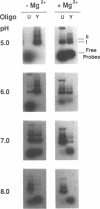
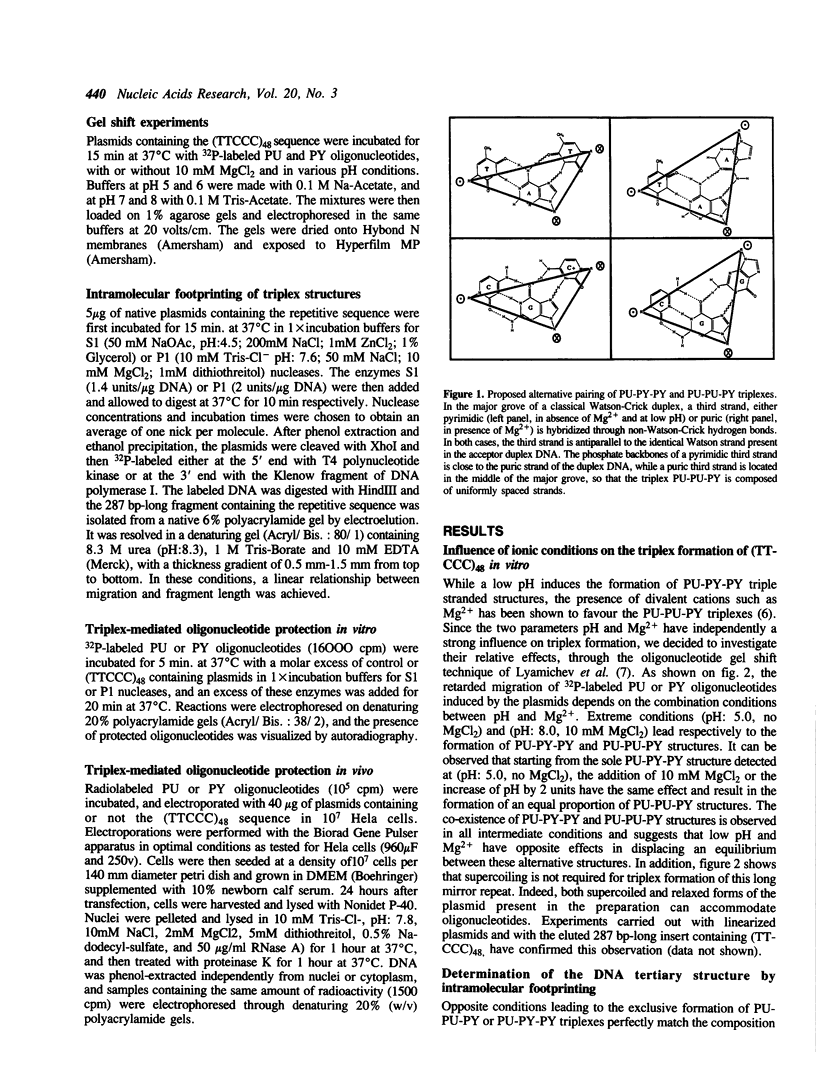
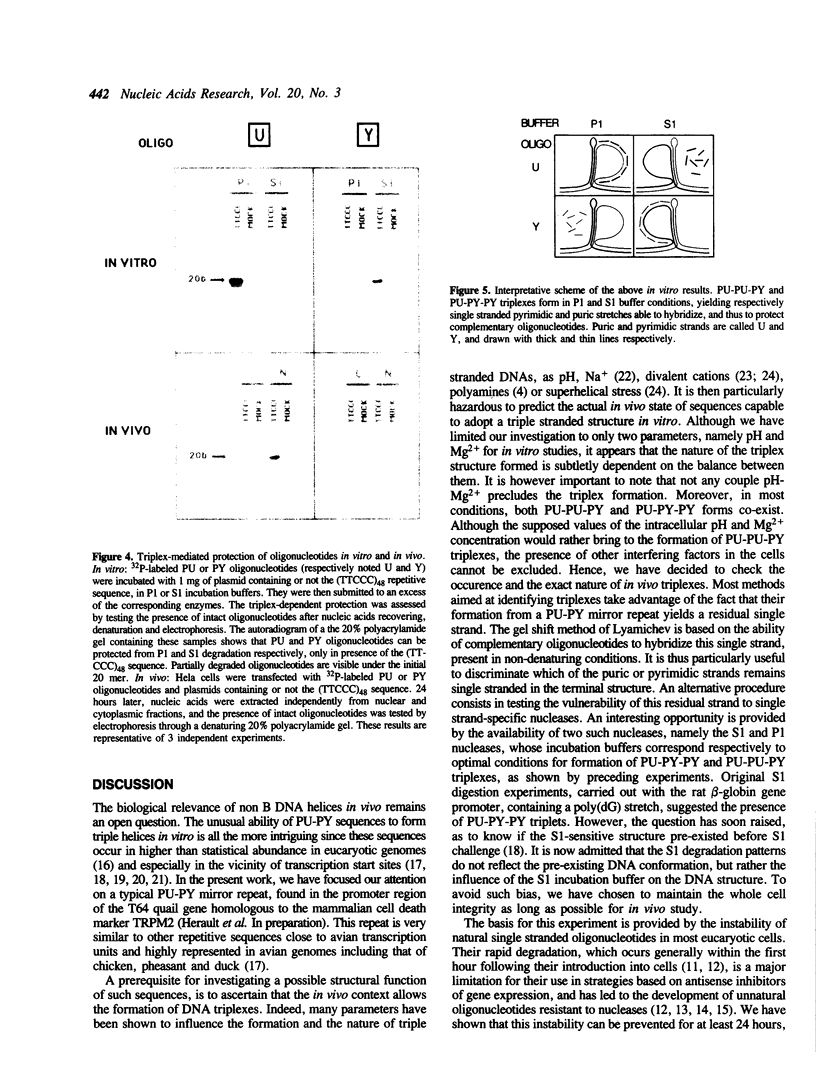
Abstract
Polypurine/polypyrimidine repetitive sequences occur with high frequency in eucaryotic genomes, particularly around transcription units. Since such sequences are known to adopt triple stranded-structures under appropriate conditions in vitro, it is of major interest to know if they occur in vivo, and thus if they can have some biological importance by inducing structural constraints in the genomic DNA. To this end, we have isolated a (TTCCC)48 sequence, present in the promoter of an avian gene, and tested its ability to form PU-PY-PY and PU-PU-PY triple helices in vitro, through the oligonucleotide gel shift technique and single strand-specific nuclease footprinting. We have then developed an oligonucleotide protection assay, which can be adapted to in vivo investigations. This strategy leads us to conclude that in vivo conditions allow preponderant formation of triplex of the PU-PU-PY class.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasty A. M., Behe M. J. An oligopurine sequence bias occurs in eukaryotic viruses. Nucleic Acids Res. 1988 Feb 25;16(4):1517–1528. doi: 10.1093/nar/16.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe D., Cabrer B., Bacolla A., Targovnik H., Pohl V., Vassart G. An unusually long poly(purine)-poly(pyrimidine) sequence is located upstream from the human thyroglobulin gene. Nucleic Acids Res. 1985 Jul 25;13(14):5127–5144. doi: 10.1093/nar/13.14.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D. A., Wells R. D. Effect of length, supercoiling, and pH on intramolecular triplex formation. Multiple conformers at pur.pyr mirror repeats. J Biol Chem. 1990 Jun 25;265(18):10652–10658. [PubMed] [Google Scholar]
- Gilmour D. S., Thomas G. H., Elgin S. C. Drosophila nuclear proteins bind to regions of alternating C and T residues in gene promoters. Science. 1989 Sep 29;245(4925):1487–1490. doi: 10.1126/science.2781290. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Thomas G. H., Siegfried E., Elgin S. C., Lis J. T. Optimal heat-induced expression of the Drosophila hsp26 gene requires a promoter sequence containing (CT)n.(GA)n repeats. J Mol Biol. 1990 Feb 20;211(4):751–761. doi: 10.1016/0022-2836(90)90075-W. [DOI] [PubMed] [Google Scholar]
- Hampel K. J., Crosson P., Lee J. S. Polyamines favor DNA triplex formation at neutral pH. Biochemistry. 1991 May 7;30(18):4455–4459. doi: 10.1021/bi00232a012. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Detection of triple-helix related structures adopted by poly(dG)-poly(dC) sequences in supercoiled plasmid DNA. Nucleic Acids Res. 1991 Aug 11;19(15):4267–4271. doi: 10.1093/nar/19.15.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y. Cationic metal-specific structures adopted by the poly(dG) region and the direct repeats in the chicken adult beta A globin gene promoter. Nucleic Acids Res. 1989 Jun 26;17(12):4493–4502. doi: 10.1093/nar/17.12.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer L. J., Hampel K., Lee J. S. Synthetic repeating sequence DNAs containing phosphorothioates: nuclease sensitivity and triplex formation. Nucleic Acids Res. 1989 Feb 25;17(4):1549–1561. doi: 10.1093/nar/17.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J., Morgan A. R. Poly(pyrimidine) . poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984 Aug 24;12(16):6603–6614. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamichev V. I., Mirkin S. M., Frank-Kamenetskii M. D., Cantor C. R. A stable complex between homopyrimidine oligomers and the homologous regions of duplex DNAs. Nucleic Acids Res. 1988 Mar 25;16(5):2165–2178. doi: 10.1093/nar/16.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor H., Rao B. S., Martin R. G. Abundance and degree of dispersion of genomic d(GA)n.d(TC)n sequences. J Mol Evol. 1988;27(2):96–101. doi: 10.1007/BF02138367. [DOI] [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus-Sekura C. J., Woerner A. M., Shinozuka K., Zon G., Quinnan G. V., Jr Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages. Nucleic Acids Res. 1987 Jul 24;15(14):5749–5763. doi: 10.1093/nar/15.14.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L., Heilig R., Dupret D., Mandel J. L. Repetitive satellite-like sequences are present within or upstream from 3 avian protein-coding genes. Nucleic Acids Res. 1983 Mar 11;11(5):1227–1243. doi: 10.1093/nar/11.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrothalassitis G. J., Watson D. K., Papas T. S. The human ETS-2 gene promoter: molecular dissection and nuclease hypersensitivity. Oncogene. 1990 Sep;5(9):1337–1342. [PubMed] [Google Scholar]
- Michel D., Gillet G., Volovitch M., Pessac B., Calothy G., Brun G. Expression of a novel gene encoding a 51.5 kD precursor protein is induced by different retroviral oncogenes in quail neuroretinal cells. Oncogene Res. 1989;4(2):127–136. [PubMed] [Google Scholar]
- Mirkin S. M., Lyamichev V. I., Drushlyak K. N., Dobrynin V. N., Filippov S. A., Frank-Kamenetskii M. D. DNA H form requires a homopurine-homopyrimidine mirror repeat. Nature. 1987 Dec 3;330(6147):495–497. doi: 10.1038/330495a0. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. DNA conformation at the 5' end of the chicken adult beta-globin gene. Cell. 1983 Dec;35(2 Pt 1):467–477. doi: 10.1016/0092-8674(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Subasinghe C., Shinozuka K., Cohen J. S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res. 1988 Apr 25;16(8):3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman-Weinberg A., Littauer U. Z., Ginzburg I. The inhibition of neurite outgrowth in PC12 cells by tubulin antisense oligodeoxyribonucleotides. Gene. 1988 Dec 10;72(1-2):297–307. doi: 10.1016/0378-1119(88)90155-2. [DOI] [PubMed] [Google Scholar]
- Thuong N. T., Asseline U., Roig V., Takasugi M., Hélène C. Oligo(alpha-deoxynucleotide)s covalently linked to intercalating agents: differential binding to ribo- and deoxyribopolynucleotides and stability towards nuclease digestion. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5129–5133. doi: 10.1073/pnas.84.15.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf T. M., Jennings C. G., Rebagliati M., Melton D. A. The stability, toxicity and effectiveness of unmodified and phosphorothioate antisense oligodeoxynucleotides in Xenopus oocytes and embryos. Nucleic Acids Res. 1990 Apr 11;18(7):1763–1769. doi: 10.1093/nar/18.7.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]