNs were identified based on location within the striatal region of each brain slice explant and upon their characteristic morphology using MZFLIII fluorescent stereomicroscopes (Leica, Wetzlar, Germany). MSNs exhibiting normal-sized cell bodies, even and continuous expression of YFP within all cell compartments, and >2 discernable primary dendrites > 2 cell bodies long were scored as “healthy” (Crittenden et al., 2010). The statistical robustness of this assay was determined and monitored using the Assay Validation Ratio (AVR) (Bresnick et al., 2003), a variant of the Z'-factor score (Zhang et al., 1999) that is used for high-throughput assays. Typical AVR values for this assay ranged from ~1.2 to ~1.8, with an overall average of 1.5 for the data included in this report. HTT fragment-containing inclusions were identified via expression of a CFP fusion tag and by immunostaining with the Htt-aggregate specific monoclonal antibody mEM48 (Yu et al., 2002) or MAB 5492 (2B4) against HTT exon-1 (Lunkes et al., 2002). Images were captured using Axiovision software (Zeiss, Thornwood, NY) with a CoolSNAP digital camera (Photometrics Tucson, AZ) on an upright Axioskop or StereoLumar fluorescence microscope (Zeiss, Thornwood, NY).
Results
Brain slice assay development and cross-validation
For this brain slice-based assay for HD, we established chronic coronal brain slice explants (Stoppini et al., 1991) containing both striatum and cortex from postnatal day 10 (P10) rat brain tissue. We have previously shown that such brain slice explants can be readily transfected using biolistics (Lo et al., 1994), and that this process can be scaled sufficiently to enable meaningful screening of targeted chemical compound libraries (Wang et al., 2006). The nearly 100% co-transfection linkage rate of biolistics (Braithwaite et al., 2010) allowed the co-transfection of DNA constructs based on human mutant HD alleles with those encoding yellow fluorescent protein (YFP) on a separate expression plasmid to create an independent and vital morphological marker for transfected neurons. The YFP marker was used to monitor the numbers and vitality of MSNs in cortico-striatal brain slice explants (Crittenden et al., 2010) as a function of time after transfection and treatment condition.
Even with the use of non-cell type specific promoters in this assay, biolistic transfection of brain slices is biased towards neurons, as can be seen in Fig. 1a. Cortical regions in these coronal brain slices were evident by the radial projection of the apical dendrites of YFP-transfected cortical pyramidal neurons, while the identity of transfected MSNs within the striatal regions of these brain slice explants was determined by their characteristic morphology (Fig. 1b) and confirmed by immunostaining against the striatal MSN marker DARPP32 (Fig. 1e–h).
Figure 1.
Neurodegeneration induced in striatal medium spiny neurons (MSNs) via biolistic transfection of HTT N-terminal-based constructs. (a) Fluorescence photomicrograph of a live hemi-coronal brain slice biolistically transfected with YFP to provide a vital marker for neuronal numbers and health. The circular region in the center portion of the slice is the striatum, while the surrounding cortex is readily identified by the radially projecting apical dendrites of transfected cortical pyramidal neurons. Scale bar, 1 mm. (b) Higher magnification view of an individual, live MSN transfected with YFP. Scale bar, 50 µm. (c–d) MSNs co-transfected with YFP and the polyglutamine expanded construct Htt-N90Q73 show the coalescence of the CFP fusion tag on the HTT construct into intracellular aggregates (d). Scale bar, 50 µm. (e–f) The striatal regions of the brain slice explants immunostain positive for the striatal marker DARPP32 (red). The striatum is at left, while at right the adjoining cortical region does not express DARPP32. Panel (f) shows the no primary antibody control with a dotted line drawn along the demarcation between the striatal (left) and cortical (right) regions of a similarly positioned brain slice. Scale bar, 300 µm. (i–l) Intracellular HTT fragment-CFP aggregates formed after transfection of Htt-N90Q73 (j, blue) immunostain positive with the HTT exon-1 antibody MAB5492 (k, red). Scale bar, 10 µm. At higher magnification, the characteristic surface-staining pattern of HTT aggregates can be seen; for clarity, the blue CFP aggregate image is displaced just to the left of the red MAB5492 immunostaining image (l). (m, n) Intracellular HTT fragment-CFP aggregates (blue) also immunostain positive for the aggregate-specific marker mEM48 (red). Scale bar, 10 µm. (o, p) Htt-N90Q73-CFP forms perinuclear aggregates. Images of striatal neurons triply transfected with Htt-N90Q73-CFP (blue), histone 2B–mCherry to label the nucleus (red), and MAP2C–YFP to label the cytoplasm and dendrites (green). Scale bar, 10 µm.
Transfection of a mutant HTT expression construct containing exon 1 of HD and including a 73 polyglutamine expansion ("Htt-N90Q73") resulted in the gradual degeneration of MSNs over a 4–7 day period; this proteolytic fragment of HTT is implicated in HD pathogenesis (Landles et al., 2010) and is similar to the transgene in the R6/2 transgenic mouse model but with a much shorter polyglutamine expansion (Mangiarini et al., 1996). Beginning 2–3 days after transfection with Htt-N90Q73, the dendritic arbors of MSNs become progressively dystrophic, leading ultimately to their complete loss and the subsequent death and clearance of the neuron; this loss was quantified by scoring the number of healthy MSNs per striatal region in each hemi-coronal brain slice as a function of time after explantation and transfection via the independent YFP marker (see Methods and (Crittenden et al., 2010)). By day 5 after transfection, a 3- to 5-fold difference between control and Htt-N90Q73-transfected brain slices was typically seen (Fig. 2a).
Figure 2.
Quantification and cross-validation of the brain slice-based HD assay. (a) Control MSNs remain viable for 5 days or more as monitored by scoring numbers of healthy MSNs (see Methods) as a function of time (light gray bars). In contrast, by day 5 after explantation and transfection, there is a 3- to 5-fold decrease in numbers of healthy MSNs co-transfected with YFP and Htt-N90Q73 (open bars). Ordinate shows the mean total numbers of viable MSNs per striatal region of each brain slice ± SEM, N=27–34 brain slices per condition, scored as 'healthy' as described in the Methods. (b) Rescue of Htt-N90Q73-transfected neurons at day 5 by single bolus addition of the pan-caspase inhibitor BOC-D-FMK ("BOC-D") to the culture medium at the time of explantation; bulk concentrations of the compound in the culture medium are shown in µM. For this and all bar graphs that follow, the light gray bar denotes the control condition transfected with YFP only and treated with vehicle, while the dark gray bars denote significant difference from the vehicle-treated, Htt-N90Q73-transfected condition by ANOVA followed by Dunnett's post hoc comparison test at the 5% confidence level. Dashed lines show 1 SD above the mean value for Htt-N90Q73. In all cases, the control condition (light gray bars) is also significantly different from the vehicle-treated, Htt-N90Q73-transfected condition by these statistical criteria. (c) Partial rescue of Htt-N90Q73-transfected neurons by 200 ng/ml recombinant human BDNF added to the culture medium at the time of explantation and fed once thereafter on day 3. This concentration of BDNF in the bulk medium was used to compensate for the limited penetrability of intact brain tissue, and has previously been demonstrated to retain selectivity for cognate TrkB receptors (Ip et al., 1993; McAllister et al., 1997; Yacoubian and Lo, 2000). (d) Several HDAC inhibitors were able to provide concentration-dependent neuroprotection to Htt-N90Q73-transfected MSNs as assessed at day 5 after single bolus compound administration at the time of explantation and transfection; note the characteristic inverted U-shaped concentration curves for these compounds. Of these HDAC inhibitors, the FDA-approved drug SAHA consistently showed the highest and most reproducible levels of neuroprotection. SAHA, suberoylanilide hydroxamic acid; TSA, trichostatin A; PBA, phenylbutyrate.
Fusion of a CFP tag to the Htt-N90Q73 construct also allowed the direct visualization of HTT-containing aggregates; as can be seen in Fig. 1c and d, transfection of HTT-based constructs containing >35 glutamines resulted in the formation of CFP-labeled aggregates. These aggregates stained positive for the HTT exon-1 antibody MAB 5492 (2B4; Fig. 1i–l) (Lunkes et al., 2002) and for the HTT aggregate-specific antibody mEM48 (Fig. 1m, n) (Yu et al., 2002), indicating similarity of these HTT-containing aggregates to those observed in human disease and in mouse models (Davies et al., 1997; DiFiglia et al., 1997; Scherzinger et al., 1997). The location of these aggregates was predominantly perinuclear (Fig. 1o, p), reminiscent of that previously reported for aggresomes of HTT and other misfolded proteins (Johnston et al., 1998; Taylor et al., 2003; Waelter et al., 2001).
Cross-validation of this brain-slice HD screening platform was done by testing a range of small molecule compounds that have been shown to be active other HD assays and, in particular, in whole animal, transgenic models for HD. To date, numerous compounds that have been reported to be active in cell-line and/or primary culture models for HD have generally shown limited activity in this brain slice based HD assay, including Congo Red, trehalose, benzothiazoles, C2–8, cardiac glycosides, and cannabinoids (Fecke et al., 2009; Varma et al., 2008). Notable exceptions were the pan-caspase inhibitor BOC-D-FMK (Aiken et al., 2004; Varma et al., 2007) (Fig. 2b) and BDNF (Zuccato and Cattaneo, 2007) (Fig. 2c). That the majority of the hits from in vitro assays did not replicate in this brain slice-based HD assay, though many of these assays incorporated HTT-based expression constructs similar to those used here, suggests that there are indeed significant physiological differences in cellular function and phenotype between dissociated neurons/cell lines and brain slice explants in which primary neurons remain in situ in their immediate tissue context.
In contrast, several compounds that did show efficacy in the brain slice-based HD were also ones for which there was in vivo data supporting their therapeutic potential in HD, most notably histone deacetylase (HDAC) inhibitors, which have been shown to be broadly efficacious across a range of in vivo models for HD (Butler and Bates, 2006; Hockly et al., 2003; Kazantsev and Thompson, 2008; Thomas et al., 2008). As can be seen in Fig. 2d, several HDAC inhibitors showed concentration-dependent protection against Htt-N90Q73-induced neurodegeneration of MSNs. Note that concentration values given are for bulk concentrations of these compounds in the supporting media; actual concentrations in the interior of the brain slices were likely to be significantly lower. Interestingly, the most consistently protective HDAC inhibitor in this assay was suberoylanilide hydroxamic acid (SAHA), which has also been shown to be protective in a number of in vitro as well as in vivo HD models (Butler and Bates, 2006), and is FDA-approved (Vorinostat) for treatment of refractory cutaneous T-cell lymphoma (Garber, 2007).
Screening of a chemical biology library of neuroprotective and anti-inflammatory drug-like compounds
Implementation of this brain slice-based assay for HD as a "high-throughput biology" screen is demonstrated in Fig. 3a, showing good consistency over extended periods of screening and the ability to clearly identify primary hits as well as compound toxicity. We describe here the results of focusing this HD screening platform on a collection of 74 drug-like compounds directed against targets implicated in cell death and inflammatory processes (these compounds and their nominal targets of action are listed in Supplemental Table 1). While several of these compounds/targets have been previously implicated in HD pathogenesis, or broadly associated with neurodegenerative processes, the rationale here was to probe directly whether intervention at each of these targets, in an hypothesis-neutral manner, was sufficient to modify the rate and/or extent of neuronal degeneration driven by Htt-N90Q73 within a neural tissue context.
Figure 3.
Use of the brain slice-based HD assay for small molecule compound screening and the identification of neuroprotective hits. (a) Example of a blinded screening run of 275 compound-concentrations assessing numbers of healthy MSNs at day 5 after co-transfection with YFP and Htt-N90Q73. Note that several compounds were toxic in certain concentration ranges; there was one strong hit in this screening run that rescued fully to control levels (solid horizontal line at a value of 315%) which was subsequently decoded and identified as BOC-D-FMK (see Fig. 2b). The second data point shows "super-rescue" by triple co-transfection with the anti-apoptotic gene Bcl-xL. (b) The IKKβ inhibitors WAY-717 ("W-717") and WAY-781 ("W-781"), but not WAY-638 ("W-638"), provided significant neuroprotection to Htt-N90Q73-transfected neurons in a concentration-dependent manner. (c) Inhibiting the IKK complex by the alternative means of an IKK NEMO binding-domain (IKK-NBD) peptide also provided significant neuroprotection. (d) The CXCR3 inhibitors Compound 6 ("Cpd 6") and T487 significantly rescued against Htt-N90Q73-induced neurodegeneration in a concentration-dependent manner. For (b) through (d), bulk compound concentrations in the culture medium are shown are in µM. Light and dark gray bars denote significant differences from the vehicle-treated, Htt-N90Q73-transfected condition by ANOVA followed by Dunnett's post hoc comparison test at the 5% confidence level.
Primary hits from this screen were confirmed by re-testing, out of which emerged 4 compound/target classes that provided significant and reproducible neuroprotection against Htt-N90Q73-induced neurodegeneration in this brain slice-based HD screening assay. Interestingly, these 4 target classes each targeted different aspects of inflammatory processes that likely contributed to Htt-N90Q73-induced neurodegeneration via direct as well as non-cell autonomous mechanisms.
IκB kinase complex (IKK)
Fig. 3b shows that 2 of 3 small molecule inhibitors of IKK were found to provide neuroprotection to MSNs in Htt-N90Q73-transfected brain slices: WAY-717 and WAY-781 are both potent inhibitors of IKKβ (IC50= 0.04 and 0.014 µM, respectively) and inhibit TNF induction by LPS in the sub- to low micromolar range in cell models. These neuroprotective effects were specific for Htt-N90Q73-induced neurodegeneration, as the survival and health of control, YFP-transfected MSNs were not affected by treatment with these compounds over the same concentration range (Supplemental Fig. 1a). This direct evidence that drug intervention in the IKK pathway can have therapeutic potential in HD supports previous reports from our work and others of the involvement of the NF-κB pathway in HD and other neurodegenerative diseases (Kaltschmidt and Kaltschmidt, 2010; Khoshnan et al., 2004; Mattson and Meffert, 2006; Thompson et al., 2009). To corroborate that the target of these small molecule compounds was IKKβ, we tested and confirmed the efficacy of an IKK-Nemo binding domain peptide (IKK-NBD, fused to the antennapedia cell permeation sequence; Fig. 3 c), which also inhibits the IKK complex but by disruption of the protein interaction between the IKK β and γ subunits (Hayden and Ghosh, 2008).
CXCR3 chemokine receptor
The two compounds shown in Fig. 3d, Compound 6c and T487, are directed against the CXCR3 chemokine receptor (Cole et al., 2006; Medina et al., 2004), with both compounds showing significant, concentration-dependent protection against Htt-N90Q73-induced neurodegeneration of MSNs. As for the IKKβ inhibitors described above, these neuroprotective effects were specific for Htt-N90Q73-induced neurodegeneration as the survival and health of control, YFP-transfected MSNs were not affected (Supplemental Fig. 1b). While these compounds were originally developed for Th1-mediated immune disorders (Cole et al., 2006; Medina et al., 2004) with good selectively and potency, there is increasing appreciation of the role that CXCR3 may play in neurodegenerative processes (Lazzeri and Romagnani, 2005; Rappert et al., 2004). In addition to their expression on microglia, CXCR3 receptor expression has also been demonstrated on neurons in cortex as well as striatum, and with increased expression in astrocytes in Alzheimer's disease (Xia et al., 2000).
c-Jun N-terminal kinase (JNK)
SP600125, a specific inhibitor of JNK, provided significant neuroprotection against the degeneration of MSNs induced by transfection of Htt-N90Q73 (Fig. 4a). Interesting, JNK is implicated in apoptosis (Dhanasekaran and Reddy, 2008), and its activation has previously been implicated in in vitro and in vivo models of HD (Apostol et al., 2006; Apostol et al., 2008; Merienne et al., 2003; Scappini et al., 2007). Recently, it has been shown that intervention in JNK-related pathways can provide benefit in a rat lentiviral model of HD (Perrin et al., 2009).
Figure 4.
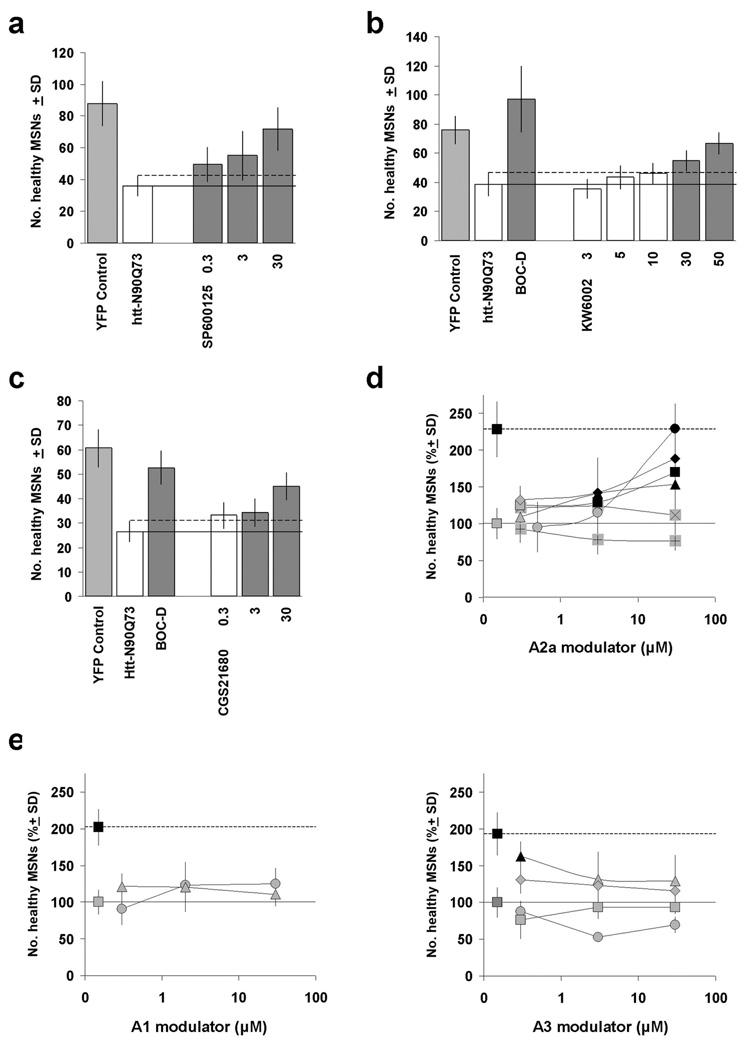
JNK and adenosine 2A (A2A) receptor modulators provide neuroprotection in the brain slice HD assay. (a) Treatment of brain slices with the small molecule JNK inhibitor SP600125 provided significant protection against Htt-N90Q73-induced neurodegeneration of MSNs. (b) The A2A receptor antagonist KW-6002 provided quantitative, concentration-dependent rescue of httN90Q73-transfected MSNs in the brain slice-based HD assay. (c) Intriguingly, the A2A receptor agonist CGS21680 also provided neuroprotection in this tissue-based assay. Light and dark gray bars denote significant differences from the vehicle-treated, Htt-N90Q73-transfected condition by ANOVA followed by Dunnett’s post hoc comparison test at the 5% confidence level. (d) Significant neuroprotection was also provided by some other A2A receptor modulators, but not by A1 or A3 receptor modulators (e). (d) A2A modulators: KW-6002 (●, antagonist); SCH58261 (X, antagonist); SCH442416 (▲, antagonist) ZM241385 (+, antagonist); 2-(1-hexyn-1-yl)adenosine-5'-n-ethyluronamide (HE-NECA, ♦, agonist); CGS 21680 (■, agonist). (e) A1 modulators: 8-cyclopentyl-1,3-dipropylxanthine (▲, antagonist); ADAC (●, agonist). A3 modulators: MRS1220 (●, antagonist); 3-propyl-6-ethyl-5-[(ethylthio)carbony]-2 phenyl-propyl-3-pyridine carboxylate (MRS1523, ■, antagonist); I-AB-MECA (▲, agonist); IB-MECA (♦, agonist). Bulk compound concentrations in the culture medium for all compounds are shown are in µM. Dark symbols denote significant differences from the vehicle-treated, Htt-N90Q73-transfected condition (set to 100%) by ANOVA followed by Dunnett's post hoc comparison test at the 5% confidence level.
Adenosine 2A (A2A) receptor
Finally, we found that the A2A receptor antagonist KW-6002 (istradefylline) provided strong neuroprotection to Htt-N90Q73-transfected MSNs (Fig. 4b); indeed, KW-6002 has been the most effective compound out of ~700 compounds tested to date in the brain slice-based HD assay. Importantly, the neuroprotective effect of KW-6002 was selective for Htt-N90Q73-induced neurodegeneration as there was no enhancement of numbers of healthy MSNs in control brain slices transfected with the YFP visual marker alone (Suppl. Fig. 1c).
To address the pharmacological action of istradefylline, we next assayed 6 additional modulators of adenosine receptors (Jacobson and Gao, 2006) in the brain slice assay (Fig. 4d) and found that a number of the A2A receptor modulators reduced MSN degeneration significantly, whereas A1 and A3 adenosine receptor modulators generally did not (Fig. 4e). Two relatively non-specific adenosine receptor antagonists, theophylline and CGS15943, also provided partial levels of neuroprotection (data not shown). Of all the adenosine receptor modulators tested, KW-6002 reproducibly afforded the greatest level MSN neuroprotection.
Surprisingly, we found that both A2A receptor antagonists (e.g., KW-6002) as well as agonists (e.g., CGS21680) could provide neuroprotection in the brain slice assay (Fig. 4c), as has also been observed in other assays and in in vivo HD models (Popoli et al., 2008). CGS21680 has previously been reported to improve motor and neurological behaviors in the R6/2 mouse, while A2A receptor antagonists ameliorate damage in excitotoxic models of striatal neurodegeneration (Chou et al., 2005; Popoli et al., 2007). In fact, we have observed that the protective effects of KW-6002 and CGS21680 are partially additive rather than opposing in the brain slice assay (data not shown).
Discussion
We have shown here that a brain tissue-based drug screening assay can be developed for HD, and that such an assay can be used for primary screening of focused compound libraries as well as for the evaluation of specific compound classes directed at particular molecular targets. A key advantage of this platform in recapitulating HD disease progression is that intact tissue explants preserve the local cellular and molecular environment of striatal neurons; indeed, recent studies have demonstrated the importance of tissue context and non-cell autonomous processes in HD-induced pathogenesis (Gu et al., 2007; Gu et al., 2005). In fact, the neuroprotective effects of the CXCR3 inhibitors shown here would not likely have been detected in conventional cell-based assays, in which microglial are absent. Thus, these studies highlight that pathological cell-cell interactions among multiple cell-types resident in intact brain tissue may be involved and/or required for striatal degeneration in HD (Gu et al., 2007; Ilieva et al., 2009).
The neuroprotective effects of the CXCR3, IKK, and JNK inhibitors in our brain slice-based HD platform are of interest and provide further evidence for the role of inflammation in the progression of neurodegenerative diseases (Bjorkqvist et al., 2008; Möller, ; Perry et al., 2007). CXCR3, IKK, and JNK are key molecular mediators in initiating and propagating inflammatory responses in the CNS and the compounds tested in the present study were selected based on their potent and selective inhibitory in vitro profiles against these molecular targets. Notably, the efficacy of such compounds or lack thereof in this tissue-based HD-assay allows the rank-ordering of potential development compounds that otherwise may have similar profiles in simple homogeneous cell-based assays; the IKKβ inhibitor WAY-638, for example, did not show neuroprotection in this assay despite its comparable in vitro potency at the enzyme level (IC50=17.9 nM). As inflammation may play critical roles in a range of neuropathological disorders including Alzheimer's disease, Parkinson's disease and traumatic brain injury, potential therapeutics discovered in the course of HD drug discovery such as those targeting CXCR3, IKK, and JNK may prove to have broad clinical utility. In fact, we have recently provided direct evidence that JNK inhibition also protects against APP-induced neurodegeneration, but downstream of Aβ processing and production (Braithwaite et al., 2010).
As A2A receptors are also expressed in microglia (Pocock and Kettenmann, 2007), the neuroprotective effects of A2A receptor modulators observed may also reflect anti-inflammatory actions, at least in part, and our observations here add to the growing evidence for the clinical relevance of these receptors in HD (Popoli et al., 2007). Clearly, however, adenosine signaling in the striatum is complex; neuronal A2A receptors in striatum are expressed both presynaptically and postsynaptically (Popoli et al., 2007; Shen et al., 2008), and further diversity in adenosine signaling is generated by receptor heterodimerization (Ferre et al., 2007). A2A receptors are selectively expressed in striatopallidal neurons (Albin et al., 1992), and are substantially reduced in the striatum of R6/2 mice and post mortem in HD (Cha et al., 1999; Glass et al., 2000). Furthermore, aberrations in A2A receptor signaling occur in the R6/2 mouse, as well as in blood cells from HD patients (Tarditi et al., 2006). Finally, the neuroprotective benefits of these A2A receptor modulators could have been mediated, at least in part, through the well-documented cross-activation of TrkB by A2A receptor activation (Diogenes et al., 2004; Lee and Chao, 2001; Mojsilovic-Petrovic et al., 2006; Pousinha et al., 2006; Rajagopal et al., 2004; Tebano et al., 2008; Wiese et al., 2007). Such heterogeneities in adenosine receptor composition, cellular and subcellular loci, downstream signaling pathways, and functional responses may contribute to the paradoxical observation here and in previous reports that both nominal A2A receptor antagonists as well as agonists can provide apparent benefit in HD models (Fig. 4b–d).
The therapeutic potential of KW-6002 for HD described here is of particular interest because of its clinical testing in Parkinson’s disease and its proximity to clinical usage (Jacobson and Gao, 2006). KW-6002 has been shown to be safe in clinical Phase I studies, and provided benefit in Phase II studies alone or in combination with L-dopa (Schwarzschild et al., 2006), although a recent Phase II monotherapy trial did not achieve its primary endpoint (Fernandez et al., 2010). Thus, KW-6002's apparent safety and tolerability (Schwarzschild et al., 2006), together with its robust efficacy shown here in the brain slice HD assay and in R6/2 mice (L. Menalled, personal communication), suggest that it merits further consideration as a potential therapeutic for HD.
Finally, the approach we have taken in developing this brain-slice based screen for HD can be generalized to other neurodegenerative disorders including Alzheimer's and Parkinson's diseases for which genetic linkages are known, at least for familial forms (Braithwaite et al., 2010). This brain-slice platform approach is also consonant with the growing emphasis in the drug discovery field on hypothesis-neutral drug screening strategies in identifying new classes of therapeutic compounds for intractable disorders, especially for those for which clinically validated drug targets are not yet known. The cost and throughput of this screening platform is such that it can be used for primary screening of drug candidates via focused drug libraries and chemical biological approaches, and can be incorporated into drug development pipelines as a secondary screening system for hit-to-lead progression and for lead optimization. In fact, we recently used this platform to help prioritize and optimize lead compound candidates and conduct target validation studies from discovery screens originating in HD cell line models (Hoffstrom et al., 2010; Varma et al., 2007). Importantly, such unbiased phenotypic/functional screens have the potential for identifying drug targets and pathways that may not be contained within the core processes of disease pathogenesis. Such potential drug targets may thus not be easily discoverable through basic mechanistic studies, but nevertheless could be capable of modifying disease progression therapeutically in the clinical context.
Supplementary Material
Acknowledgements
Our grateful thanks to Carl Johnson, Allan Tobin, Ethan Signer, Nancy Wexler, Jim Wang, Larry Park, Vahri Beaumont, and Robert Pacifici for their encouragement and support, and to Max Wallace who made this work possible. Many thanks also to Martin Digrandi and Perry Hall for providing the IKK inhibitors used in this study. Supported by the High Q Foundation, CHDI Foundation, Inc., the Hereditary Disease Foundation, and NIH grant NS048181.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiken CT, et al. A cell-based screen for drugs to treat Huntington's disease. Neurobiology of Disease. 2004;16:546–555. doi: 10.1016/j.nbd.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Albin RL, et al. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington's disease. Annals of Neurology. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- Apostol BL, et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Human Molecular Genetics. 2006;15:273–285. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- Apostol BL, et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Molecular and Cellular Neuroscience. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist M, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. The Journal of Experimental Medicine. 2008;205:1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell-Pages M, et al. Huntington's disease: from huntingtin function and dysfunction to therapeutic strategies. Cellular & Molecular Life Sciences. 2006;63:2642–2660. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, et al. Inhibition of c-Jun kinase provides neuroprotection in a model of Alzheimer's disease. Neurobiology of Disease. 2010;39:311–317. doi: 10.1016/j.nbd.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick JN, et al. Identification of signal transduction pathways used by orphan g protein-coupled receptors. Assay & Drug Development Technologies. 2003;1:239–249. doi: 10.1089/15406580360545053. [DOI] [PubMed] [Google Scholar]
- Butler R, Bates GP. Histone deacetylase inhibitors as therapeutics for polyglutamine disorders. Nature Reviews Neuroscience. 2006;7:784–796. doi: 10.1038/nrn1989. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, et al. Normal huntingtin function: an alternative approach to Huntington's disease. Nature Reviews Neuroscience. 2005;6:919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- Cha JH, et al. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington's disease. Philosophical Transactions of the Royal Society of London -Series B: Biological Sciences. 1999;354:981–989. doi: 10.1098/rstb.1999.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, et al. CGS21680 attenuates symptoms of Huntington's disease in a transgenic mouse model. Journal of Neurochemistry. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- Cole AG, et al. Identification and initial evaluation of 4-N-aryl-[1,4]diazepane ureas as potent CXCR3 antagonists. Bioorganic & Medicinal Chemistry Letters. 2006;16:200–203. doi: 10.1016/j.bmcl.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, et al. CalDAG-GEFI down-regulation in the striatum as a neuroprotective change in Huntington's disease. Human Molecular Genetics. 2010;19:1756–1765. doi: 10.1093/hmg/ddq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SW, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Diogenes MJ, et al. Activation of Adenosine A2A Receptor Facilitates Brain-Derived Neurotrophic Factor Modulation of Synaptic Transmission in Hippocampal Slices. Journal of Neuroscience. 2004;24:2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecke W, et al. Small molecule drug discovery for Huntington's Disease. Drug Discovery Today. 2009;14:453–464. doi: 10.1016/j.drudis.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, et al. Istradefylline as monotherapy for Parkinson disease: Results of the 6002-US-051 trial. Parkinsonism & Related Disorders. 2010;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ferre S, et al. Adenosine Receptor Heteromers and Their Integrative Role in Striatal Function. TheScientificWorldJOURNAL. 2007 doi: 10.1100/tsw.2007.211. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber K. HDAC inhibitors overcome first hurdle. Nature Biotechnology. 2007;25:17–19. doi: 10.1038/nbt0107-17. [DOI] [PubMed] [Google Scholar]
- Glass M, et al. The pattern of neurodegeneration in Huntington's disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington's disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- Gu X, et al. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington's disease. Mol Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 2005;46:433–444. doi: 10.1016/j.neuron.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Gusella JF, Macdonald ME. Huntington’s disease: seeing the pathogenic process through a genetic lens. Trends in Biochemical Sciences. 2006;31:533–540. doi: 10.1016/j.tibs.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared Principles in NF-kappaB Signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hockly E, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstrom BG, et al. Inhibitors of protein disulfide isomerase suppress apoptosis induced by misfolded proteins. Nature Chemical Biology. 2010;6:900–906. doi: 10.1038/nchembio.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Ilieva H, et al. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. The Journal of Cell Biology. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imarisio S, et al. Huntington's disease: from pathology and genetics to potential therapies. Biochemical Journal. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- Ip NY, et al. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nature Reviews Drug Discovery. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, et al. Aggresomes: A Cellular Response to Misfolded Proteins. The Journal of Cell Biology. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C. NF-kappaB in the Nervous System. Cold Spring Harbor Perspectives in Biology. 2010:2. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Khoshnan A, et al. Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. Journal of Neuroscience. 2004;24:7999–8008. doi: 10.1523/JNEUROSCI.2675-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landles C, et al. Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease. Journal of Biological Chemistry. 2010;285:8808–8823. doi: 10.1074/jbc.M109.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Current Drug Targets - Immune Endocrine & Metabolic Disorders. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- Lee FS, Chao MV. Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo DC, et al. Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron. 1994;13:1263–1268. doi: 10.1016/0896-6273(94)90412-x. [DOI] [PubMed] [Google Scholar]
- Lunkes A, et al. Proteases Acting on Mutant Huntingtin Generate Cleaved Products that Differentially Build Up Cytoplasmic and Nuclear Inclusions. Molecular Cell. 2002;10:259–269. doi: 10.1016/s1097-2765(02)00602-0. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- McAllister AK, et al. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Medina J, et al. Discovery and development of a CXCR3 antagonist T487 as therapy for Th1-mediated immune disorders. 29th National Medicinal Chemistry Symposium. 2004 [Google Scholar]
- Menalled L, et al. Systematic behavioral evaluation of Huntington's disease transgenic and knock-in mouse models. Neurobiology of Disease. 2009;35:319–336. doi: 10.1016/j.nbd.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merienne K, et al. Polyglutamine expansion induces a protein-damaging stress connecting heat shock protein 70 to the JNK pathway. Journal of Biological Chemistry. 2003;278:16957–16967. doi: 10.1074/jbc.M212049200. [DOI] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J, et al. Protecting Motor Neurons from Toxic Insult by Antagonism of Adenosine A2a and Trk Receptors. Journal of Neuroscience. 2006;26:9250–9263. doi: 10.1523/JNEUROSCI.1856-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller T. Neuroinflammation in Huntington's disease. Journal of Neural Transmission. 117:1001–1008. doi: 10.1007/s00702-010-0430-7. [DOI] [PubMed] [Google Scholar]
- Perrin V, et al. Implication of the JNK pathway in a rat model of Huntington's disease. Experimental Neurology. 2009;215:191–200. doi: 10.1016/j.expneurol.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Perry VH, et al. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends in Neurosciences. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Popoli P, et al. A Critical Evaluation of Adenosine A2a Receptors as Potentially "Druggable" Targets in Huntington's Disease. Current Pharmaceutical Design. 2008;14:1500–1511. doi: 10.2174/138161208784480117. [DOI] [PubMed] [Google Scholar]
- Popoli P, et al. Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington's disease. Progress in Neurobiology. 2007;81:331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pousinha PA, et al. Triggering of BDNF facilitatory action on neuromuscular transmission by adenosine A2A receptors. Neuroscience Letters. 2006;404:143–147. doi: 10.1016/j.neulet.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, et al. Transactivation of Trk Neurotrophin Receptors by G-Protein-Coupled Receptor Ligands Occurs on Intracellular Membranes. Journal of Neuroscience. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappert A, et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. Journal of Neuroscience. 2004;24:8500–8509. doi: 10.1523/JNEUROSCI.2451-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scappini E, et al. Intersectin enhances huntingtin aggregation and neurodegeneration through activation of c-Jun-NH2-terminal kinase. Human Molecular Genetics. 2007;16:1862–1871. doi: 10.1093/hmg/ddm134. [DOI] [PubMed] [Google Scholar]
- Scherzinger E, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, et al. Targeting adenosine A2A receptors in Parkinson's disease. Trends in Neurosciences. 2006;29:647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Shen H-Y, et al. A Critical Role of the Adenosine A2A Receptor in Extrastriatal Neurons in Modulating Psychomotor Activity as Revealed by Opposite Phenotypes of Striatum and Forebrain A2A Receptor Knock-Outs. Journal of Neuroscience. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, et al. A simple method for organotypic cultures of nervous tissue. Journal of Neuroscience Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Tarditi A, et al. Early and transient alteration of adenosine A2A receptor signaling in a mouse model of Huntington disease. Neurobiology of Disease. 2006;23:44–53. doi: 10.1016/j.nbd.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Taylor JP, et al. Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Human Molecular Genetics. 2003;12:749–757. doi: 10.1093/hmg/ddg074. [DOI] [PubMed] [Google Scholar]
- Tebano MT, et al. Adenosine A2a receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. 2008;Vol. 104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- Thomas EA, et al. The HDAC inhibitor 4b ameliorates the disease phenotype and transcriptional abnormalities in Huntington's disease transgenic mice. 2008;Vol. 105:15564–15569. doi: 10.1073/pnas.0804249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LM, et al. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. Journal of Cell Biology. 2009;187:1083–1099. doi: 10.1083/jcb.200909067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma H, et al. High Throughput Screening for Neurodegeneration and Complex Disease Phenotypes. Combinatorial Chemistry & High Throughput Screening. 2008;11:238–248. doi: 10.2174/138620708783877753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma H, et al. Selective inhibitors of death in mutant huntingtin cells. Nature Chemical Biology. 2007;3:99–100. doi: 10.1038/nchembio852. [DOI] [PubMed] [Google Scholar]
- Waelter S, et al. Accumulation of Mutant Huntingtin Fragments in Aggresome-like Inclusion Bodies as a Result of Insufficient Protein Degradation. Molecular Biology of the Cell. 2001;12:1393–1407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wang JK, et al. Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10461–10466. doi: 10.1073/pnas.0600930103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese S, et al. Adenosine receptor A2A–R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proceedings of the National Academy of Sciences. 2007;104:17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia MQ, et al. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer's disease. Journal of Neuroimmunology. 2000;108:227–235. doi: 10.1016/s0165-5728(00)00285-x. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nature Neuroscience. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Yu ZX, et al. Huntingtin inclusions do not deplete polyglutamine-containing transcription factors in HD mice. Human Molecular Genetics. 2002;11:905–914. doi: 10.1093/hmg/11.8.905. [DOI] [PubMed] [Google Scholar]
- Zhang JH, et al. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Progress in Neurobiology. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.