Abstract
Metastatic and primary bone cancers are usually accompanied by severe pain that is difficult to manage. In light of the adverse side effects of opioids, manipulation of the endocannabinoid system may provide an effective alternative for the treatment of cancer pain. The present study determined that a local, peripheral increase in the endocannabinoid 2-arachidonoylglycgerol (2-AG) reduced mechanical hyperalgesia evoked by the growth of a fibrosarcoma tumor in and around the calcaneous bone. Intraplantar (ipl) injection of 2-AG attenuated hyperalgesia (ED50 of 8.2 μg) by activation of peripheral CB2 but not CB1 receptors and had an efficacy comparable to that of morphine. JZL184 (10 μg, ipl.), an inhibitor of 2-AG degradation, increased the local level of 2AG and mimicked the antihyperalgesic effect of 2-AG, also through a CB2 receptor-dependent mechanism. These effects were accompanied by an increase in CB2 receptor protein in plantar skin of the tumor-bearing paw as well as an increase in the level of 2AG. In naïve mice, intraplantar administration of the CB2 receptor antagonist AM630 did not alter responses to mechanical stimuli demonstrating that peripheral CB2 receptor tone does not modulate mechanical sensitivity. These data extend our previous findings with anandamide in the same model and suggest that the peripheral endocannabinoid system is a promising target for the management of cancer pain.
Keywords: Endocannabinoid, Monoglycerol lipase, 2-arachidonoyl glycerol, cancer, cannabinoid receptor, pain
1 INTRODUCTION
Over 60% of individuals with primary or metastatic bone cancer suffer from severe pain [1] making pain a major factor contributing to diminished quality of life in these patients. Typically, bone cancer pain is treated with opioid therapy which produces adverse side-effects including nausea, respiratory dysfunctions, and physical dependence [2]. Moreover, because some patients do not attain sufficient analgesia with opioids, cancer pain management remains a therapeutic challenge. Studies of the endocannabinoid system are unveiling the relevance of this system to the management of pain associated with tissue damage [3,4,5].
The endocannabinoid system includes cannabinoid (CB) receptors (CB1 and CB2), their endogenous ligands and the enzymes responsible for their synthesis and degradation. In addition to anandamide (AEA), 2-arachidonoyl glycerol (2-AG) has been characterized as an endocannabinoid (reviewed by [5]). 2-AG is synthesized by diacylglycerol lipase [6] and hydrolyzed to arachidonic acid and glycerol by monoacylglycerol lipase (MGL) [7,8] as well as serine hydrolase α-β-hydrolase domain 6 (ABHD6) [9,10]. Basal levels of 2-AG are higher than those of AEA in brain and skin [11], and 2-AG acts as a full agonist of CB1 and CB2 receptors in multiple assay systems (reviewed by [12]).
Peripheral anti-hyperalgesic effects of 2-AG have been demonstrated in models of tissue injury. Injection of 2-AG near the site of injury decreases nocifensive behavior in rat models of inflammatory [13,14] and neuropathic pain [15]. Whether the anti-hyperalgesic effect of 2-AG in the periphery is mediated by CB1 or CB2 receptors is dependent on the model and the behavioral assay: The effect of 2-AG in the formalin model of inflammatory pain is selectively blocked by local administration of a CB2 receptor antagonist [13], but both CB1 and CB2 receptor antagonists block the anti-allodynic effect of 2-AG in a model of neuropathic pain [15].
An alternative approach to injection of 2-AG to increase its level in tissue is to inhibit the degradation of what is synthesized endogenously. Whereas MGL accounts for the majority of the degradation of 2-AG in neurons within the brain, the contribution of ABHD6 ranges from 15% [9] to 40% [10]. Early studies used the MGL inhibitor URB602 to increase the level of endogenous 2-AG locally in the brain [16] or in vitro [17]. A local injection of URB602 in the periphery attenuates inflammatory and neuropathic hyperalgesia ([13,15] respectively). However, in tissue homogenates URB602 inhibits fatty acid amide hydrolase, the enzyme that degrades AEA [18]. This observation in conjunction with the low potency of the compound impugns the selectivity of URB602 in vivo in the absence of measures of endocannabinoids under the same experimental conditions. Recently, a more selective inhibitor of MGL has been generated: JZL184 elevates levels of 2AG but not AEA following acute systemic administration [19,20].
We have used a murine model of bone cancer pain that mimics metastatic bone cancer pain in humans [21,22] to address the efficacy of synthetic cannabinoid receptor agonists to reduce tumor-evoked pain [23,24,25]. We also determined that mechanical hyperalgesia in the tumor-bearing paw is associated with a decrease in the level of the endocannabinoid AEA in the associated plantar skin, and treatments that increase the level of AEA locally alleviate the hyperalgesia [26]. In the present study, we extend our investigation of the endocannabinoid system in bone cancer pain to address whether increasing the level of 2-AG locally through intraplantar administration of 2-AG or the MGL inhibitor JZL184 reduces tumor-related mechanical hyperalgesia. Following determination that both pharmacological approaches produced an anti-hyperalgesic effect that was mediated by the CB2 receptor, tissue levels of 2AG and the CB2 receptor were investigated. Although the intraplantar injection of a selective CB2 receptor antagonist did not alter sensitivity to a mechanical stimulus in naïve mice, indicating that basal CB2 receptor activity does not modulate the nociceptive mechanical threshold, increasing the level of 2-AG at the site of sensory transduction may be advantageous in the management of tumor-evoked pain in humans.
2 METHODS
2.1 Subjects
Adult male C3H/HeNCr MTV− mice (National Cancer Institute; 25–30 g) were used throughout this study. Mice were housed 4 per cage, allowed free access to food and water, and maintained on a 12-hour light/dark schedule. All behavioral testing was performed during the light cycle. Experiments adhered to the guidelines set forth by the Committee for Research and Ethical Issues of the IASP, and procedures were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
2.2 Maintenance and implantation of fibrosarcoma cells
NCTC clone 2472 fibrosarcoma cells (American Type Culture Collection, Manassas, VA, USA) were maintained as described previously [26]. This clone was derived from a connective tissue tumor in a C3H mouse, rendering the fibrosarcoma cells syngeneic with C3H/He mice [21]. Fibrosarcoma cells (2×105 cells in 10 μl of phosphate buffered saline, pH 7.3) were injected into and around the calcaneus bone of the animal’s left hind paw while the mouse was anesthetized with isoflurane (2%). Histological studies conducted previously documented that this approach produces a tumor with bone osteolysis [21].
2.3 Measurement of mechanical sensitivity in naïve mice
Mechanical sensitivity was measured using graded von Frey monofilaments with bending forces of 3.9, 5.9, 9.8, 13.7, 19.6 and 39.2 mN. Monofilaments were applied individually to the plantar surface of the hind paw in order of increasing force [16]. Each monofilament was applied 10 times and the withdrawal frequency was calculated.
2.4 Measurement of mechanical hyperalgesia
Response to mechanical stimuli was selected as the dependent measure in the study because this measure is highly reproducible in our hands and effective in resolving sensitivity to cannabinoid receptor ligands. Moreover, touch-evoked pain is prominent in human pain syndromes [27]. Mechanical hyperalgesia in the tumor-bearing paw was defined as an increase in withdrawal frequency in response to a standard mechanical stimulus: a von Frey monofilament that delivers a force of 3.9 mN (0.4 g). Animals were placed on an elevated wire mesh platform, covered individually with glass containers and allowed to acclimate for 30 minutes prior to testing. The monofilament was applied to the plantar surface of each hind paw ten times, and the withdrawal frequency was calculated for each paw as the (number of withdrawal responses/total stimuli) × 100%.
The baseline (pre-tumor) withdrawal frequency for each hind paw was measured on 3 consecutive days preceding implantation of fibrosarcoma cells. The average baseline withdrawal frequency evoked by the 3.9 mN monofilament across several experiments was 13%. Following implantation, the development of mechanical hyperalgesia was monitored daily. Consistent with previous studies [21] an increase in the withdrawal frequency occurred in response to the test stimulus in the tumor-bearing paw. By 10 days after fibrosarcoma cell implantation, the average paw withdrawal frequency increased to 78% in the tumor-bearing paw across several experiments. Approximately 15% of mice did not display mechanical hyperalgesia after implantation of fibrosarcoma cells. On the day of drug injections (day 10 or 11 after implantation), only mice that exhibited a withdrawal frequency ≥70% were used in the experiments. Following intraplantar (ipl) drug injections, the withdrawal frequency of each hind paw was measured every 30 minutes for 3.5 hours. The individual scoring behavioral responses following injection of drugs was blinded to the treatment of the animal in all experiments, and at least 2 drug groups were tested in each session.
2.5 Drug solutions and administration
A stock solution of the endocannabinoid 2-AG (Tocris, Ellisville, MO, USA) was prepared in ethanol (10 μg/μl). JZL184 (Cayman Chemical, Ann Arbor, MI, USA) was prepared in DMSO:Tocrisolve™100 (1:12.5, 12.5 μg/μl). The CB1 receptor antagonist AM281 [1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-mo rpholinyl-1H-pyrazole-3-carboxamide; Tocris] and the CB2 receptor antagonist AM630 [6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl)methanone; Tocris] were dissolved in DMSO (10 μg/μl). Each receptor antagonist exhibits more than a 100-fold difference in affinity for CB1 and CB2 receptors [28,29].
All drugs were diluted to the final dose in saline for injection in a volume of 10 μl. The highest concentrations of organic solvents in a dose were used as vehicle controls. Drugs or vehicles were injected subcutaneously into the plantar surface of the hind paw.
2.6 Analysis of behavioral data
The effect of each drug on mechanical hyperalgesia in tumor-bearing mice was calculated as a percent of the maximum possible effect on hyperalgesia.
Additionally, the percent maximum drug effect was calculated for use in the dose response analysis.
Calculating maximum drug effect produced a limited number of values greater than 100 or less than 0, which occurred at high and low doses, respectively. These values were adjusted to 100 and 0, respectively, to determine the anti-hyperalgesic effect of the drug.
2.7 Measurement of AEA and 2-AG
In order to determine whether the tumor condition altered tissue levels of 2-AG, tumor-bearing mice were euthanized by decapitation under isoflurane anesthesia and lumber DRG L3-L5 and samples of plantar paw skin ipsilateral to tumors were collected. Parallel samples were collected from naïve mice. To determine the selectivity and efficacy of JZL184 on endocannabinoid levels in the periphery, samples of paw skin were collected upon euthanasia 2 hr after injection of JZL184 (10 μg, ipl.). Upon removal, samples were weighed, frozen in liquid nitrogen, and kept frozen at −80°C until the time of processing. Endogenous AEA and 2AG were measured as previously described [26]. On the first day of processing, tissues were minced and extracted with 5 volumes of chloroform at 4°C overnight. On the second day of processing, samples were then homogenized with an equal volume of methanol:Tris-HCl 50 mM (1:1) containing 5 pmol of deuterated d8-AEA and 100 pmol of deuterated d8-2-AG as internal standards. Homogenates were centrifuged at 2500 xg for 15 min (4°C); the aqueous phase plus debris were collected and extracted again with 1 volume of chloroform. The organic phases were pooled and evaporated with a gentle stream of nitrogen gas. Vials containing the dried samples were weighed for determination of total lipid weight and were stored at −80°C until analyzed. Targeted isotope-dilution HPLC/atmospheric pressure chemical ionization/mass spectrometry was conducted on each sample. A ZORBAX SB-C18 (0.5 ×150 mm) column was used. The column was maintained at 40°C. The mobile phase A was 0.1% formic acid in 2 mM of ammonium acetate, and phase B was 0.1% formic acid in acetonitrile. The flow rate was 10 μl/min with a gradient that began with 50% A:50% B. The AEA and 2-AG levels in unknown samples were estimated from the ratio of the area of the signals of deuterated and non-labeled AEA (0.2–200 pmol), or 2-AG (2–2000 pmol) standards. Data are expressed as pmol AEA or 2-AG per g tissue weight or total lipid extracted from samples. On three occasions, insufficient recovery of deuterated compounds or an unusual amount of extracted lipids resulted in amounts of endocannabinoids that were more than two standard deviations beyond the mean for the group. These values were deleted from the data set for statistical analysis.
2.8 Western blot analysis of CB2 receptor protein
Samples of plantar paw skin, tibial nerve (~1 cm,) and L3–L5 dorsal root ganglia (DRG) from naive and tumor-bearing mice were dissected, frozen on dry ice, and stored at −80°C until time of processing. Samples of nerve and DRGs were pooled from 3 mice. On the day of processing, samples were sonicated in single-detergent lysis buffer (50 mM Tris-HCl, pH 8.0 with 1% Triton X-100, 150 mM NaCl, 0.02% Na azide, 100 μg/ml PMSF, and 1 μg/ml protease inhibitor mixture (Sigma), and the supernatant was obtained after centrifugation at 800 xg for 10 min. The supernatant was concentrated using an Amicon Ultra-0.5 centrifugal filter (Millipore Corporation, Billerica, MA, USA). Western blot analysis was performed on 30 μg of protein/sample. Samples were loaded onto a 10% SDS-PAGE gel, subjected to electrophoresis and then transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Philadelphia, PA, USA). Samples of one tissue type were processed on the same gel. Nonspecific binding to membranes was blocked by incubation in phosphate-buffered saline with 3% defatted dry milk for 1 h at room temperature. The membranes were probed with a rabbit anti-CB2 receptor antibody (1:500, Cayman) overnight at 4°C. Detection of the primary antibody was performed using a peroxidase conjugate of goat anti-rabbit IgG (1:10,000; Amersham Biosciences, Pittsburgh, PA, USA). Immunoreactivity was visualized using the enhanced chemifluorescence detection reagent (Pierce, Rockford, IL) and X-ray film (Eastman Kodak Company, Rochester, NY, USA). The gel was treated with 0.01% phenylhydrazine for 10 min after detection of CB2 receptor immunoreactivity in order to neutralize the peroxidase activity associated with this antigen. Actin immunoreactivity (rabbit anti-actin antibody,1:500, Sigma) within each sample was then quantified as a loading control. Multiple exposures were done of each film after each antibody detection to insure that measures of density of silver grains with respect to immunoreactivity were within the linear range of the response of the X-ray film. The density of silver grains was quantified using Metamorph (v5.07, Molecular Devices, Sunnyvale, CA, USA). Specificity of the CB2R antibody was confirmed using plantar skin from CB2R−/− mice (B6.129P2-Cnr2tm1Dgen/J Jackson Laboratory, Bar Harbor, ME, USA).
2.9 Statistical Analyses
All data are presented as the group mean ± S.E.M. Results were compared between groups and across time using Student’s t-test, one-way and two-way analyses of variance (with repeated measures when applicable) followed by the Bonferroni t-test for comparisons between groups. For all statistical analyses, a probability value of <0.05 was considered significant. The dose-response data were analyzed using Prism (GraphPad v. 5.01).
3 RESULTS
3.1 2-AG attenuated tumor-evoked mechanical hyperalgesia
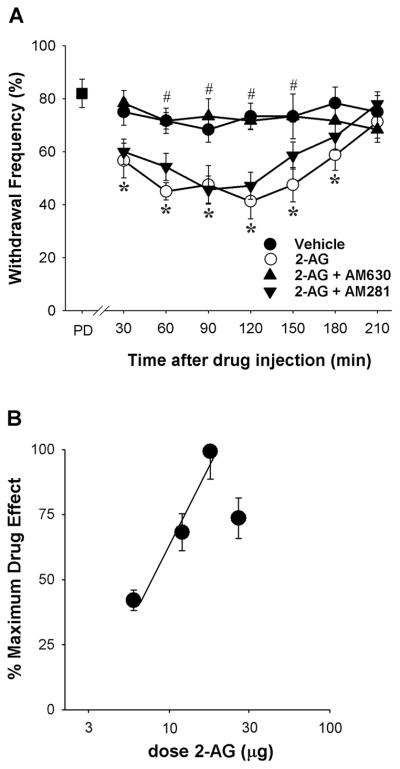
Peripheral administration of 2-AG (18 μg, ipl., ipsilateral to the tumor) decreased mechanical hyperalgesia in tumor-bearing mice in a time-dependent manner (Fig. 1A). A reduction in mechanical hyperalgesia occurred at the earliest time point measured (30 min) and persisted through 3 h. The vehicle (20% ethanol in saline) did not alter the withdrawal frequency of tumor-bearing mice. To determine which cannabinoid receptor subtype mediated the antihyperalgesic effect of 2-AG, 2-AG (18 μg, ipl.) was co-injected with either the CB2 receptor antagonist AM630 (4 μg) or the CB1 receptor antagonist AM281 (10 μg). The cannabinoid receptor selectivity of each antagonist at the dose used was validated in previous studies using the same route of administration in the same model [26,30]. AM630 blocked the antihyperalgesic effect of 2-AG (Fig. 1A), however, the CB1 receptor antagonist AM281 had no effect on 2-AG (p=0.454, 2-way ANOVA). Neither dose of AM630 or AM281 administered alone (ipl.) ipsilateral to the tumor reduced mechanical hyperalgesia in tumor-bearing mice (p=0.313 and p=0.662, respectively, n=5 mice/group, two-way ANOVA). Together, these data indicate that CB2 receptors play a principal role in 2-AG-mediated peripheral anti-hyperalgesia.
Figure 1.
Effect of intraplantar injection of 2-AG on mechanical hyperalgesia A. 2-AG attenuated tumor-evoked mechanical hyperalgesia by a CB2-dependent mechanism (F3,26=33.06, p<0.001, two-way ANOVA). Mechanical hyperalgesia was confirmed before drug administration (PD=pre-drug). The dose of 2-AG was 18 μg (i.pl.), the dose of AM630 was 4 μg (i.pl.), and the dose of AM281 was 10 μg (i.pl.). *Different from vehicle at p<0.01; #different from 2-AG at p<0.001 (n=6–8 mice/group; two-way ANOVA with Bonferroni’s multiple comparisons test). B. The effect of 2-AG was dose-dependent (r2= 0.57, DF=16); dose is plotted on a log scale.
The effect of 2-AG was also dose-dependent (Fig. 1B). A dose of 18 μg was the maximally effective dose and inhibited mechanical hyperalgesia by 68±4.8%; the ED50 was 8.5 μg (5.8–11,2, 95% CI).
In order to establish whether 2-AG reduced mechanical hyperalgesia by a systemic or local mechanism, 2-AG (18 μg, ipl.) was injected into the paw contralateral to the tumor. There was no change in the withdrawal frequency of the tumor-bearing paw compared to the pre-drug response (p=0.761, One-way ANOVA with repeated measures, n=4 mice), indicating that 2-AG exerted its anti-hyperalgesic effect locally.
3.2 Intraplantar injection of an MGL inhibitor mimicked the effect of 2-AG
Injection of JZL184 (10 μg, ipl.), an inhibitor of MGL, into the tumor-bearing paw also attenuated the mechanical hyperalgesia (Fig. 2A). The reduction in mechanical hyperalgesia was first noted 60 min after drug injection, and the effect was no longer evident by 3 hr post injection. The maximum inhibition of mechanical hyperalgesia by JZL184 was 34±7%, which was less than the maximal effect of 2-AG (p<0.005, Student’s t-test). A lower dose of JZL184 (4 μg) had no effect on mechanical hyperalgesia and the anti-hyperalgesic effect of a higher dose (40 μg) occurred only at 120 min. Because solubility of the drug in the vehicle (DMSO/Tocrisolve ™100/saline) restricted the range of doses we could administer, an ED50 could not be determined.
Figure 2.
Effect of intraplantar injection of JZL184 on mechanical hyperalgesia. A. JZL184 attenuated tumor-evoked mechanical hyperalgesia (F3,169=18.42, p<0.001, two-way ANOVA). Mechanical hyperalgesia was confirmed before drug administration (PD=pre-drug). B. Co-administration of the CB2 receptor antagonist AM630 (4 μg) with JZL184 (10 μg) eliminated the anti-hyperalgesia produced by JZL184. Co-administration with the CB1 receptor antagonist AM281 (10 μg) had no effect on the antihyperalgesic effect of JZL184. *Different at p<0.05, one-way ANOVA with Bonferroni’s t-test, number inside bar represents sample size).
The involvement of cannabinoid receptor subtypes in JZL184-induced anti-hyperalgesia was investigated by co-injection of JZL184 (10 μg, ipl.) and cannabinoid receptor antagonists ipsilateral to the tumor. The CB2 receptor antagonist, AM630 (4 μg), blocked the anti-hyperalgesic effect of JZL184 (120 min post-drug administration reported in Fig. 2B). Co-administration of the CB1 receptor antagonist AM281 (10 μg) with JZL184 did not diminish the anti-hyperalgesic effect of JZL184 alone. Therefore, in parallel with 2-AG, the anti-hyperalgesia produced by JZl184 was also mediated by CB2 receptors.
To determine whether the anti-hyperalgesic effect of intraplantar injection of JZL184 was mediated by a local mechanism, mechanical hyperalgesia in the tumor-bearing paw was determined following injection of JZL184 (10 μg, ipl.) into the paw contralateral to the tumor. At 120 min post drug injection the mean withdrawal frequency in the tumor-bearing paw was 87±3% compared to the pre-drug value of 93±3% (p=0.919, n=3; One-way ANOVA for repeated measures). These data indicate that the anti-hyperalgesic effect observed following intraplantar injection if JZL184 was likely mediated by a local mechanism.
3.3 Levels of 2-AG and AEA in paw skin following treatments
In comparison to AEA, the level of 2-AG was more than 60-fold higher in plantar skin of naïve mice (Table 1; values for skin reported per g of tissue). This difference is consistent with previous reports of the relative amounts of AEA and 2-AG in skin [31,32]. The tumor condition elicited different changes in the levels of 2-AG and AEA in the plantar skin of the tumor-bearing paw and related DRGs. Consistent with our earlier report [26], the level of AEA was lower in the plantar skin and DRGs of tumor bearing mice compared to samples from naïve mice. In contrast, the level of 2-AG was almost 3-fold higher in paw skin ipsilateral to the tumor, but no change occurred in the related DRGs.
Table 1.
Effect of tumors on the levels of AEA and 2AG in skin and DRGs.
| Sample | Treatment | AEA | 2-AG |
|---|---|---|---|
| Plantar paw skin# | Naive | 26.1 ± 2.1 (4) | 1572 ± 354 (4) |
| Tumor-bearing | 10.7 ± 0.2 (6)* | 4284 ± 586 (6)** | |
| DRG† | Naive | 0.9 ± 0.05 (4) | 4.6 ± 0.7 (4) |
| Tumor-bearing | 0.5 ± 0.03 (4)** | 7.0 ± 1.7 (4) |
Data for skin are expressed as pmol/g tissue;
data for DRGs are expressed as pmol/DRG; L3-L5 DRG ipsilateral to the tumor were pooled from 1 mouse and comparable samples were collected from naive mice.
Different from naive within the same endocannabinoid at p<0.05,
different at p<0.001 (Student’s t test; AEA data for skin were converted to log10 for statistical analysis). Numbers in parentheses represent the same size.
In order to address the selectivity of JZL184 in disrupting the degradation of 2-AG over AEA, the levels of 2-AG and AEA were measured in plantar paw skin ipsilateral to the injection of drug. Samples were collected between 100–120 min following drug administration in order to measure 2-AG during the time of the CB2-dependent anti-hyperalgesic effect. Vehicle did not alter the relative amounts of 2-AG and AEA or the effect of the tumor condition (Table 2, values reported per g total lipid). The level of 2-AG increased more than 4-fold following injection of JZL184 in naive mice; a 2-fold increase occurred in skin from the tumor-bearing hind paw. Although the proportional change in 2AG in the skin from tumor-bearing mice was smaller than that in skin from naive mice due to the higher basal level of 2AG, the absolute amount of 2AG that accumulated following drug administration was larger in the skin from tumor-bearing mice. No change occurred in the level of AEA in response to JZL184 in naive or tumor-bearing mice. These data confirm the efficacy and selectivity of JZL184 for inhibition of MGL over fatty acid amide hydrolase in murine skin under the condition in which anti-hyperalgesia was observed.
Table 2.
Effect of JZL184 on levels of AEA and 2-AG in hind paw skin.
| Condition | Treatment | AEA pmol | 2-AG nmol |
|---|---|---|---|
| Naïve | Vehicle | 550 ± 104 (8) | 45 ± 6 (9) |
| JZL184 | 455 ± 85 (5) | 208 ± 32# (5) | |
| Tumor-bearing | Vehicle | 135 ± 51* (5) | 190 ± 113* (4) |
| JZL184 | 230 ± 23 (5) | 409 ± 62# (5) |
Endocannabinoid values were normalized to the g of lipid extracted from the sample.
Statistical analyses were conducted on the log10 of the individual values.
Different from naïve/vehicle at p<0.05,
different from vehicle in corresponded group at p<0.05, one-way ANOVA within endocannabinoid with Bonferroni’s t test. Numbers in parentheses represent the same size.
3.4 CB2 receptor tone in naïve mice
Several lines of evidence indicate that CB1 receptors contribute to the threshold for nociception: First, genetic deletion of CB1 receptors in nociceptors results in thermal and mechanical hyperalgesia compared to wild type mice [33]. Secondly, mechanical hyperalgesia occurs in naïve mice following intraplantar injection of a CB1 receptor antagonist [26]. Therefore, intraplantar injection of the CB2 receptor antagonist AM630 was used to determine whether basal CB2 receptor tone in skin regulates sensitivity to mechanical stimuli. We used the dose of AM630 (4 μg) that blocked the effect of 2-AG on mechanical hyperalgesia in tumor-bearing mice. Responses to monofilaments of 3.9 to 39.2 mN (0.4–4 g) were measured at 2 h following intraplantar injection of AM630, a time point at which intraplantar injection of AM630 blocked the anti-hyperalgesic effect of 2-AG (Fig. 1A). Compared to the vehicle control (20% DMSO), AM630 did not alter the response to any mechanical stimulus within the range tested at 2 hr post drug administration (Fig. 3). Although the basal level of 2-AG in skin of naïve mice is more than 60-fold greater than that of AEA (see above), these data suggest that CB2 receptors do not modulate mechanical sensitivity in naïve mice.
Figure 3.
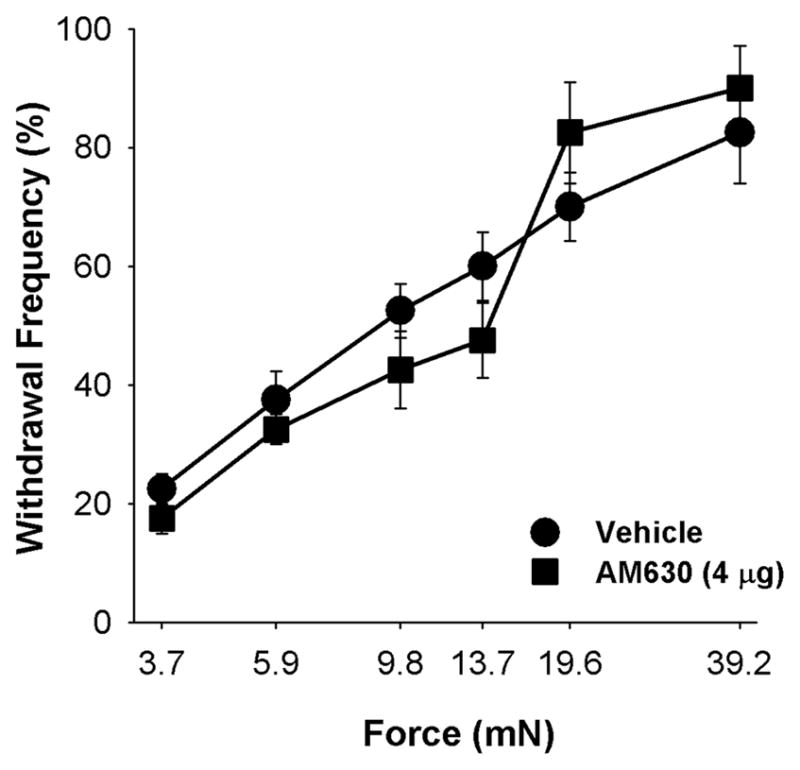
The CB2 receptor antagonist AM630 did not alter sensitivity to mechanical stimuli in naive mice at 2 h following injection of 4 μg (ipl.) ipsilateral to the testing site (F20,96=1.14, p=0.325, two-way ANOVA, n=4 mice/group).
3.5 Expression of CB2 receptor protein in tumor-bearing mice
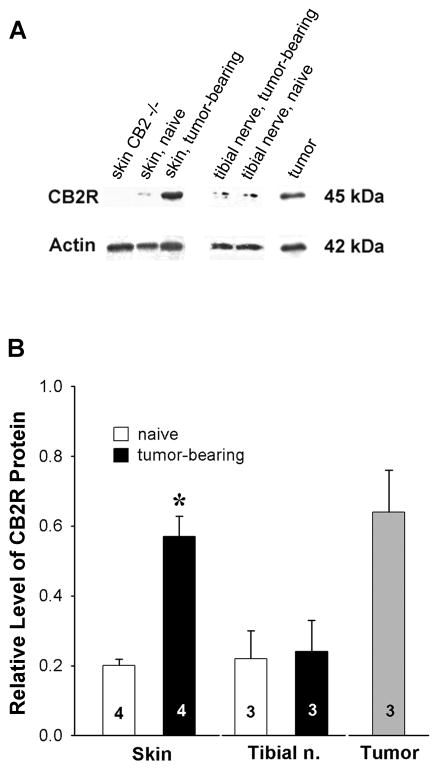
Given that the anti-hyperalgesic effects of 2-AG was mediated by local CB2 receptors, the expression of CB2 receptor protein was determined by analyzing Western blots of peripheral tissues from naïve and tumor-bearing mice. The absence of an immunoreactive band at 45 kD in plantar skin from CB2−/− mice confirmed the selectivity of the antibody used to detect CB2 receptor protein (Fig. 4A). The amount of CB2 receptor in DRGs from both naïve and tumor-bearing mice was at the limit of detection, so reliable conclusions could not be drawn for these samples. There was no difference in the level of CB2 receptor protein in tibial nerve ipsilateral to tumors compared to nerve from naïve mice. However, samples of the tumor included significant amounts of CB2 receptor protein, and CB2 receptor protein was higher in plantar paw skin ipsilateral to tumors compared to skin from naïve mice (Fig. 4B). On the basis of these data it is likely that effects of CB2 receptor agonists on mechanical hyperalgesia are mediated by non-neuronal cells which may include keratinocytes as well as fibrosarcoma and immune cells.
Figure 4.
Expression of CB2 receptor protein increased in plantar skin ipsilateral to tumors in tumor-bearing mice. Western blot analysis was used to determine the expression of CB2 receptor protein; the amount of CB2 receptor protein was normalized to the amount of actin within each sample. A. Representative examples of CB2 receptor (CB2R) and actin protein detected by Western blot. Note the absence of CB2 receptor-immunoreactivity in skin from the CB2−/− mouse. B. In densitometric analyses of images of blots, the amount of CB2 receptor-immunoreactivity was normalized to the amount of actin-immunoreactivity within each sample. *Different from plantar skin of naive mice at p<0.05 (Student’s t-test). Values inside the bars represent the sample size.
4 DISCUSSION
Pain related to tumor growth is often difficult to manage, and approximately two-thirds of patients experience pain with advanced disease [34], particularly with metastases to bone [1]. These data compel novel approaches to the management of tumor-related pain. The present results demonstrate that 2-AG inhibited mechanical hyperalgesia in a murine model of bone cancer pain by a local CB2 receptor-dependent mechanism. The effect was mimicked by JZL184, an inhibitor of MGL, which increased the endogenous level of 2-AG in tumor-bearing mice. The maximum effect of 2AG was comparable to intraplantar administration of morphine which reduced mechanical hyperalgesia by 53% in this model [30]. It is likely that the anti-hyperalgesic effect is mediated by the activation of CB2 receptors on non-neuronal cells in the skin, but the cellular mechanism underlying the effect remains to be resolved.
4.1 Pharmacology of the anti-hyperalgesic effect of 2-AG
Evidence that the anti-hyperalgesic effect of 2-AG was mediated by activation of CB2 and not CB1 receptors is noteworthy in light of reports that 2-AG is a full agonist at both CB1 and CB2 receptors [35], and 2-AG activates CB1 receptors in the brain [16,36]. Conversely, AEA administered by the same route is anti-hyperalgesic by activation of CB1 and not CB2 receptors in this model [26]. The apparent selective effect of 2-AG for peripheral murine CB2 receptors is consistent with the anti-nociceptive effect of this endocannabinoid in rats in the formalin model of nocifensive behavior [13]. However, the data contrast with a report in a more relevant model of persistent hyperalgesia: Both CB1 and CB2 receptors contribute to the anti-hyperalgesic effect of 2-AG in assays of mechanical and thermal sensitivity in a rat model of neuropathic pain [15]. The variability in observations most likely reflects underlying differences in the pathophysiology of the models of peripheral injury and accompanying neurochemical changes in somatosensory neurons [37]. We [26,38,39] and others [40] have shown that chemicals released from cancer cells modify the neurochemistry and excitability of DRG neurons in vitro. Importantly, the anti-hyperalgesia following 2-AG in the present study was not mediated systemically because 2-AG injected into the contralateral paw did not alter mechanical hyperalgesia exhibited by the tumor-bearing paw.
In addition to the receptor selectivity of 2-AG in reducing mechanical hyperalgesia, it was curious that the anti-hyperalgesic effect of 2-AG (18 μg, equivalent to 48 nmol) occurred within the context of a level of 2-AG in plantar skin of the tumor-bearing hind paw that was approximately 3-fold higher than that in skin from naïve mice. The higher level of 2AG in the skin of tumor bearing mice can be attributed in part to the synthesis of 2AG by fibrosarcoma cells (Khasabova, unpublished observation). Evidence that exogenous 2-AG as well as inhibition of MGL with a dose of JZL184 that increased levels of 2-AG more than 2-fold had the same anti-hyperalgesic effect supports the biological relevance of the observation. The tissue chemistry underlying this apparent conundrum, however, is not known. There may be compartmentalization of 2-AG within cells, such as the fibrosarcoma cells, and not in the interstitial fluid where CB2 receptors mediating the anti-hyperalgesic effect are localized. Using in vivo microdialysis in nucleus accumbens, JZL184 was shown to increase recovery of 2-AG in the dialysate following neuronal depolarization [19]. Alternatively, the increased expression of CB2 receptors in the tumor-bearing hind paw may reflect the induction of a receptor with a lower affinity for 2-AG thereby requiring a higher level of 2-AG for its activation.
Finally, exogenous 2-AG was less effective in reducing mechanical hyperalgesia at doses higher than 18 μg. Decreased efficacy at a high dose also occurs with the synthetic CB2 receptor agonist AM1241 [30] and is most likely related to CB2 receptors expressed by cells at the injection site. CB2 agonists promote the recruitment of eosinophils resulting in the release of inflammatory mediators [12,41] that would cause pronociceptive effects to counter-balance the anti-hyperalgesic effect of 2-AG. Alternatively, we cannot exclude the possibility that elevated tissue levels of 2-AG following exogenous administration of the endocannabinoid results in increased hydrolysis of the 2-AG and the generation of intermediates such as leukotrienes and prostaglandin E2 (PGE2) which promote hyperalgesia [42,43,44].
4.2 Anti-hyperalgesia mediated by JZL184
This is the first evidence that JZL184 promotes anti-hyperalgesia through a peripheral mechanism in a model of persistent pain. Importantly, we demonstrated that JZL184 (10 μg) elevated the level of 2-AG and not AEA near the site of injection at the dose that was maximally effective in reducing mechanical hyperalgesia in the tumor-bearing paw. The absence of an effect of JZL184 on AEA is consistent with evidence that the anti-hyperalgesic effect of AEA by the same route of administration in this model was mediated solely by CB1 receptors [26], and the anti-hyperalgesic effect of JZL184 was not blocked by the CB1 receptor antagonist at any time-point during its effectiveness. It is noteworthy that the anti-hyperalgesic effect was mediated by CB2 receptors because two recent studies have demonstrated that systemic administration of JZL184 is anti-hyperalgesic in a murine model of neuropathic pain by a CB1 receptor-dependent mechanism [36,45]. This effect following systemic drug administration is most likely mediated centrally because the dose of JZL184 (16 mg/kg, equivalent to approximately 400 μg/mouse [19] was 40 times that used in the present study, and the authors documented a 5–6 fold increase in 2-AG within the central nervous system. Our data also differ from a recent report that intraplantar injection of JZL184 had no effect on the acute mechanical hyperalgesia produced by intraplantar injection of capsaicin in rats [46]. Effectiveness of the peripheral dose of JZL184 in rats was established by its inhibition of capsaicin-induced nocifensive behaviors and thermal hyperalgesia. Whereas the difference in species may contribute to the difference in results, it is also likely that long-term changes underlying sensory transduction in tumor-bearing mice are more relevant (see below).
Although systemic administration of JZL184 increases the level of 2-AG in brain for more than 8 hr [19], the anti-hyperalgesic effect following intraplantar administration was no longer evident 3 h after drug administration. The effect was likely specific to JZL184 as mechanical hyperalgesia was still reduced at 3 h following exogenous administration of 2-AG. The reason for the short duration of action of JZL184 is not known. In addition to the increased generation of arachidonic acid by ABHD6 when MGL is inhibited, JZL184 may promote the accumulation of yet to be defined intermediates that counter-balance its effect on mechanical hyperalgesia.
4.3 CB2 receptors and anti-hyperalgesia
In the periphery CB2 receptors are predominately expressed by keratinocytes [47,48] and immune cells [49,50]. Compared to skin from naive mice, CB2 receptor protein was higher in plantar skin ipsilateral to tumors and was present in tumors, but the cellular location remains to be determined. The antibody used for detection of protein by Western blot was not specific in immunohistochemistry. In contrast to reports of increased expression of CB2 receptor in DRG following peripheral nerve injury [51,52], this did not occur in tumor-bearing mice. Even though nerve injury has been shown in this model [53], the level of CB2 receptor protein was unchanged in the distal portion of the tibial nerve ipsilateral to the tumor-bearing paw. The relatively low level of CB2 receptor protein detected in DRGs was consistent with levels of CB2 receptor mRNA that were also at the limit of detection using quantitative real-time PCR (Seybold, unpublished observation). These data contrast with an increase in CB1 receptor mRNA in DRGs and receptor protein in tibial nerve ipsilateral to tumors in tumor-bearing mice [26]. Thus, in this murine model of tumor pain, CB2 receptors on keratinocytes as well as tumor and immune cells most likely reduce hyperalgesia indirectly by inhibiting the secretion of algogenic substances that increase the excitability of nociceptors. We speculate that CB2 receptors on keratinocytes [47,48] may inhibit the release of ATP. An increase in the interstitial level of ATP is associated with the development of tumors in skin and nocifensive behavior that is reduced by a P2X receptor antagonist in a murine model of skin cancer pain [54]. Additional support for a role of ATP in tumor-related pain is evidence of increased expression of the P2X3 receptor on epidermal nerve fibers in the murine model used in the present experiments [55].
Peripheral administration of the CB2 receptor antagonist did not alter mechanical sensitivity indicating that this sensory modality is not modulated in naïve mice by a basal level of CB2 receptor activation. These data are interesting in light of the high levels of 2-AG in skin relative to AEA and evidence that basal activation of CB1 receptors affects the threshold for nociception in naïve mice [26,33]. Moreover, peripheral CB2 receptors do not play a tonic role in modulating mechanical hyperalgesia in tumor bearing mice as local administration of the CB2 receptor antagonist did not alter mechanical hyperalgesia (Khasabova and Seybold, unpublished observation). Similarly, genetic deletion of CB2 receptors did not alter development of mechanical or thermal allodynia in a murine model of neuropathic pain [36].
4.4 Conclusion
Taken together, the data demonstrate that peripheral 2-AG signaling may be a significant target to exploit for the management of cancer pain. In contrast to AEA, which inhibits nociception through CB1 receptors on DRG neurons [3,33], CB2 receptors occurred in skin but were not associated with somatosensory neurons of tumor-bearing mice. Thus, peripheral effects of 2-AG on mechanical hyperalgesia are most likely mediated by keratinocytes, fibrosarcoma and (or) immune cells. Dual pharmacological modulation of peripheral AEA and 2-AG signaling that directly and indirectly affects DRG neurons may be a novel approach to reducing cancer pain without the side effects associated with systemic cannabinoid administration.
Acknowledgments
This work was supported by grants from the National Institute for Drug Abuse (DA011471, DAS) and the National Cancer Institute [CA091007 (DAS), CA138684 (VSS)]. The authors are grateful to P. Villalta and the University of Minnesota Cancer Center for assistance in the quantification of endocannabinoids.
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
anandamide
- ACPA
arachidonylcyclopropylamide
- AM1241
(2-iodo-5-nitrophenyl)-(1-(1-methylpiperidin-2-ylmethyl)-1H-indol-3-yl)methanone
- AM281
1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- AM630
6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-y l](4-methoxyphenyl)methanone
- CB
cannabinoid
- DMSO
dimethylsulfoxide
- DRG
dorsal root ganglion
- ED50
effective dose for 50% effect
- MGL
monoacylglycerol lipase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Iryna A. Khasabova, Email: khasa003@umn.edu.
Anisha Chandiramani, Email: chan0708@umn.edu.
Catherine Harding-Rose, Email: hardi006@umn.edu.
Donald A. Simone, Email: simon003@umn.edu.
Virginia S. Seybold, Email: vseybold@umn.edu.
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–49s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Cherny NI, Portenoy RK. Cancer pain management. Current strategy. Cancer. 1993;72:3393–415. doi: 10.1002/1097-0142(19931201)72:11+<3393::aid-cncr2820721606>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–70. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guindon J, Beaulieu P. The role of the endogenous cannabinoid system in peripheral analgesia. Curr Mol Pharmacol. 2009;2:134–9. doi: 10.2174/1874467210902010134. [DOI] [PubMed] [Google Scholar]
- 5.Sagar DR, Gaw AG, Okine BN, Woodhams SG, Wong A, Kendall DA, Chapman V. Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain. 2009;8:5–59. doi: 10.1186/1744-8069-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 9.Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–57. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, et al. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br J Pharmacol. 2007;150:766–81. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–46. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mecs L, Tuboly G, Toth K, Nagy E, Nyari T, Benedek G, Horvath G. Peripheral antinociceptive effect of 2-arachidonoyl-glycerol and its interaction with endomorphin-1 in arthritic rat ankle joints. Clin Exp Pharmacol Physiol. 2010;37:544–50. doi: 10.1111/j.1440-1681.2009.05346.x. [DOI] [PubMed] [Google Scholar]
- 15.Desroches J, Guindon J, Lambert C, Beaulieu P. Modulation of the anti-nociceptive effects of 2-arachidonoyl glycerol by peripherally administered FAAH and MGL inhibitors in a neuropathic pain model. Br J Pharmacol. 2008;155:913–24. doi: 10.1038/bjp.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 17.King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, et al. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–65. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandevoorde S, Jonsson KO, Labar G, Persson E, Lambert DM, Fowler CJ. Lack of selectivity of URB602 for 2-oleoylglycerol compared to anandamide hydrolysis in vitro. Br J Pharmacol. 2007;150:186–91. doi: 10.1038/sj.bjp.0706971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–9. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, et al. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–66. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Mouedden M, Meert TF. Evaluation of pain-related behavior, bone destruction and effectiveness of fentanyl, sufentanil, and morphine in a murine model of cancer pain. Pharmacol Biochem Behav. 2005;82:109–19. doi: 10.1016/j.pbb.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Hamamoto DT, Giridharagopalan S, Simone DA. Acute and chronic administration of the cannabinoid receptor agonist CP 55,940 attenuates tumor-evoked hyperalgesia. Eur J Pharmacol. 2007;558:73–87. doi: 10.1016/j.ejphar.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, et al. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–86. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 25.Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN 55, 212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, et al. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci. 2008;28:11141–52. doi: 10.1523/JNEUROSCI.2847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–7. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Lan R, Gatley J, Lu Q, Fan P, Fernando SR, Volkow ND, et al. Design and synthesis of the CB1 selective cannabinoid antagonist AM281: a potential human SPECT ligand. AAPS PharmSci. 1999;1:E4. doi: 10.1208/ps010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–72. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khasabova IA, Gielissen J, Chandiramani A, Harding-Rose C, Abu Odeh D, Simone DA, Seybold VS. CB1 and CB2 receptor agonists promote analgesia through synergy in a murine model of tumor pain. Behavioral Pharm. doi: 10.1097/FBP.0b013e3283474a6d. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–81. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu P, Bisogno T, Punwar S, Farquhar-Smith WP, Ambrosino G, Di Marzo V, et al. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur J Pharmacol. 2000;396:85–92. doi: 10.1016/s0014-2999(00)00226-0. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptor. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:312–20. doi: 10.1016/j.pain.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 35.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 36.Kinsey SG, Long JZ, Cravatt BF, Lichtman AH. Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti-allodynic effects in mice through distinct cannabinoid receptor mechanisms. J Pain. 2010;11:1420–28. doi: 10.1016/j.jpain.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–98. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 38.Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, et al. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;27:10289–98. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chizhmakov I, Mamenko N, Volkova T, Khasabova I, Simone DA, Krishtal O. P2X receptors in sensory neurons co-cultured with cancer cells exhibit a decrease in opioid sensitivity. Eur J Neurosci. 2009;29:76–86. doi: 10.1111/j.1460-9568.2008.06556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schweizerhof M, Stösser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15:802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 41.Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006;177:8796–805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- 42.Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry. 2001;40:861–866. doi: 10.1021/bi002303b. [DOI] [PubMed] [Google Scholar]
- 43.Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, et al. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–86. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- 44.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–10. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spradley JM, Guindon J, Hohmann AG. Inhibitors of monoacylglycerol lipase, fatty-acid amide hydrolase and endocannabinoid transport differentially suppress capsaicin-induced behavioral sensitization through peripheral endocannabinoid mechanisms. Pharmacol Res. 2010;62:249–58. doi: 10.1016/j.phrs.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casanova ML, Blázquez C, Martínez-Palacio J, Villanueva C, Fernández-Aceñero MJ, Huffman JW, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA. 2005;102:3093–8. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 50.Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukoc Biol. 2003;74:486–96. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- 51.Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–80. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–45. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, et al. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001;21:9367–76. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujita M, Andoh T, Sasaki A, Saiki I, Kuraishi Y. Involvement of peripheral adenosine 5′-triphosphate and P2X purinoceptor in pain-related behavior produced by orthotopic melanoma inoculation in mice. Eur J Neurosci. 2010;31:1629–36. doi: 10.1111/j.1460-9568.2010.07185.x. [DOI] [PubMed] [Google Scholar]
- 55.Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR, et al. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005;1044:197–205. doi: 10.1016/j.brainres.2005.02.081. [DOI] [PubMed] [Google Scholar]