Abstract
In a study of performance-enhancing substance use among 231 experienced young male weightlifters, we found that 27 (12%) reported illicit use of human growth hormone (HGH) or its bioactive derivative, insulin-like growth factor-1 (IGF-I). All of these 27 men also reported use of anabolic-androgenic steroids (AAS) and 22 (81%) met criteria for current or past AAS dependence. Fifteen (56%) also reported current or past dependence on opioids, cocaine, and/or ecstasy. These findings suggest that among young male weightlifters, illicit HGH use has become a common form of substance abuse, frequently associated with both AAS dependence and classical substance dependence.
Introduction
Human growth hormone (HGH), once an expensive performance-enhancing drug used primarily by elite athletes,1,2 has now become cheaply available over the Internet.3–6 However, few studies have as yet assessed the prevalence or correlates of illicit HGH use. Anonymous surveys have produced disparate results; an American study claimed that 11 (5%) of 224 10th-grade boys and one (0.5%) of 208 girls had used HGH,7 but a German survey of 2287 adolescent students found a prevalence of only 0.5% in boys and 0.3% in girls.8 An anonymous survey of 100 anabolic-androgenic steroid (AAS) users in Wales found that 12 (12%) users reported having tried HGH,9 and a similar Welsh study nine years later found that the prevalence had risen to 25 (24%) of 102 AAS users.10 An interview study found only one HGH user (0.6%) among 176 self-declared AAS users (171 men, 5 women) in Wales,11 and another found only one (4%) HGH user among 25 American women who had used AAS.12 A subsequent interview study of experienced male weightlifters found that 3 (6%) of 48 AAS users reported lifetime HGH use, and one additional AAS user reported use of the bioactive derivative of HGH, insulin-like growth factor-1 (IGF-I).13 None of the 45 comparison weightlifters in this study reported use of HGH or IGF-I.
Notably, the above studies were conducted at a time when HGH was more expensive than it is today.6 By contrast, a recent interview study of 32 AAS users seeking treatment at a Swedish addiction clinic (30 male, 2 female) reported that 15 (47%) had used HGH and 5 (16%) IGF-I.14 These individuals used many classical drugs of abuse, including cannabis, opioids, and amphetamines – suggesting that AAS and HGH were often part of a larger pattern of polysubstance abuse in this population.
There is substantial evidence that long-term supraphysiologic levels of HGH may cause adverse effects – as suggested by studies of acromegaly, a naturally occurring disorder characterized by prolonged supraphysiologic levels of HGH. Acromegalic patients show standardized mortality rates about twice that of the general population,15 and the magnitude and duration of HGH elevation are the primary determinants of survival.16,17 Acromegaly is particularly associated with adverse cardiovascular effects, including cardiomyopathy, hypertension, valve dysfunction, and arrhythmias.18–20 Supraphysiologic levels of HGH may also lead to diabetes mellitus,21 impaired respiratory function,22 and possibly various malignancies.23,24 The risk of these outcomes in illicit HGH users remains to be determined. However, given the potential for adverse physical consequences associated with long-term HGH use, it is imperative to explore the phenomenon of illicit HGH use. Therefore, in an effort to augment the limited data on the characteristics of illicit HGH users, we present preliminary findings on HGH and IGF-I use obtained in an ongoing study of American male weightlifters.
Methods
Since 2005, we have evaluated male weightlifters and other athletes in a study exploring risk factors for AAS use. We have recruited men aged 18–40 in Florida, Massachusetts, and California, using methods designed to obtain a representative sample of experienced weightlifters while attempting to minimize selection bias, as described in detail previously.13,25 In particular, the study's focus on AAS and other performance-enhancing drugs is not disclosed during the recruitment process. Study participants receive demographic questions; the Structured Clinical Interview for DSM-IV (SCID);26 a computerized battery of psychological rating scales; physiological measures including fat-free mass index (FFMI);27 and detailed questions regarding history of use of both classical and performance-enhancing drugs – the latter including not only AAS, but also HGH, IGF-I, thyroid hormones, clenbuterol, and others. We have described these methods in greater detail elsewhere.25
For this paper, we compared 1) all men reporting lifetime use of HGH/IGF-I with 2) men reporting lifetime AAS use but no HGH/IGF-I use and 3) men reporting no AAS or HGH/IGF-I use. We performed these comparisons using linear and logistic regression (using ranked data in cases of skewed distributions) with adjustment for study site (Florida, Massachusetts, or California), age (modeled as quintiles of the distribution), ethnicity (white versus non-white), and years of regular weightlifting (defined as attending a commercial gymnasium ≥ 3 days per week). Alpha was set at 0.05, 2-tailed. Note that we did not adjust for the effect of multiple comparisons and that therefore some differences, especially those of marginal significance, might be attributable to chance.
Results
Of 248 participants seen as of June 2009, 231 yielded evaluable data; 17 were excluded because of incomplete data (N = 2), presence of AAS (N = 6) or other drugs (N = 5) in urine or hair samples despite denial by the participant, or implausibly high muscularity and low body fat despite denial of AAS use (N = 4; see operational criteria for this exclusion previously reported (25)). Of the 231 evaluable men, 100 (43%) reported lifetime AAS use and 131 (57%) did not. Twenty-six men who reported lifetime HGH use and one additional man reported use of IGF-I, but not HGH. Since IGF-I is the bioactive derivative of HGH, we classified this man with the other HGH users, bringing the total to 27. Strikingly, all of these 27 men were also AAS users (Figure 1); none of the 131 non-AAS-users reported use of HGH or IGF-I (p < 0.001, by Fisher’s exact test). All of the HGH users first tried HGH after having already tried AAS; the mean (SD) latency from first AAS use to first HGH use was 4.5 (4.1) years (range 0.5–19 years).
Figure 1.
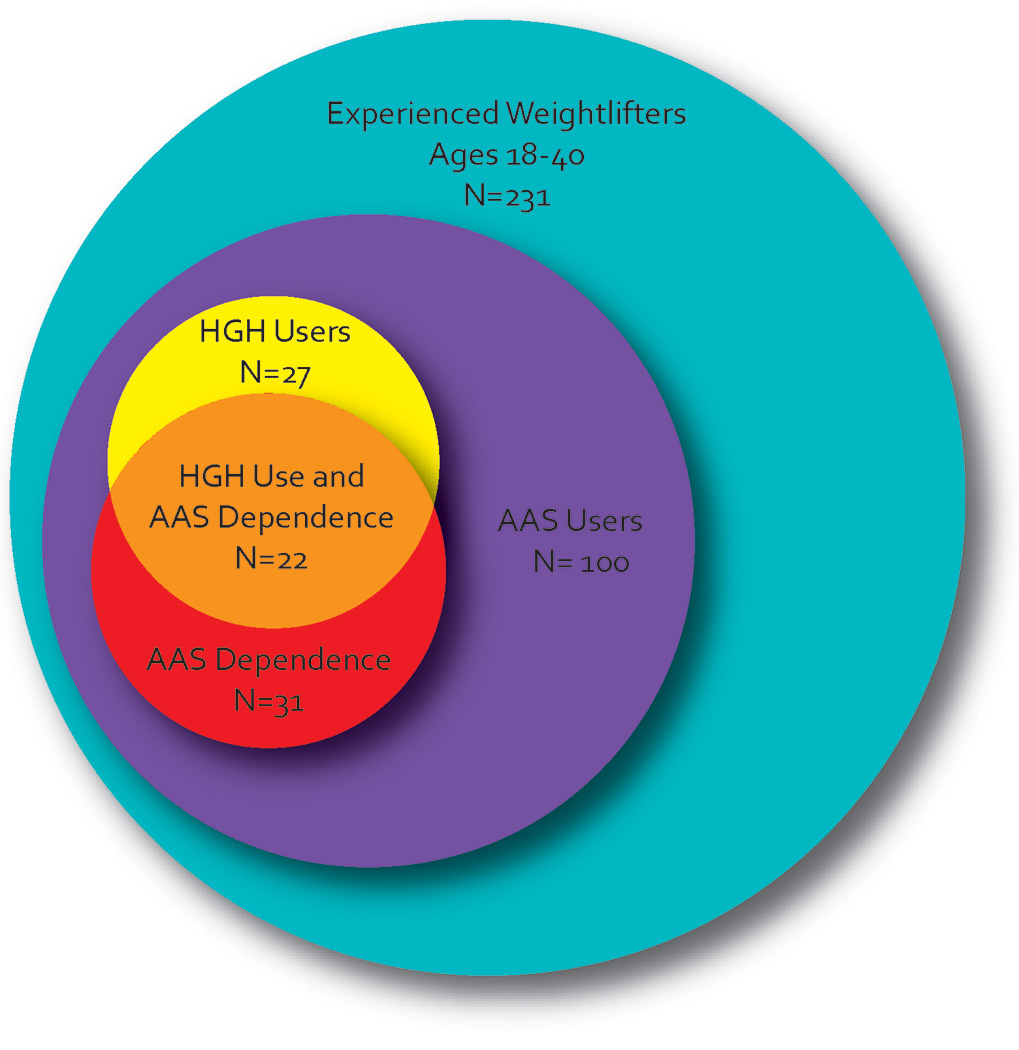
Association between anabolic-androgenic steroid use, anabolic-androgenic steroid dependence, and human growth hormone use in 231 young male weightlifters.
The 26 men who had specifically taken HGH reported a median (interquartile range) lifetime duration of HGH use of 23 (10, 55) weeks; the 7 men reporting use of IGF-I (6 of whom had also used HGH) reported a median IGF-I use of 9 (8, 10) weeks. Users typically reported using 15–20 units of HGH per week and 50–75 µg/day of IGF-I, but these estimates should be considered approximate, especially given the uncertain authenticity of illicitly obtained preparations. Notably, the 27 HGH/IGF-I users typically reported very long-term AAS use, with a median total lifetime AAS use of 173 (91, 390) weeks. By comparison, the 73 AAS users without HGH/IGF-I use reported a median of only 24 (10, 42) total weeks of AAS use (mean estimated difference in ranks [95% confidence interval] 31.8 [20.8, 42.7]; p < 0.001). Indeed, 22 (81%) of the HGH/IGF-I users had a history of current or past AAS dependence, as diagnosed by modified DSM-IV criteria that we have recently published.28 By contrast only 9 (12%) of the 73 AAS users without HGH/IGF-I use displayed a history of AAS dependence (odds ratio [95% confidence interval]: 28.1 [6.4, 123.2]; p < 0.001).
The HGH/IGF-I users were older, had lifted weights for longer, and were strikingly more muscular (i.e., higher in FFMI) than either comparison group (Table 1). The HGH/IGF-I users were the least well educated of the three groups, with only 19% having graduated from college despite their greater mean age. All groups showed a substantial lifetime prevalence of non-alcohol substance dependence, with the HGH/IGF-I users showing the highest prevalence of all. This contrast became even more striking when we deleted cases of cannabis dependence, the most common form of non-alcohol substance dependence. More than half of the 27 HGH/IGF-I users reported a history of dependence on at least one drug other than cannabis or alcohol, including opiates (N = 8), methylenedioxymethamphetamine ("ecstasy") (8), cocaine (7), stimulants (1), and/or polysubstances (1). The prevalence of these forms of substance dependence among the HGH/IGF-I users was slightly higher than among AAS users who had not used HGH/IGF-I, and markedly higher than among weightlifters who had used neither AAS nor HGH/IGF-I (Table 1).
Table 1.
Demographic Features of HGH Users, Other AAS Users, and Comparison Weightlifters
| Group | Between-Group Comparisons | |||||
|---|---|---|---|---|---|---|
| I. HGH plus AAS Use (N = 27) |
II. AAS but no HGH use (N = 73) Mean (SD) |
III. No AAS or GH Use (N=131) |
Group I vs. Group II | Group I vs. Group III | Group II vs. Group III | |
| Age, Yrs. | 32.5 (4.7) | 29.2 (6.2) | 27.8 (5.8) | 0.01a | < 0.001a | 0.09a |
| Mean Difference (95% confidence interval)b | ||||||
| Years of regular weightlifting | 11.6 (4.7) | 9.8 (6.1) | 8.4 (5.0) | −0.5 (−2.3, 1.2) | 0.4 (−1.3, 2.1) | 0.9 (−0.2, 2.1) |
| Fat-free mass index, kg/m2 | 26.2 (2.8) | 23.3 (2.3) | 22.8 (1.9) | 2.8*** (1.8, 3.8) | 3.4*** (2.5, 4.3) | 0.6 (0.0, 1.2) |
| N (%) | Odds Ratio (95% confidence interval)b | |||||
| Income > $30,000 | 21 (78) | 45 (62) | 71 (54) | 1.3 (0.4, 3.8) | 1.6 (0.6, 4.3) | 1.2 (0.6, 2.3) |
| Never married | 15 (56) | 56 (77) | 105 (80) | 0.6 (0.2, 1.8) | 0.7 (0.2, 1.8) | 1.1 (0.5, 2.5) |
| Grad 4-yr college | 5 (19) | 20 (27) | 62 (47) | 0.5 (0.2, 1.6) | 0.2** (0.1, 0.6) | 0.4** (0.2, 0.8) |
| Alcohol dependencec | 4 (15) | 17 (23) | 19 (15) | 0.5 (0.1, 1.7) | 0.7 (0.2, 2.5) | 1.5 (0.7, 3.1) |
| Substance dependenced | 19 (70) | 41 (56) | 50 (38) | 2.1 (0.8, 5.5) | 4.2** (1.6, 10.8) | 2.0* (1.1, 3.7) |
| Non-cannabis substance dependencee | 15 (56) | 31 (42) | 22 (17) | 1.6 (0.6, 4.1) | 56*** (2.1, 15.0) | 3.6*** (1.7, 74) |
By t test, two-tailed.
Estimates adjusted for age, study site, and ethnicity (see full text of paper).
p < 0.05;
p < 0.01;
p < 0.001
Lifetime history of alcohol dependence.
Lifetime history of dependence on any classical drug of abuse other than alcohol (excludes AAS dependence).
Lifetime history of dependence on any classical drug other than alcohol or cannabis (excludes AAS dependence).
Discussion
In an ongoing study of 231 experienced male weightlifters aged 18–40, we found that illicit HGH use is common, often prolonged, and closely associated with abuse of or dependence upon both AAS and classical drugs – a finding consistent with the European data cited earlier (10, 14). In this context, it is difficult to determine whether HGH abuse arises solely as a comorbid substance abuse disorder in a population with high rates of AAS use and of classical substance dependence, or whether prolonged HGH use might develop into a true chemical dependency in its own right. Although HGH does not produce a “reward” of acute intoxication in the manner of classical dependence-inducing drugs such as alcohol or opioids, the possibility remains that its metabolic effects, or perhaps even subtle hedonic effects, might themselves be sufficiently reinforcing to induce a dependence syndrome in some individuals. Further studies of the neurobiology underlying HGH abuse will be needed to understand this distinction more fully.
Notably, the 27% prevalence of lifetime HGH/IGF-I use among AAS users in the present study was significantly greater than the 8% prevalence among 48 AAS users in our similar previous study of weightlifters in 200313 (p = 0.009 by Fisher's exact test) – likely reflecting the increasing availability and decreasing price of HGH in recent years. When interpreting these findings however, it should be recognized that young male weightlifters, while probably the largest consumers of HGH6,10,14 are not the only population that uses this hormone illicitly. In particular, many non-weightlifters, often older than age 40, receive prescriptions for HGH from “anti-aging” clinics, compounding pharmacies, and other possibly illegal sources.29 These individuals likely differ from the population evaluated in the present study.
Despite its apparently growing popularity, there is little evidence that supraphysiologic HGH produces anabolic effects in non-HGH-deficient individuals30–33 – although it might have such effects when used in conjunction with AAS (30, 31) or shortly after stopping AAS use.34,35 Conversely, as reflected in the acromegaly literature, there is substantial evidence that long-term supraphysiologic levels of HGH may adverse cardiovascular,18–20 metabolic,21 and respiratory effects22 as well as increase the risk for certain types of malignancy.23,24 Importantly, long-term AAS use also impairs cardiac function36,37 – raising the ominous possibility of additive cardiotoxic effects,38 given the overlap between illicit HGH and AAS use.
Our findings regarding HGH abuse have implications not only for public health in general, but for practicing clinicians as well. Most notably, given the observed overlap between HGH abuse, AAS use, and classical substance use, individuals with chemical dependencies, especially those reporting AAS use, should be routinely questioned about HGH use. Identification and awareness are essential first steps to curb the increasing numbers of HGH-using individuals. In addition, future investigation into the underlying neurobiology of HGH abuse will be necessary to better understand potential adverse effects, delineate possible reinforcing effects, and to explore potential treatment interventions.
We acknowledge several limitations to this study. First, like almost all studies of substance abusers in the field, our findings rely on retrospective self-reports by individuals using illicit substances of uncertain potency or authenticity. Thus, the possibilities of recall bias and other forms of information bias must be recognized. Second, despite our methods to minimize selection bias described above, the possibility remains that the HGH users in this study were not fully representative of the source population from which they were drawn. Although selection bias and information bias cannot be entirely eliminated in naturalistic studies of illicit substance abusers, it will be important to retest our findings in other populations of weightlifters and other individuals at risk for HGH abuse. Third, as previously noted, we did not adjust for the effect of multiple comparisons in our analysis. Although there are reasons to favor this approach,39,40 the greater possibility of chance associations must be considered when evaluating the tests of significance that we have reported.
In summary, our preliminary observations suggest that illicit HGH abuse has become common among young American male weightlifters, and is often associated with polysubstance abuse, embracing both performance-enhancing and classical drugs. With the declining price and greater availability of HGH, future years may see even larger numbers of users, ingesting HGH for even longer periods at higher doses. Escalating abuse of HGH may eventually pose significant health problems,18–24 given evidence that long-term supraphysiologic levels of HGH may be associated with elevated morbidity and mortality.
Acknowledgments
This study was supported in part by Grants T32-DA07252 (Dr. Brennan) and RO1-DA016744 (Drs. Pope, Kanayama, and Hudson) from the National Institute on Drug Abuse, Bethesda, MD. Dr. Brennan was supported by the Clinical Investigator Training Program (CITP) through the Beth Israel Deaconess Medical Center – Harvard/MIT Division of Health Sciences and Technology in collaboration with Pfizer Inc. and Merck & Co.
Footnotes
Declaration of Interest
Dr. Brennan has received research grant support from Eli Lilly. Dr. Hudson has received research grant support from Eli Lilly, Ortho-McNeil Janssen Scientific Affairs, and Forest Laboratories, and has been a consultant for Eli Lilly and Pfizer. Dr. Pope has received research grant support from Solvay Pharmaceuticals and has provided expert testimony in several legal cases involving the related issue of anabolic-androgenic steroid abuse. Dr. Kanayama declares no conflicts of interest. The authors alone are responsible for the writing and content of this paper.
References
- 1.Baseball's week of drug news. Associated Press; 2009. Feb 14, [Google Scholar]
- 2.Dahlberg T. Marion Jones can't stop running with a lie. Associated Press. 2008 October 29; [Google Scholar]
- 3.Elitefitness.com Jintropin HGH: Real or Fake? [Accessed August 26, 2009]; Available online at: http://www.elitefitness.com/forum/counterfeit-anabolic-steroids/jintropin-hgh-real-fake-please-help-644006.html. [Google Scholar]
- 4.Llewellyn W. Anabolics. 9th ed. Jupiter, Florida: Molecular Nutrition; 2009. [Google Scholar]
- 5.EC21 Global B2B Marketplace. [Accessed August 26, 2009]; Available at: http://www.ec21.com/ec-market/Growth_hormone.html. [Google Scholar]
- 6.Graham MR, Evans P, Davies B, Baker JS. AAS, growth hormone, and insulin abuse: psychological and neuroendocrine effects. Therapeutics and clinical risk management. 2008;4:587–597. doi: 10.2147/tcrm.s2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rickert VI, Pawlak-Morello C, Sheppard V, Jay MS. Human growth hormone: a new substance of abuse among adolescents? Clin Ped. 1992;31:723–726. doi: 10.1177/000992289203101206. [DOI] [PubMed] [Google Scholar]
- 8.Wanjek B, Rosendahl J, Strauss B, Gabriel HH. Doping, drugs and drug abuse among adolescents in the State of Thuringia (Germany): prevalence, knowledge and attitudes. Int J Sports Med. 2007;28:346–353. doi: 10.1055/s-2006-924353. [DOI] [PubMed] [Google Scholar]
- 9.Evans NA. Gym and tonic: a profile of 100 male steroid users. Br J Sports Med. 1997;31:54–58. doi: 10.1136/bjsm.31.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JS, Graham MR, Davies B. Steroid and prescription medicine abuse in the health and fitness community: A regional study. Euro J Intern Med. 2006;17:479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Pates R, Barry C. Steroid use in Cardiff: a problem for whom? Journal of Performance-Enhancing Drugs. 1996;1:92–97. [Google Scholar]
- 12.Gruber AJ, Pope HG., Jr Psychiatric and medical effects of anabolic-androgenic steroid use in women. Psychotherapy and Psychosomatics. 2000;69:19–26. doi: 10.1159/000012362. [DOI] [PubMed] [Google Scholar]
- 13.Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 14.Skarberg K, Nyberg F, Engstrom I. Multisubstance use as a feature of addiction to anabolic-androgenic steroids. Euro Addict Res. 2009;15:99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- 15.Ayuk J, Sheppard MC. Does acromegaly enhance mortality? Rev Endocr Metab Disord. 2008;9:33–39. doi: 10.1007/s11154-007-9067-8. [DOI] [PubMed] [Google Scholar]
- 16.Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006;355:2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 17.Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 18.Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol. 2008;69:347–358. doi: 10.1111/j.1365-2265.2008.03292.x. [DOI] [PubMed] [Google Scholar]
- 19.Colao A, Vitale G, Pivonello R, et al. The heart: an end-organ of GH action. Eur J Endocrinol. 2004;151 Suppl 1:S93–S101. doi: 10.1530/eje.0.151s093. [DOI] [PubMed] [Google Scholar]
- 20.Lombardi G, Galdiero M, Auriemma RS, Pivonello R, Colao A. Acromegaly and the cardiovascular system. Neuroendocrinology. 2006;83:211–217. doi: 10.1159/000095530. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen JO, Moller L, Krag M, Billestrup N, Christiansen JS. Effects of growth hormone on glucose and fat metabolism in human subjects. Endocrinol Metab Clin North Am. 2007;36:75–87. doi: 10.1016/j.ecl.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Luboshitzky R, Barzilai D. Hypoxemia and pulmonary function in acromegaly. Am Rev Respir Dis. 1980;121:471–475. doi: 10.1164/arrd.1980.121.3.471. [DOI] [PubMed] [Google Scholar]
- 23.Matano Y, Okada T, Suzuki A, et al. Risk of colorectal neoplasm in patients with acromegaly and its relationship with serum growth hormone levels. Am J Gastroenterol. 2005;100:1154–1160. doi: 10.1111/j.1572-0241.2005.40808.x. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins PJ. Cancers associated with acromegaly. Neuroendocrinology. 2006;83:218–223. doi: 10.1159/000095531. [DOI] [PubMed] [Google Scholar]
- 25.Kanayama G, Hudson JI, Pope HG. Demographic and psychiatric features of men with anabolic-androgenic steroid dependence: a comparative study. Drug Alcohol Depend. 2009;102:130–137. doi: 10.1016/j.drugalcdep.2009.02.008. NIHMS 116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 27.Kouri EM, Pope HG, Jr, Katz DL, Oliva P. Fat-free mass index in users and nonusers of anabolic-androgenic steroids. Clin J Sport Med. 1995;5:223–228. doi: 10.1097/00042752-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Issues for DSM-V: Clarifying the diagnostic criteria for anabolic-androgenic steroid dependence. Am J Psychiatry. 2009;166:642–644. doi: 10.1176/appi.ajp.2009.08111699. NIHMSID 109288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olshansky SJ, Perls TT. New developments in the illegal provision of growth hormone for "anti-aging" and bodybuilding. JAMA. 2008;299:2792–2794. doi: 10.1001/jama.299.23.2792. [DOI] [PubMed] [Google Scholar]
- 30.Ehrnborg C, Rosen T. Physiological and pharmacological basis for the ergogenic effects of growth hormone in elite sports. Asian Journal of Andrology. 2008;10:373–383. doi: 10.1111/j.1745-7262.2008.00403.x. [DOI] [PubMed] [Google Scholar]
- 31.Gibney J, Healy ML, Sonksen PH. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocrine Reviews. 2007;28:603–624. doi: 10.1210/er.2006-0052. [DOI] [PubMed] [Google Scholar]
- 32.Graham MR, Baker JS, Evans P, et al. Potential benefits of recombinant human growth hormone (rhGH) to athletes. Growth Horm IGF Res. 2009;19:300–307. doi: 10.1016/j.ghir.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Graham MR, Davies B, Grace FM, Kicman A, Baker JS. Anabolic steroid use: patterns of use and detection of doping. Sports Medicine (Auckland, NZ) 2008;38:505–525. doi: 10.2165/00007256-200838060-00005. [DOI] [PubMed] [Google Scholar]
- 34.Graham MR, Baker JS, Evans P, et al. Physical effects of short-term recombinant human growth hormone administration in abstinent steroid dependency. Hormone Research. 2008;69:343–354. doi: 10.1159/000117390. [DOI] [PubMed] [Google Scholar]
- 35.Graham MR, Baker JS, Evans P, et al. Short-term recombinant human growth hormone administration improves respiratory function in abstinent anabolic-androgenic steroid users. Growth Horm IGF Res. 2007;17:328–335. doi: 10.1016/j.ghir.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Weiner RB, Kanayama G, Hudson JI, et al. Abstract 3048: Chronic anabolic-androgenic steroid use is associated with left ventricular systolic and diastolic dysfunction. Circulation. 2009;120:S741. [Google Scholar]
- 37.Kanayama G, Hudson JI, Pope HG., Jr Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cittadini A, Berggren A, Longobardi S, et al. Supraphysiological doses of GH induce rapid changes in cardiac morphology and function. J Clin Endocrinol Metab. 2002;87:1654–1659. doi: 10.1210/jcem.87.4.8363. [DOI] [PubMed] [Google Scholar]
- 39.Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142:904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]


