Abstract
Interleukin 6 (IL-6) is an inflammatory cytokine overexpressed in obese individuals that contributes to the development of diseases such as insulin resistance, type 2 diabetes, and cardiovascular disease. This study investigated the inhibitory effect of an extract from the bamboo Phyllostachys edulis (BEX) on lipotoxicity-induced over-production of IL-6 in metabolic cell lines. Palmitic acid (PA, 0.4 mM) was used to induce lipotoxicity in murine C2C12, 3T3-L1, and Hepa6 cells. Both intra- and extra-cellular protein concentrations of IL-6 were measured in the three cell lines after PA treatment with or without the presence of BEX using cytometric bead assays. IL-6 mRNA levels were quantified using real-time PCR, and nuclear concentrations of c-fos, p50 and p65 proteins were measured using DNA-binding ELISA in 3T3-L1 cells. Lipotoxicity increased IL-6 protein concentration in both cytosol and media collected from myoblast and myotube C2C12, as well as preadipose and adipose 3T3-L1, and the presence of BEX (0.5%, v/v) effectively inhibited this overproduction. IL-6 protein expression in hepatic Hepa6 cells was less affected by lipotoxicity. BEX significantly ameliorated PA-induced upregulation of IL-6 mRNA, which correlated with a reduction in nuclear translocation of p50, p65, and c-fos proteins with the presence of BEX, indicating inhibition of NF-κB and AP-1 activation. In summary, BEX inhibits lipotoxicity-induced IL-6 overproduction in muscle and adipose cell lines through the NF-κB and AP-1 pathways, implicating a potential application of this natural product as a cost-effective anti-inflammation nutraceutical.
Keywords: Lipotoxicity, IL-6, NFκB, AP-1, Bamboo extract
Introduction
Interleukin-6 (IL-6) is a pro-inflammatory cytokine. Its biological activities are initiated by binding to a high-affinity receptor complex, which consists of two membrane and one soluble glycoproteins (1). Unlike other cytokines that function via paracrine or autocrine mechanisms, IL-6 is a predominantly circulatory molecule (2). Approximately 25–30% of circulating IL-6 levels is secreted from adipose tissue, with a greater contribution from visceral than subcutaneous fat (3, 4). Besides adipose tissue, skeletal muscle is another significant source of this cytokine (5).
IL-6 is one of the key biochemical risk factors in the pathogeneses of insulin resistance, type 2 diabetes and cardiovascular disease, which are associated with chronic low-grade inflammation (6–8). In healthy men and women, the production and circulating concentration of IL-6 increase with adiposity (3), and plasma IL-6 concentrations positively correlate with fasting insulin and insulin resistance (9). In the newly identified “normal-weight obese syndrome”, systemic concentrations of IL-6 increase in subjects with over 30% of body weight from fat (10), which preludes the development of insulin resistance (11). IL-6 may also induce endothelial expression of chemokines and adhesion molecules, therefore facilitating atherogenesis (12), and it has been reported that elevated concentrations of IL-6 predict total and cardiovascular mortality (13).
Adiposity is associated with the onset of lipotoxicity that results from elevated levels of free fatty acids (FFA) in the peripheral circulation (14). Long-chain saturated FFA such as myristic, palmitic, and stearic acids are known ligands to Toll-Like Receptor 4 (TLR4) (15), an upstream signaling component of both NF-κB and AP-1, two of the major transcriptional regulatory pathways for IL-6 (16, 17).
Bamboo Phyllostachys edulis is one of the fastest growing plants in the world, with large biomass and wide geographic distribution, signifying that it is an abundant and sustainable natural resource. In this study, we investigated the regulatory effect of an ethanolic extract from Phyllostachys edulis on lipotoxicity-induced overproduction of IL-6 in metabolic cells. Three murine cell lines were used in this study, i.e. myoblast C2C12, pre-adipose 3T3-L1, and hepatic Hepa6. C2C12 and 3T3-L1 cells were also differentiated into myotubes and adipocytes, respectively. IL-6 protein concentrations were measured in both cytosol and culture media. IL-6 mRNA, and nuclear concentrations of c-fos, p50, and p65 proteins were further measured in 3T3-L1 cells to investigate the transcriptional regulation of this cytokine. To determine whether the IL-6 response was specific to lipotoxicity or due to more general cytotoxic mechanisms, 3T3-L1 pre-adipocytes were treated with a variety of apoptosis-inducing drugs to see if they had any effect on IL-6 secretion.
Experimental Methods
Bamboo extract (BEX)
The BEX used in this study was provided by Golden Basin LLC (Kailua, HI). It is made from fresh leaves and small branches of bamboo Phyllostachys edulis in Hunan Province, China, through a patented ethanol/water extraction procedure (Chinese invention patent, CN 1287848A). Freeze-dried BEX powder was further extracted with 100% ethanol (100 mg/ml) at room temperature for 4 h, and centrifuged at 10,000 × g at 4°C for 10 min. The ethanol soluble portion (supernatant, 12 g dry mass/L), was used in the studies.
Cell line maintenance
C2C12, Hepa6, and 3T3-L1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA), and maintained in Dulbecco's Modified Eagle Medium (DMEM, ATCC catalog number 30-2202) with the supplement of 10% Fetal Bovine Serum (Biomeda, Foster City, CA, catalog number 22685), and 50ug/mL penicillin/ streptomycin (Gibco/Invitrogen, Carlsbad, CA, Catalog number 15140-122). The cells were incubated in a cell culture incubator at 37°C with an atmosphere of 95% air, 5% carbon dioxide, and 55% humidity.
Myotube differentiation
C2C12 cells were allowed to reach confluency and then incubated in DMEM with a supplement of 2.5% horse serum (Sigma, St. Louis, MA, catalog number H1138) for 10 days.
Adipocyte differentiation
3T3-L1 pre-adipocytes were allowed to reach confluency for 2 days, and then treated with 0.5 mM methylisobutylxanthine (Sigma, catalog number I5879), 10 μg/ml insulin (Sigma, catalog number I0516), and 1 μM dexamethasone (Sigma, catalog number D4902) for 2 days, followed by treatment with10μg/ml insulin for 6 days (18).
Lipotoxic treatment of cells
Palmitic acid (PA, purchased from Sigma, catalog number P5585) was dissolved in dimethyl sulfoxide (DMSO, purchased from Sigma, catalog number D4540) at a concentration of 0.4 M. This stock solute was further diluted in complete culture medium at a 1:1000 ratio to reach the final concentration of 0.4 mM. Sonication was applied to disperse PA. Each of the cell lines was treated with: (i) complete medium; (ii) complete medium containing 0.4 mM PA; (iii) complete medium containing 0.4 mM PA and 0.5% (v/v) ethanol-soluble fraction of BEX; and (iv) complete medium containing 0.4 mM PA and 0.5% (v/v) ethanol as a solvent control. To further understand the influences of solvents and BEX on IL-6 production outside the context of PA, 3T3-L1 pre-adipocytes were treated with: (v) 0.1% (v/v) DMSO; (vi) 0.1% DMSO and 0.5% ethanol; (vii) 0.5% ethanol; (viii) 0.5% ethanol-soluble fraction of BEX.
Apoptotic chemical treatment of cells
3T3-L1 pre-adipocytes were treated with 10μM paclitaxel (PTX), 10mM 5-fluorouracil (5FU), 5mM hydrogen peroxide (H2O2), and 1mg/mL geneticin (G418). Concentrations were determined by assaying the maximum drug concentration at which cell death occurred after 24 hours of treatment, but did not prematurely detach and die before 20 hours.
Quantitative Real-time PCR (qRT-PCR)
Total RNA was extracted from cells after treatments using RNeasy Mini Kit (Qiagen, Valencia, CA, catalog number 74104). cDNA was synthesized using the SuperScript III First-Strand Synthesis System (Invitrogen, catalog number 18080-051). 18s ribosomal RNA (18s), hypoxanthineguanine phosphoribosyltransferase (HPRT), and ubiquitin c (UBC) were used as housekeeping genes. The sequences of the primers are: 18s Fwd: GCAATTATTCCCCATGAACG; 18s Rev: GGGACTTAATCAACGCAAGC; HPRT Fwd: TCCTCCTCAGACCGCTTTT; HPRT Rev: CCTGGTTCATCATCGCTAATC; UBC Fwd: GACCAGCAGAGGCTGATCTT; UBC Rev: CCTCTGAGGCGAAGGACTAA. IL-6 primers were purchased from SABiosciences (Frederick, MD, catalog number PPM03015A-200). qRT-PCR was performed in quadruplicates (5 uL each reaction) in 384-well plates in a Light cycler 480 II real-time PCR machine (Roche Applied Sciences, Indianapolis, IN). Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen, catalog number 11733-038) was used to carry out the qPCR reactions.
Fractionation of cell lysate
Cytosolic and nuclear cellular fractions were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA, catalog number 40010). The protein concentration of each fraction was measured using a Bradford assay (Bio-rad, Hercules, CA, catalog number 500-0006).
IL-6 protein detection
Cell culture media were collected and concentrated using Vivaspin 2 concentrators (GE Healthcare, Piscataway, NJ, catalog number 28-9322-45). IL-6 protein concentration was measured in the cell cytosolic fraction and the concentrated culture medium using a Cytometric Bead Array (CBA) - Mouse IL-6 Flex Set (BD Biosciences, San Jose, CA, catalog number 558301). The flow cytometer used was a BD FACSCalibur (BD Biosciences).
Quantification of p50, p65, and c-fos nuclear translocation
TransAM NFκB Family Kit and TransAM AP-1 Family Kit (Active Motif, Carlsbad, CA, catalog numbers 43296 and 44296) were used to measure the concentrations of p50, p65, and c-fos in nuclear fractions of the cells via colorimetric DNA-binding ELISA.
Statistical analyses
Software used was Prism 4.0a (GraphPad Software, Inc.). Methods employed in the data analyses included one-way ANOVA with Tukey's multiple comparison test, one-way ANOVA with Bonferroni post test. p ≤ 0.05 was considered statistically significant.
Results
BEX inhibits PA-induced overproduction of IL-6 protein in 3T3-L1 and C2C12 cells
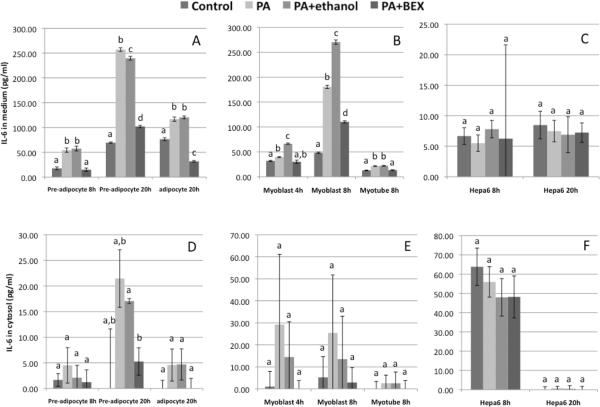
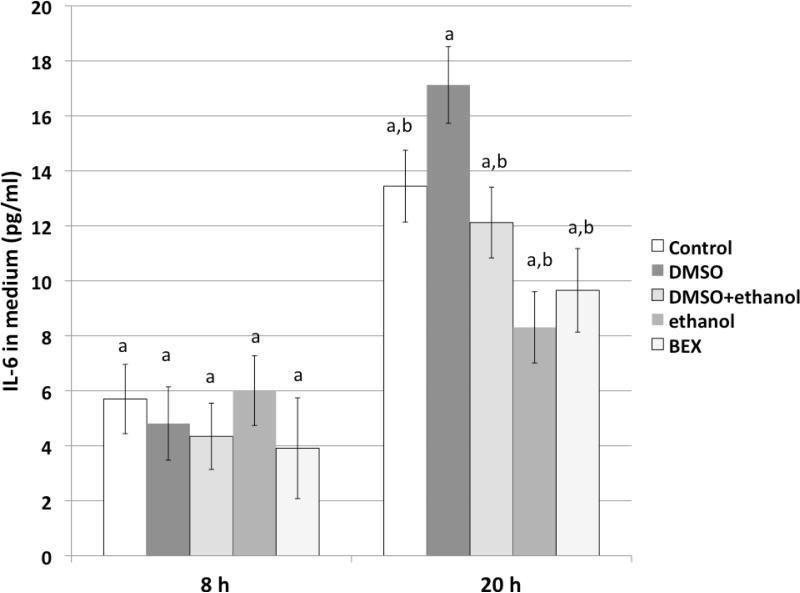
Figures 1A & 1B show IL-6 protein concentrations in the culture media of 3T3-L1 and C2C12 cells at different time points. PA treatment significantly increased the secretion of IL-6 from pre-adipocyte, adipocyte, myoblast, and myotube cells, and this increase was effectively ameliorated by the presence of BEX. After 20 hours of treatment, the media of pre-adipocytes contained noticeably more IL-6 protein than those from adipocytes (Figure 1A), which is in line with a previous study reporting that expression of IL-6 is greater in pre-adipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice (19). Meanwhile, Figure 1B also shows that at 8 hours, myoblasts secreted more IL-6 protein in comparison to myotubes. On the other hand, the amounts of IL-6 protein retained in the cytosol fractions were much lower (Figure 1D & 1E), implicating that most of the IL-6 synthesized in adipose and muscle tissues may be secreted. In contrast, the IL-6 production in Hepa6 cells was less influenced by the PA treatment, and the amount of secreted IL-6 was much lower than those from 3T3-L1 and C2C12. Treatment of 3T3-L1 cells with solvent vehicles did not significantly alter levels of secreted IL-6, nor did treatment of 3T3-L1 cells with BEX in the absence of PA (Figure 2).
Figure 1. IL-6 protein concentrations in culture media and cytosol fractions of 3T3-L1, C2C12, and Hepa6 cells.
The cells were treated with normal medium (control), 0.4 mM PA, PA+0.5% ethanol, or PA+0.5% BEX. Mean and SE are shown. Means without a common letter differ (comparisons were among the four treatments on the same cell type at the same time point), P<0.05, one-way ANOVA, Bonferroni post test.
Figure 2. The effects of solvents and BEX on IL-6 secretion from 3T3-L1 cells in the absence of palmitic acid.
The cells were treated with normal medium (control), 0.1% (v/v) DMSO, 0.1% DMSO+0.5% (v/v) ethanol, 0.5% ethanol, and 0.5% BEX for 8 h and 20 h. Mean and SE are shown. Means without a common letter differ (comparisons were among the treatments at the same time point), P<0.05, one-way ANOVA, Bonferroni post test.
BEX inhibits IL-6 overproduction at the transcriptional level
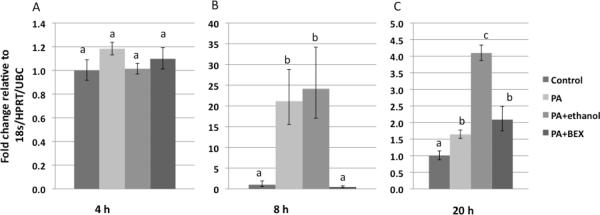
Transcription of the IL-6 gene was further studied in pre-adipose 3T3-L1 cells through relative quantification of IL-6 mRNA. Figure 3 shows that PA treatment upregulated the gene expression of IL-6, and this upregulation was significantly inhibited by BEX. Interestingly, PA increased IL-6 mRNA approximately 20 fold at 8 hours, in comparison to less dramatic changes before at 4 hours and after at 20 hours, demonstrating a dynamic change of IL-6 transcriptional regulation in response to PA.
Figure 3. IL-6 mRNA expression in 3T3-L1 cells.
The cells were treated with control medium, 0.4 mM PA, PA+0.5% ethanol, or PA+0.5% BEX. Mean and SE are shown. Means without a common letter differ (comparisons were among the four treatments on the same cell type at the same time point), P<0.05, one-way ANOVA, Bonferroni post test.
BEX inhibits nuclear translocation of p50, p65, and c-fos
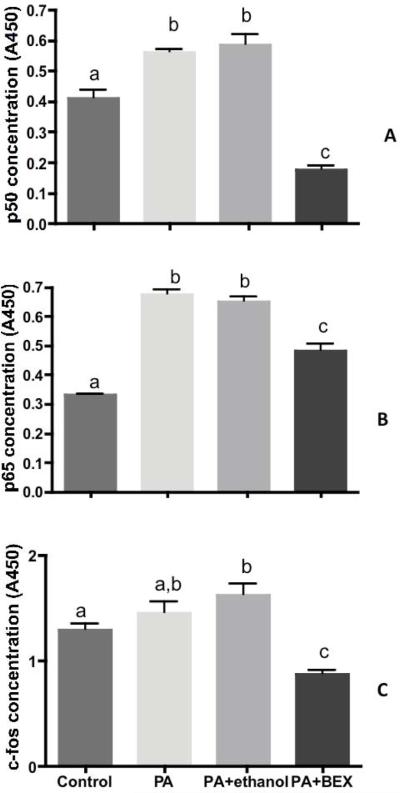
The concentrations of p50, p65 (the two most abundant subunits of the NF-κB complex) and c-fos (a subunit of the AP-1 complex) in the nuclear fractions were measured after 2, 4, and 8 hours of treatments. PA treatment enhanced nuclear translocation of both p50 and p65 as early as 2 hours (Figure 4A & 4B), and these increases were attenuated by BEX. On the other hand, a lipotoxicity-induced increment of c-fos in the nuclear fraction was observed at 4 hours, and the presence of BEX showed a significant inhibitory effect at this time point (Figure 4C). These results indicate that both and AP-1 pathways were involved in the PA-induced upregulation of IL-6 transcription, and BEX effectively inhibited the activation of both pathways.
Figure 4. The concentrations of p50, p65, and c-fos in the nuclear fraction of 3T3-L1 cells.
The cells were treated with control medium, 0.4 mM PA, PA+0.5% ethanol, or PA+0.5% BEX. The concentrations of p50 and p65 were measured after 2 hours of treatment, and that of c-fos after 4 hours of treatment. Mean and SE are shown. Means without a common letter differ (comparisons were among the four treatments on the same cell type at the same time point), P<0.05, one-way ANOVA with Tukey's multiple comparison test.
3T3-L1 pre-adipocytes secrete IL-6 in response to apoptosis-inducing chemicals
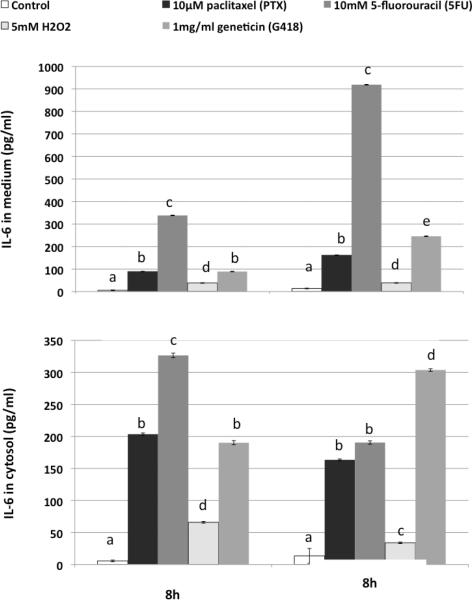
All cytotoxic chemical treatments significantly increased levels of secreted (Figure 5A) and intracellular IL-6 (Figure 5B). 5FU was particularly effective in inducing IL-6 secretion. H2O2 induced lower levels of intracellular and secreted IL-6 than the rest of the drugs, but still significantly more than untreated pre-adipocytes.
Figure 5. Cytotoxicity/apoptosis-inducing reagents upregulates IL-6 protein concentrations in culture media (A) and cytosol (B) of 3T3-L1 cells.
The cells were treated with normal medium (control), 10μM paclitaxel (PTX), 10mM 5-fluorouracil (5FU), 5mM hydrogen peroxide (H2O2), and 1mg/mL geneticin (G418) for 8 h and 20 h. Means without a common letter differ (comparisons were among the treatments at the same time point), P<0.05, one-way ANOVA, Bonferroni post test.
Discussion
This study investigated the inhibitory effect of BEX on lipotoxicity-induced overproduction of inflammatory cytokine IL-6 in adipose, muscle, and liver cell lines at the levels of protein, mRNA, and transcriptional regulation, and revealed the involvement of both NF-κB and AP-1 in the regulatory pathways. Our previous publication has demonstrated that the presence of BEX in culture media did not affect cellular uptake of FFA, and protected a variety of cell lines from PA-induced cytotoxicity (20). These findings indicate that the anti-inflammatory effect of BEX is not due to the blockage of FFA absorption, nor due to compromised cellular function. The molecular events further upstream of NF-κB and AP-1 activation are to be investigated. Future fractionation and purification of BEX is going to allow us to identify the major functional compound(s), and investigate potential synergistic effects among different components of this complex extract.
Other than PA, the apoptosis-inducing reagents tested in this study also induced the upregulation of IL-6 secretion in 3T3-L1 cells, implicating that IL-6 not only responses to lipotoxicity, but also responses to generic cytotoxic/apoptotic stimuli. This provides a new insight towards further mechanistic investigation in the anti-inflammatory effect of BEX.
The anti-inflammatory effect of BEX, especially the attenuation of IL-6, has been reported for several other natural products, including tomato-derived lycopene (27), cortex lycii radicis extracts (21), tart cherry (26), blueberry (22), grape seed procyanidins (23), cinnamon extract (24), garlic 1,2-vinyldithiin (25), and resveratrol (28). Due to the use of different models and experimental procedures, and variable purity of the extracts, it is difficult to perform accurate comparison between the efficacy of BEX and other reported natural products. However, the on-going effort to purify functional compound(s) from BEX will allow us to compare the efficiency of these compounds versus other known anti-inflammation compounds in the near future. The extraordinary abundance of the raw material is a major advantage of BEX, as Phyllostachys edulis is one of the fastest growing land plants in the world, growing 1 to 1.6 feet per day during the initial growth phase. It is a “running bamboo” that can be easily propagated, and also a tolerant plant adaptable to 5 of the 8 climate zones. This rich and sustainable natural resource holds potential of yielding a cost-effective nutraceutical.
In summary, this study demonstrated a potent anti-inflammatory effect of BEX on cellular and molecular levels, implicating a potential application of this cost-effective natural product in the prevention of obesity-induced chronic inflammation. Safety and efficacy of BEX for human consumption are currently under investigation.
Acknowledgements
We thank Golden Basin LLC for providing the bamboo extract. This study was made possible by grant numbers R21 AT003874-02 (Panee) from the National Center for Complementary and Alternative Medicine (NCCAM), R21 AT005139-01 (Panee) from NCCAM and the Office of Research on Women's Health (ORWH), 5G12RR003061-23 from the National Center for Research Resources (NCRR), and 5P20 MD000173-08 from the National Center on Minority Health and Health Disparities (NCMHD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ORWH, NCRR, NCMHD or the National Institute of Health (NIH).
Sources of financial support: This study was made possible by grant numbers R21 AT003874-02 (Panee) and R21 AT005139-01 (Panee) from the National Center for Complementary and Alternative Medicine (NCCAM) and Office of Research on Women's Health (ORWH), 5G12RR003061-23 from the National Center for Research Resources (NCRR), and 5P20 MD000173-08 from the National Center on Minority Health and Health Disparities (NCMHD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, NCRR, NCMHD or the National Institute of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest: The authors declare that there are no conflicts of interest.
References
- 1.Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 2.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 4.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Real JM. Genetic predispositions to low-grade inflammation and type 2 diabetes. Diabetes Technol Ther. 2006;8:55–66. doi: 10.1089/dia.2006.8.55. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, et al. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 10.De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? The American journal of clinical nutrition. 2007;85:40–45. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Di Renzo L, Bertoli A, Bigioni M, Del Gobbo V, Premrov MG, Calabrese V, et al. Body composition and -174G/C interleukin-6 promoter gene polymorphism: association with progression of insulin resistance in normal weight obese syndrome. Curr Pharm Des. 2008;14:2699–2706. doi: 10.2174/138161208786264061. [DOI] [PubMed] [Google Scholar]
- 12.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 13.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 14.van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.11.049. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Kanazawa A, Tsukada S, Maeda S. PKCepsilon induces interleukin-6 expression through the MAPK pathway in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:707–712. doi: 10.1016/j.bbrc.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 18.Ntambi JM, Kim YC. Adipocyte Differentiation and Gene Expression. J Nutr. 2000;130:3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 19.Harkins JM, Moustaid-Moussa N, Chung YJ, K.M. P, Pestka JJ, North CM, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 20.Panee J, Liu W, Lin Y, Gilman C, Berry MJ. A novel function of bamboo extract in relieving lipotoxicity. Phytother Res. 2008;22:675–680. doi: 10.1002/ptr.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Z, Huang Q, Ni HX, Wang D. Cortex Lycii Radicis extracts improve insulin resistance and lipid metabolism in obese-diabetic rats. Phytother Res. 2008;22:1665–1670. doi: 10.1002/ptr.2552. [DOI] [PubMed] [Google Scholar]
- 22.DeFuria J, Bennett G, Strissel KJ, Perfield JW, Milbury PE, Greenberg AS, et al. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139:1510–1516. doi: 10.3945/jn.109.105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chacón MR, Ceperuelo-Mallafré V, Maymó-Masip E, Mateo-Sanz JM, Arola L, Guitiérrez C, et al. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine. 2009;47:137–142. doi: 10.1016/j.cyto.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Qin B, Dawson H, Polansky MM, Anderson RA. Cinnamon extract attenuates TNF-alpha-induced intestinal lipoprotein ApoB48 overproduction by regulating inflammatory, insulin, and lipoprotein pathways in enterocytes. Horm Metab Res. 2009;41:516–522. doi: 10.1055/s-0029-1202813. [DOI] [PubMed] [Google Scholar]
- 25.Keophiphath M, Priem F, Jacquemond-Collet I, Clément K, Lacasa D. 1,2-vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J Nutr. 2009;139:2055–2060. doi: 10.3945/jn.109.105452. [DOI] [PubMed] [Google Scholar]
- 26.Seymour EM, Lewis SK, Urcuyo-Llanes DE, Tanone II, Kirakosyan A, Kaufman PB, et al. Regular tart cherry intake alters abdominal adiposity, adipose gene transcription, and inflammation in obesity-prone rats fed a high fat diet. J Med Food. 2009;12:935–942. doi: 10.1089/jmf.2008.0270. [DOI] [PubMed] [Google Scholar]
- 27.Markovits N, Ben Amotz A, Levy Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr Med Assoc J. 2009;11:598–601. [PubMed] [Google Scholar]
- 28.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. The American journal of clinical nutrition. 2010 doi: 10.3945/ajcn.2009.28435. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bataille R, Klein B. C-reactive protein levels as a direct indicator of interleukin-6 levels in humans in vivo. Arthritis Rheum. 1992;35:982–984. doi: 10.1002/art.1780350824. [DOI] [PubMed] [Google Scholar]