Abstract
Nesfatin-1 reduces food intake when injected centrally in rodents. We recently described wide distribution of nucleobindin2 (NUCB2)/nesfatin-1 immunoreactivity in rat brain autonomic nuclei activated by various stressors. We used C57BL/6 mice to localize brain NUCB2/nesfatin-1 immunoreactivity and assessed activation of NUCB2/nesfatin 1 neurons after water avoidance stress (WAS). Gastric emptying of a non-nutrient liquid was also determined. NUCB2/nesfatin-1 immunoreactivity was detected in cortical areas including piriform, insular, cingulate and somatomotor cortices, the limbic system including amygdaloid nuclei, hippocampus and septum, the basal ganglia, bed nucleus of the stria terminalis, the thalamus including paraventricular and parafascicular nuclei, the hypothalamus including supraoptic, periventricular, paraventricular (PVN), arcuate nuclei and ventromedial and lateral hypothalamic areas. Intensely labeled NUCB2/nesfatin-1 neurons were detected in a previously undefined region which we named intermediate dorsomedial hypothalamus. In the brainstem, NUCB2/nesfatin-1 immunoreactivity was detected in the raphe nuclei, Edinger-Westphal nucleus, locus coeruleus (LC), lateral parabrachial nucleus, ventrolateral medulla (VLM) and dorsal vagal complex. WAS induced Fos expression in 35% of NUCB2/nesfatin-1-immunoreactive neurons in the PVN, 50% in the LC, 54% in the rostral raphe pallidus, 58% in the VLM, 39% in the middle part of the nucleus of the solitary tract (NTS) and 33% in the caudal NTS. Nesfatin-1 injected intracerebroventricularly significantly decreased gastric emptying. These data showed that NUCB2/nesfatin-1 immunoreactivity is distributed in mouse brain areas involved in the regulation of stress response and visceral functions activated by an acute psychological stressor suggesting that nesfatin-1 might play a role in the efferent component of the stress response.
Keywords: Fos, hypothalamus, medulla, NUCB2, gastric emptying, water avoidance stress
Introduction
The recently discovered 82 amino acid polypeptide nesfatin-1 and its precursor nucleobindin2 (NUCB2) were first identified in the rat brain (Oh-I et al., 2006). Initial reports provided compelling evidence that nesfatin-1 reduces nocturnal feeding in rats upon forebrain or hindbrain injection (Maejima et al., 2009; Oh-I et al., 2006; Stengel et al., 2009b; Yosten and Samson, 2010). In addition, a number of consistent neuroanatomical studies in rats have localized nesfatin-1 immunoreactivity and its co-expression with other transmitters in the brain, prominently in cell bodies of hypothalamic nuclei regulating food intake and autonomic brain centers (Brailoiu et al., 2007; Foo et al., 2008; Goebel et al., 2009a; Oh-I et al., 2006). Such a widespread distribution of NUCB2/nesfatin-1 in other brain areas supported a potentially broader spectrum of centrally mediated biological actions linked with autonomic regulation of visceral function (Stengel et al., 2009b; Yosten and Samson, 2009; Yosten and Samson, 2010) in addition to those originally described on food intake (review in Stengel et al., 2010b). In particular, a potential implication in the stress response is underscored by recent reports that intracerebroventricular (icv) nesfatin-1 activates corticotropin-releasing factor (CRF)-positive neurons and increases plasma adrenocorticotropic hormone and corticosterone levels (Konczol et al., 2010; Yoshida et al., 2010) and that various acute stressors including wrap restraint stress (Goebel et al., 2009b; Konczol et al., 2010; Xu et al., 2010; Yoshida et al., 2010), abdominal surgery (Stengel et al., 2010d) or injection of lipopolysaccharide (Bonnet et al., 2009) activate nesfatin-1-immunoreactive (ir) neurons in the rat brain.
However, less is known about the brain distribution and biological actions of nesfatin-1 in mice. So far studies have focused on hypothalamic nuclei regulating food intake, the dorsal vagal complex (DVC), and Edinger-Westphal (EW) nucleus where NUCB2/nesfatin-1 has been detected similarly (Goebel et al., 2011; Okere et al., 2010) as established in rats (Brailoiu et al., 2007; Foo et al., 2008; Goebel et al., 2009a). Functional reports in mice also indicate that icv injection of nesfatin-1 at doses ranging from 100–300 pmol/mouse is an anorexigenic modulator of dark phase food intake and reduces the orexigenic response to a fast in the light phase although such an effect only occurred at higher (1 nmol) and not at lower doses (0.1 or 0.3 nmol/mouse) effective in the dark phase (Atsuchi et al., 2010; Goebel et al., 2011; Maejima et al., 2009)..
Therefore, in the present study, we first established the pattern of NUCB2/nesfatin-1 distribution in the mouse brain obtained during both the light and dark phase as the anorexigenic effect of nesfatin-1 is more readily observed during the nocturnal phase in rodents (Stengel and Taché, 2010). Next, in view of the responsiveness of NUCB2/nesfatin-1 neurons to acute stress in rats (Goebel et al., 2009b; Konczol et al., 2010; Xu et al., 2010; Yoshida et al., 2010), we investigated whether an acute stressor in mice would activate brain neurons immunoreactive for NUCB2/nesfatin-1. We selected water avoidance (WAS) as an acute psychological stress model that has been reported to impact on visceral function and pain in both rats and mice (Cameron and Perdue, 2005; Larauche et al., 2010; Larsson et al., 2009; Melgar et al., 2008; Mönnikes et al., 1993). However unlike in rats, (Bonaz and Taché, 1994; Million et al., 2000), brain nuclei activated by exposure to WAS in mice have not been delineated. This was achieved by immunohistochemical detection of the immediate early gene product Fos (Dragunow and Faull, 1989; Sagar et al., 1988) combined with double labeling with NUCB2/nesfatin-1. Lastly, based on our present immunohistochemical data in mice showing that NUCB2/nesfatin-1 immunoreactivity is densely expressed in brain nuclei involved in vagal regulation of gastric motor functions (Taché et al., 1995), combined with recent evidence that icv injection of nesfatin-1 decreased gastric antral motility in fed mice (Atsuchi et al., 2010), we assessed at the functional level whether icv nesfatin-1 would alter gastric emptying in mice.
2. Results
2.1. Specificity of the antibody to label mouse brain tissue
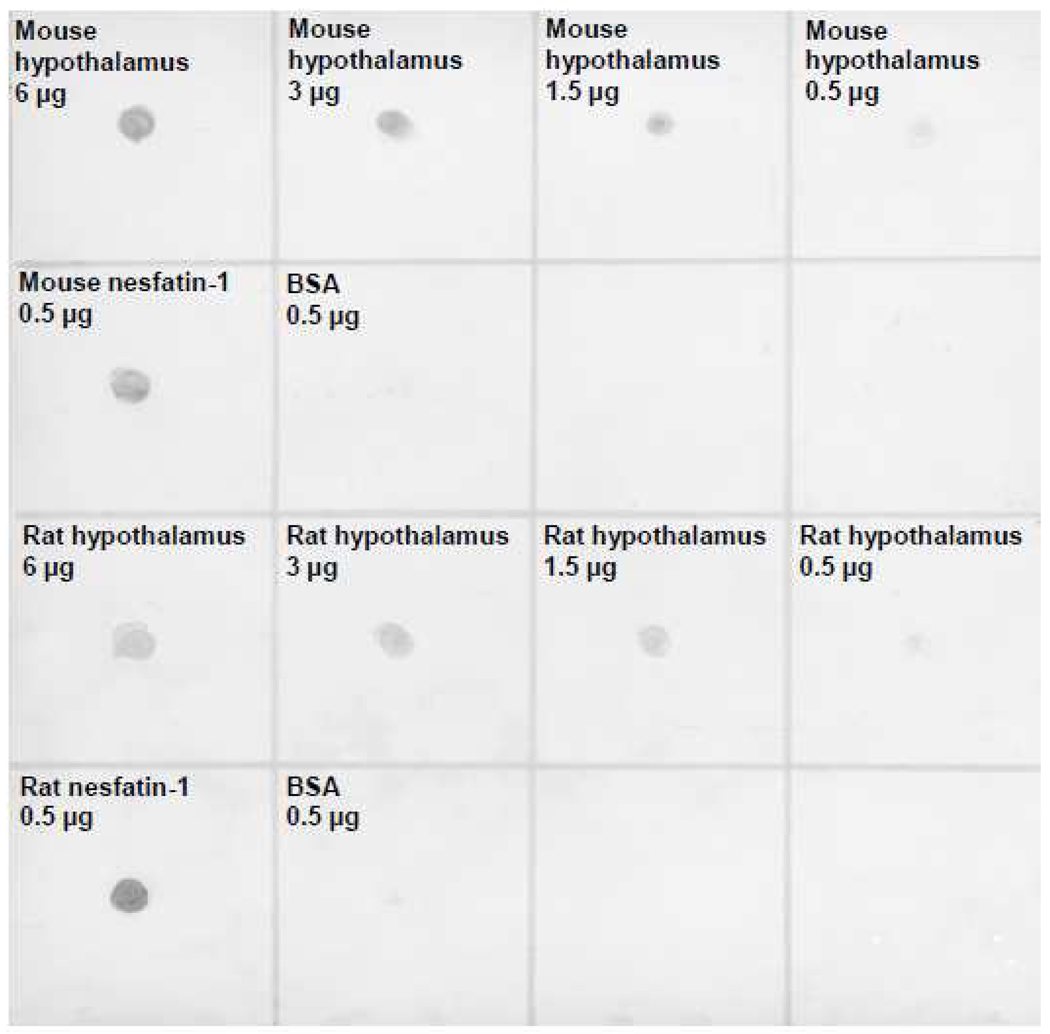
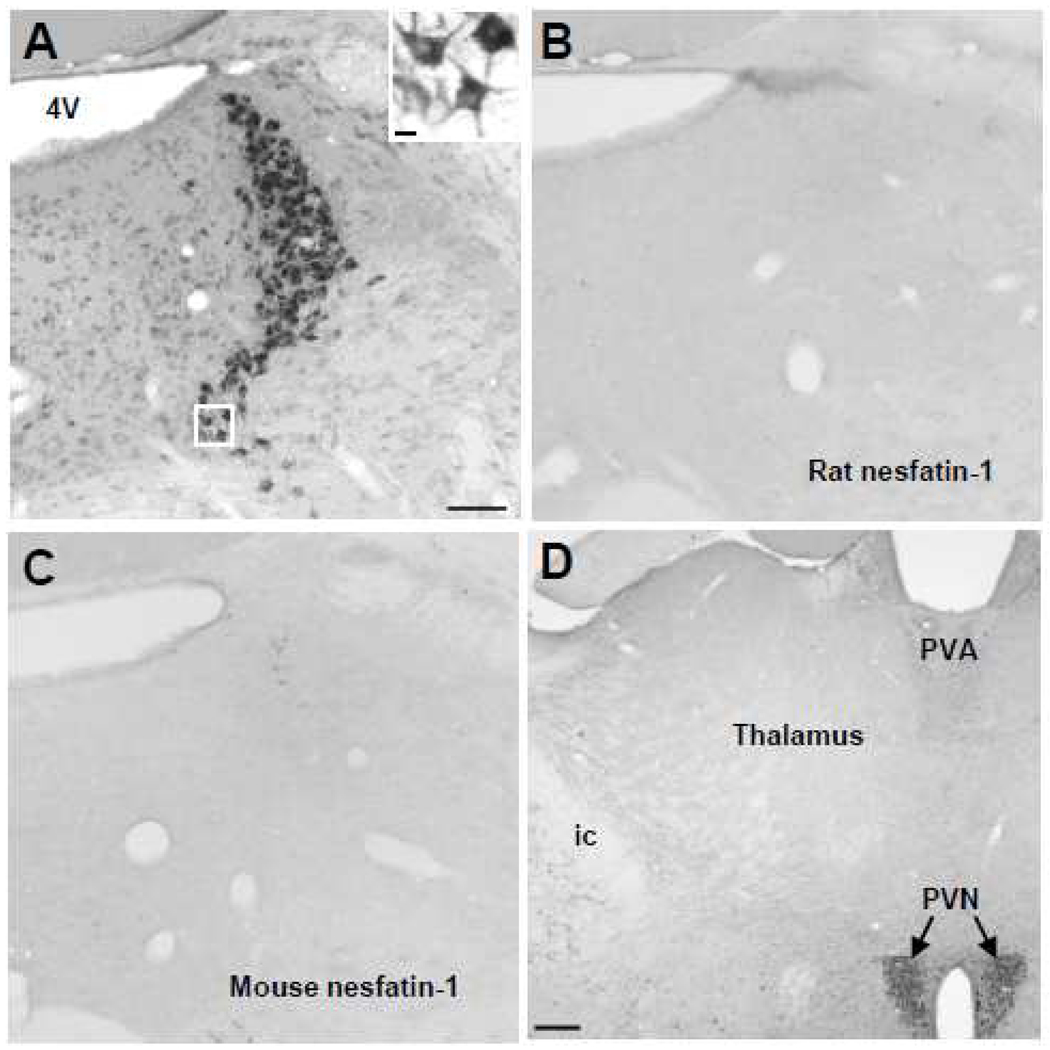
The dot blot showed that the rat nesfatin-1 antibody equally and specifically recognizes rat and mouse nesfatin-1 while BSA protein was not stained (Fig. 1). The rat nesfatin-1 antibody stained both rat and mouse hypothalamic protein loaded at different concentrations (0.5, 1.5, 3.0 and 6.0 µg) with an intensity related to the concentration (Fig. 1). Following pre-absorption of the rat anti-nesfatin-1 antibody by rat nesfatin-1 polypeptide (Fig. 2B) or mouse nesfatin-1 (Fig. 2C), no immunostaining could be detected in the mouse LC while intense immunostaining was observed with the non-preabsorbed rat nesfatin-1 antibody (Fig. 2A). These data established the specificity of the rat nesfatin-1 antibody to perform mapping studies in mice brain.
Fig. 1.
Specificity of the rat nesfatin-1 antibody in mice assessed by dot blot. Shown is a dot blot containing rat and mouse nesfatin-1 polypeptide as well as bovine serum albumin (BSA) as a negative control (0.5 µg each) together with rat and mouse hypothalamic protein in four different concentrations (6 µg, 3.0 µg, 1.5 µg, 0.5 µg). The nesfatin-1 antibody specifically stains rat and mouse nesfatin-1 polypeptide and hypothalamus protein but not BSA.
Fig. 2.
Specificity of the rat nesfatin-1 antibody shown by immunostaining and pre-absorption. Immunohistochemical staining of the locus coeruleus (LC) with the nesfatin-1 antibody (A, insert) and after preabsorption with either rat (B) or mouse (C) nesfatin-1 polypeptide. No immunostaining can be detected in the mouse LC after the rat nesfatin-1 antibody was pre-absorbed with rat or mice nesfatin-1 polypeptide. (D) shows site specificity with the lack of staining in most part of mouse thalamus by the nesfatin-1 antibody. Scale bar 100 µm in A is representative for B and C. The scale bar in the insert in A is 10 µm in C is 200 µm. Abbreviations: 4V, fourth brain ventricle; ic, internal capsule; PVA, anterior paraventricular thalamic nucleus; PVN, paraventricular nucleus of the hypothalamus.
2.2. Nesfatin-1-immunoreactivity in the mouse forebrain
NUCB2/nesfatin-1 immunoreactivity assessed during the light or dark phase in mice did not vary in distribution and density pattern. Therefore, all immunostaining studies are described in mouse brain collected during the light phase. Immunostaining showed a widespread brain distribution of NUCB2/nesfatin-1 immunoreactivity with most of the brain areas displaying some levels of NUCB2/nesfatin-1 immunoreactivity compared with pre-absorption control. However there is a brain specific distribution as selective areas are devoid of staining, namely the lamina IV of the cortex, thalamus (Fig. 2D) and medial and lateral geniculate bodies.
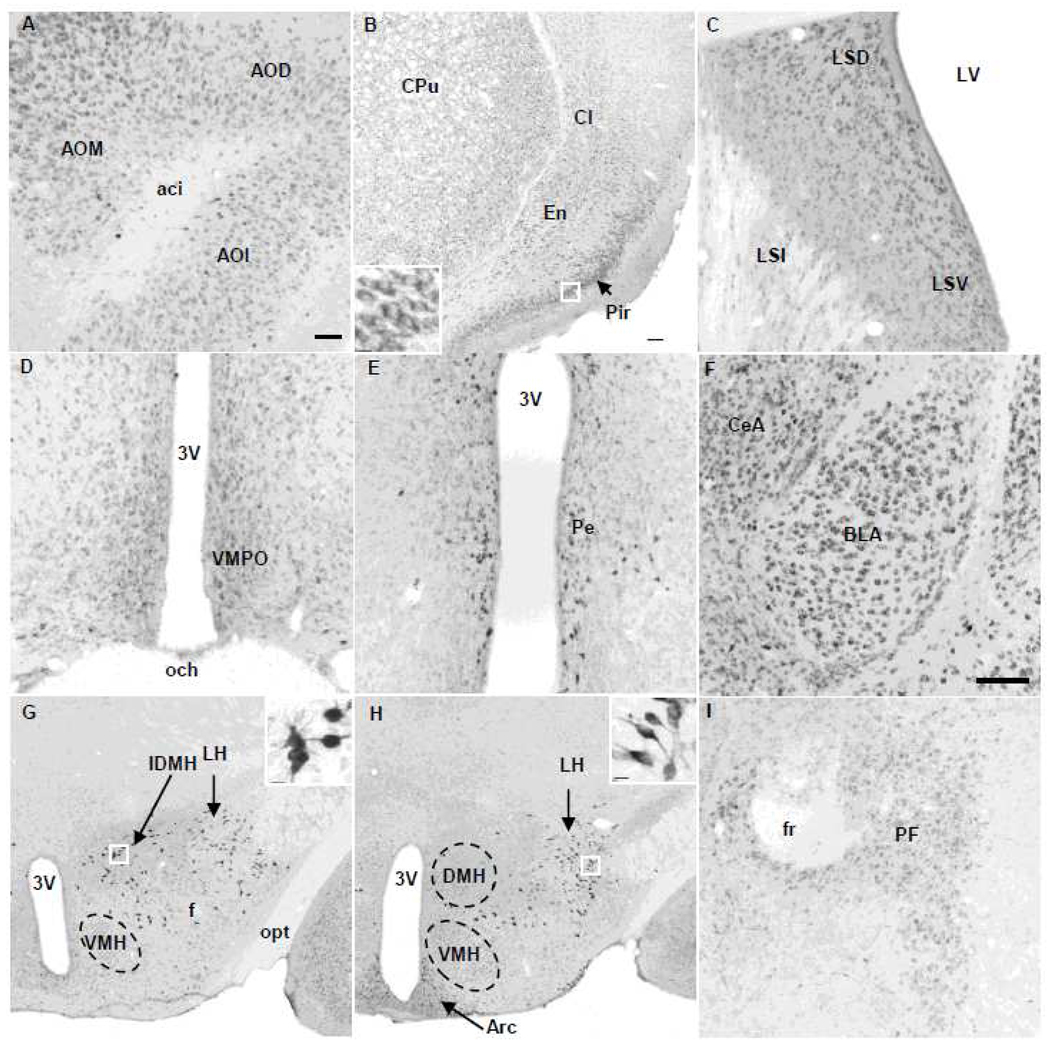
We focused the brain distribution on densely and moderately immunolabeled neuronal groups. Abundant and moderately stained NUCB2/nesfatin-1 immunoreactivity was found in all parts of the anterior olfactory nucleus (AO, Fig. 3A), accumbens nucleus (Table 1), somatomotor, piriform, insular and cingulate cortices and the endopiriform nucleus (Fig. 3B), the basal ganglia including caudate putamen and claustrum (Fig. 3B), lateral septal nucleus (Fig. 3C) and bed nucleus of the stria terminalis (BNST, Table 1). In the amygdala, nesfatin-1-ir neurons were found in all subnuclei (Table 1) with higher density in the basolateral and central nucleus (Fig. 3F). Similar to rats, the mouse parafascicular thalamic nucleus (PF) contained nesfatin-1-ir neurons (Fig. 3I). In the hippocampus (Hi), very clear nesfatin-1 immunolabeling was observed in the pyramidal cell layer (Fig. 4A). Similar to rats, in the hypothalamus, most of the nesfatin-1-ir cells showed intense staining. Nesfatin-1 labeling was found in neurons of the ventromedial preoptic nucleus (Fig. 3D) and the periventricular nucleus (Pe) that surround the third brain ventricle (Fig. 3E). We confirmed nesfatin-1 immunoreactivity that we previously reported in mouse hypothalamic feeding regulatory brain nuclei (Goebel et al., 2011) including the supraoptic nucleus (SON, Table 1), PVN (Fig. 5A, Table 1) and arcuate nucleus (Arc, Fig. 3H, Table 1). In addition, specific intense nesfatin-1 immunolabeling was also present in cell bodies and proximal segments of processes in the lateral hypothalamic area (LH, Fig. 3G, H). A similar type of intensively immunostained neurons as those observed in the LH was located in an area located rostral-caudally between the PVN and dorsomedial nucleus of the hypothalamus (DMH, Fig. 3H) which we named intermediate dorsomedial area of the hypothalamus (IDMH; Fig. 3G). The ventromedial hypothalamus (VMH) displayed less intensely immunostained NUCB2/nesfatin-1 neurons (Fig. 3H).
Fig. 3.
Representative microphotographs of nesfatin-1 immunoreactive cells in the forebrain and hypothalamus in a naïve mouse. (A) Olfactory nuclei. (B) Basolateral forebrain structure including piriform (Pir) cortex, endopiriform nucleus (En) and basal ganglia with caudate putamen (CPu) and claustrum (Cl). The insert shows higher magnification of neurons of the Pir. (C) Nesfatin-1-ir neurons of the lateral septal nucleus. (D) Ventromedial preoptic area (VMPO). (E) Periventricular nucleus (Pe) around the third brain ventricle (3V). (F) Central (CeA) and basolateral amygdaloid (BLA) nuclei. (G, H) Hypothalamus posterior to the paraventricular nucleus including the ventromedial hypothalamus (VMH), intermediate dorsomedial hypothalamus (IDMH), dorsomedial hypothalamus (DMH) and lateral hypothalamic area (LH). The inserts in G and H illustrate the profile of neurons with higher magnification of the framed area. (I) Parafascicular thalamic nucleus (PF). Scale bar in (A) is 100 µm and representative for C, D, E and I. Scale bar in (B) is 100 µm and representative for G and H and 10 µm in the inserts (B, G, H). Scale bar in (F) is 100 µm. Other abbreviations: aca: anterior commissure, anterior part; aci: anterior commissure, intrabulbar part; AOD: anterior olfactory nucleus, dorsal part; AOM: anterior olfactory nucleus, medial part; AOL: anterior olfactory nucleus, lateral part; Arc: arcuate nucleus; f: fornix; fr: fasciculus retroflexus; LSD: lateral septal nucleus, dorsal part; LSI: lateral septal nucleus, intermediate part; LSV: lateral septal nucleus, ventral part; LV: lateral ventricle; och: optic chiasm; opt: optic tract.
Table 1.
Mouse brain structures displaying NUCB2 / nesfatin-1 immunoreactivity
| Brain Structure | Density | Intensity | |
|---|---|---|---|
| Forebrain | Anterior olfactory nucleus | +++ | M |
| Accumbens nucleus | ++ | M | |
| Piriform cortex | +++ | M | |
| Insular cortex | ++ | M | |
| Cingulate cortex | + | M | |
| Somatomotor cortex | ++ | M | |
| Lateral septal nucleus | + - ++ | M | |
| Bed nucleus of the stria terminalis | +++ | M | |
| Caudate putamen | +++ | M | |
| Other cortical areas | + - ++ | M | |
| Amygdaloid nucleus | |||
| Anterior cortical nucleus | + | M | |
| Medial amygdaloid nucleus | ++ | M | |
| Central nucleus | +++ | M | |
| Basolateral nucleus | +++ | M | |
| Thalamus | Paraventricular thalamic nucleus, anterior part |
+ - ++ | M |
| Parafascicular thalamic nucleus | ++ | M | |
|
Hypo thalamus |
Medial preoptic nucleus | +++ | I |
| Supraoptic nucleus | ++++ | I | |
| Paraventricular nucleus | |||
| Parvicellular anterior | + | I | |
| Parvicellular medial | + | I | |
| Ventral | + | I | |
| Magnocellular medial | ++ | I | |
| Magnocellular lateral | +++ | I | |
| Posterior | + | I | |
| Lateral hypothalamic area | ++ | I | |
| Ventromedial hypothalamic nucleus |
++ | M | |
| Intermediate dorsomedial hypothalamic nucleus |
++ | I | |
| Arcuate nucleus | +++ | I | |
| Cerebellum | Purkinje cells | ++ | M |
|
Hippo campus |
Pyramidal cell layer | +++ | M |
| Midbrain | Edinger-Westphal nucleus | +++ | I |
| Dorsal raphe nuclei | +++ | M | |
| Pons | Lateral parabrachial nucleus | +++ | M |
| Dorsal tegmental nucleus | + | M | |
| Dorsolateral tegmental nucleus | + | M | |
| Dorsal raphe, interfascicular part | ++ | M | |
| Locus coeruleus | +++ | I | |
| Barrington’s nucleus | + | M | |
| A5 | + | M | |
| Medulla | Nucleus of the facial nerve | ++ | M |
| Nucleus gigantocellularis | + | M | |
| Raphe magnus | + | I | |
| Raphe pallidus | ++ | I | |
| Raphe obscurus | ++ | I | |
| Area postrema | + | M | |
| Nucleus of the solitary tract | ++ | M | |
| Dorsal motor nucleus of the vagus nerve |
+++ | M | |
| Nucleus ambiguus | + - ++ | M | |
| Nucleus of the hypoglossal nerve | ++ | M | |
| Lateral reticular nucleus | + | M | |
| A1/C1 (ventrolateral medulla) | ++ | M | |
| Inferior olivary nucleus | + | M | |
“+” approximately 1–4 cells, “++” 5–10, “+++” 10–20 and “++++” >20 immunoreactive cells in a 100 µm×100 µm area of an ocular grid when the objective was 10×. Intensity: M: moderate, I: intensive relative to the established intense staining of the locus coeruleus (see representative nucleus in Fig. 2A).
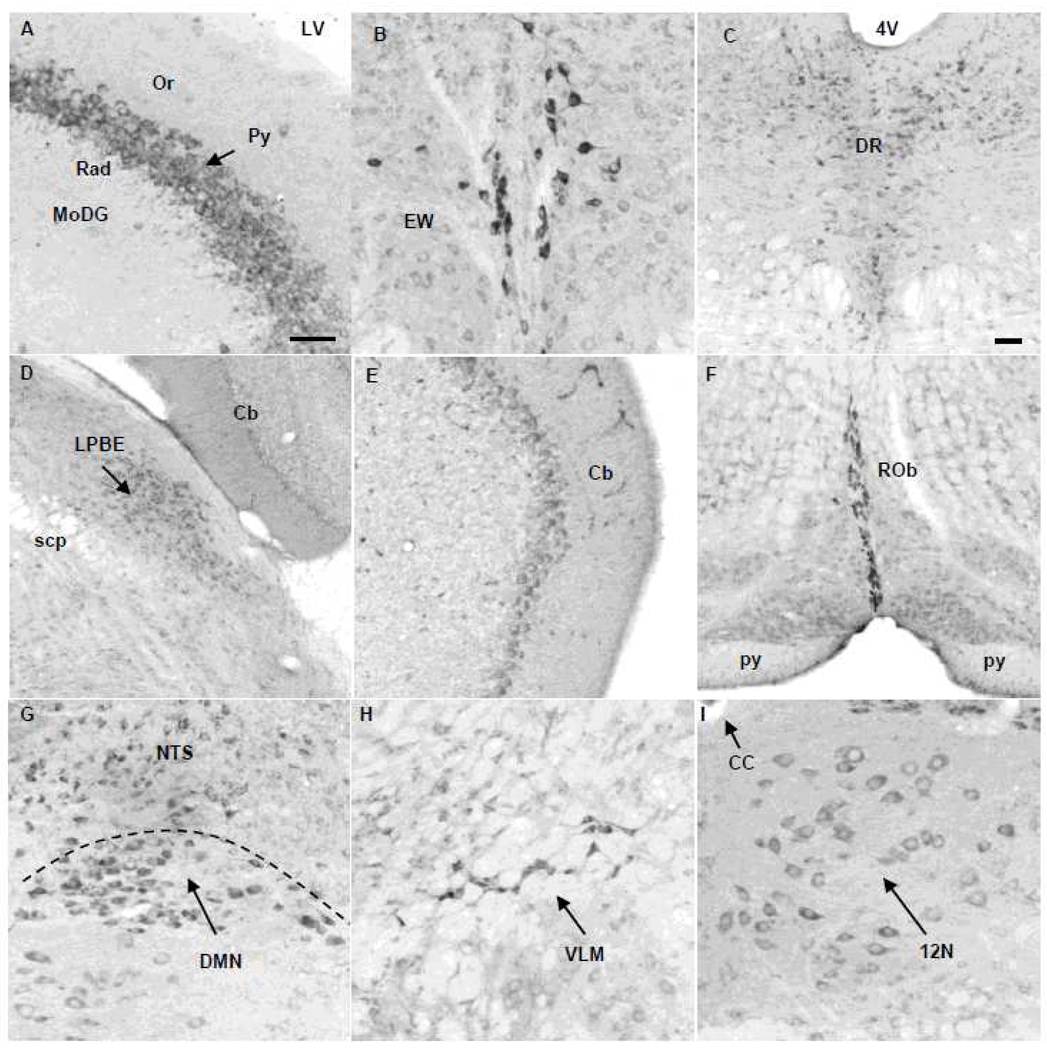
Fig. 4.
Nesfatin-1 immunoreactive cells in the hippocampus, midbrain, pons, medulla and cerebellum in naïve mice. (A) In the hippocampus, selective nesfatin-1-immunoreactive neurons are found in the pyramidal cell layer (Py). (B) Edinger-Westphal nucleus (EW). (C) Dorsal raphe nucleus (DR). (D) Lateral parabrachial nucleus, external part (LPBE). (E) Purkinje-cells in the cerebellum (Cb). (F) Raphe obscurus (ROb). (G) Dorsal motor nucleus of the vagus nerve (DMN) and nucleus of the solitary tract (NTS). (H) ventrolateral medulla (VLM, also known as A1/C1). (I) Hypoglossal nucleus (12N). Scale bar in (A) is 100 µm and representative for B, E, F, G, H, I. Scale bar in C is 100 µm and representative for (D). Other abbreviations: 4V: fourth brain ventricle; CC: central canal; LV: lateral brain ventricle; MoDG: molecular layer of the dentate gyrus; Or: oriens layer of the hippocampus; py: pyramid tract; Py: pyramidal cell layer of the hippocampus; Rad: radiatum layer of the hippocampus; scp: superior cerebellar peduncle.
Fig. 5.
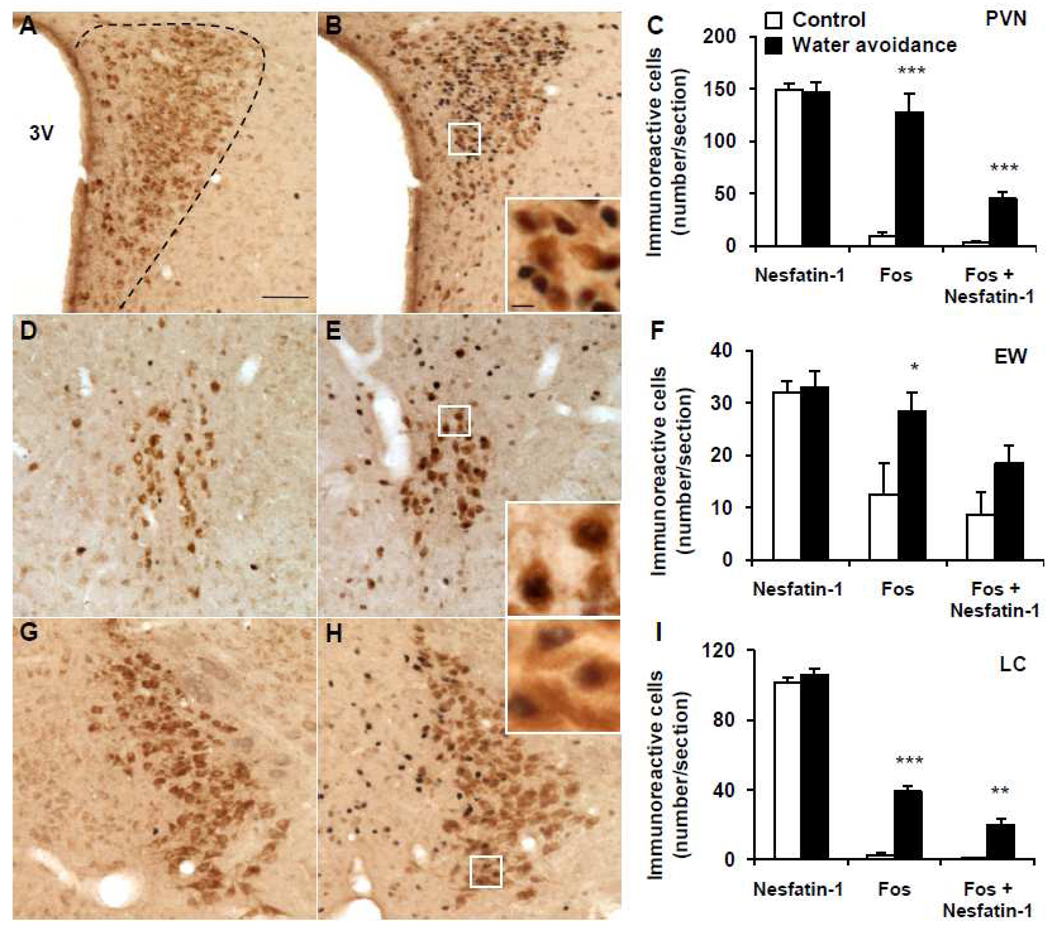
Representative double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the paraventricular nucleus (PVN), Edinger-Westphal nucleus (EW) and locus coeruleus (LC) in control mice (A, D, G) and after water avoidance stress (B, E, H). The inserts in B, E and H show higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity in the PVN (insert B), EW (insert E) and LC (insert H). The scale bar in A is 100 µm representing the scale for all the panels and 10 µm in the inserts. Unilateral cell count/section in the PVN (C), EW (F) and LC (I) as mean ± SEM of 4 mice/group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control. Other abbreviations: 3V: third brain ventricle.
2.3. Nesfatin-1 immunoreactivity in the mouse midbrain and hindbrain
Intensely labeled NUCB2/nesfatin-1 neurons were observed in the EW (Fig. 4B), LC, (Fig. 2A), raphe magnus (Table 1), raphe obscurus (Rob, Fig. 4F) and rostral raphe pallidus (RPa, Fig. 6A). Neurons with moderate staining were found in all subnuclei of the dorsal raphe (ventral, dorsal and ventrolateral, Fig. 4C), external subnuclei of lateral parabrachial nucleus (LPBE, Fig. 4D), area postrema (AP), nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus nerve (DMN, Fig. 4G), ambiguus nucleus, ventrolateral medulla (VLM, also named A1/C1, Fig. 4H) and inferior olivary nucleus (Table 1) as well as hypoglossal nucleus (12N, Fig. 4I) and facial nerve nucleus (Table 1). In addition, the Purkinje-cells in the cerebellum were stained for nesfatin-1 in mice (Fig. 4E).
Fig. 6.
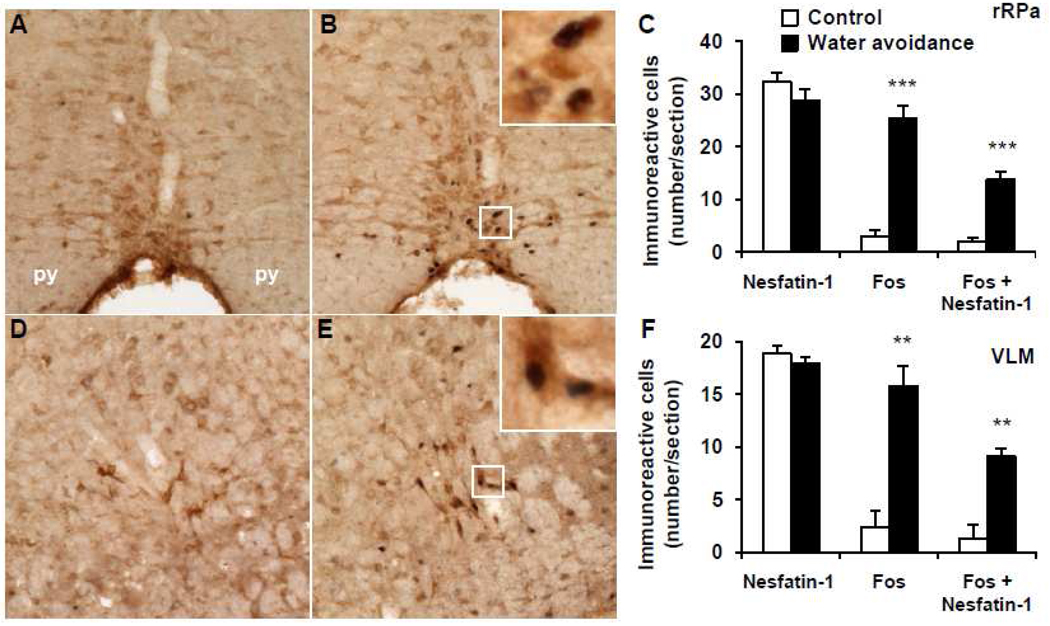
Representative double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the rostral raphe pallidus (rRPa) and ventrolateral medulla (VLM) in control mice (A, D) and after water avoidance stress (B, E). The inserts in B and E show higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity in the rRPa (insert B) and VLM (insert E). The scale bar in A is 100 µm representing the scale for all the panels and 10 µm in the inserts. Unilateral cell count/section in the rRPa (C) and VLM (F) as mean ± SEM of 4 mice/group. **p < 0.01, ***p < 0.001 compared with control. Other abbreviations: py: pyramidal tract.
2.4. Water avoidance stress activates NUCB2/nesfatin-1-immunoreactive neurons in the mouse brain
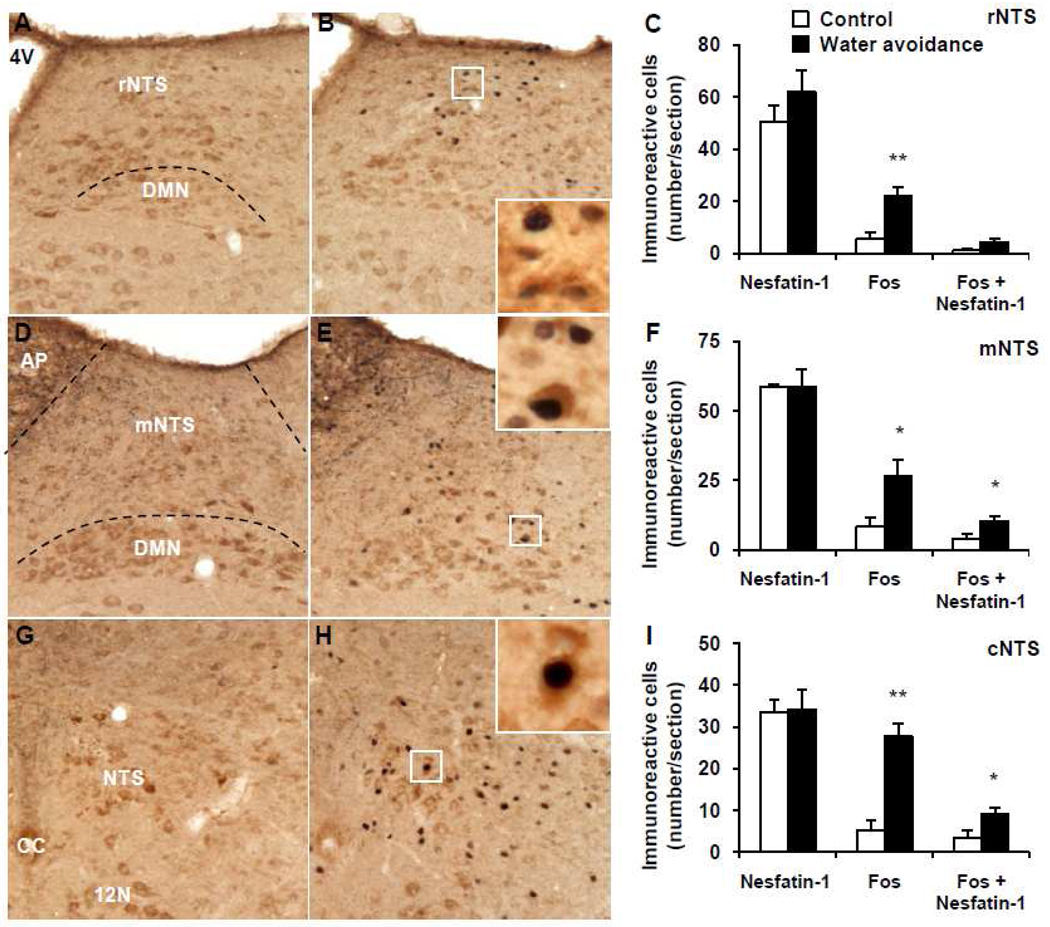
In the brain of undisturbed control mice that were group-housed until euthanasia, Fos immunostaining was low. and NUCB2-nesfatin-1-ir neurons were prominently localized (number/section) in the PVN (149.2 ± 5.6, Fig. 5A, C), EW (32.1 ± 2.1, Fig. 5D, F), LC (101.7 ± 2.9, Fig. 5G, I), rRPa (32.3 ± 1.8, Fig. 6A, C), VLM (18.9 ± 0.8, Fig. 6D, F), rNTS (50.4 ± 6.4, Fig. 7A, C), mNTS (58.8 ± 1.0, Fig. 7D, F) and cNTS (33.5 ± 3.0, Fig. 7G, I).
Fig. 7.
Representative double immunohistochemical staining for Fos (dark blue) and nesfatin-1 (brown) in the nucleus of the solitary tract at the caudal (cNTS), middle (mNTS) and rostral level (rNTS) with respect to the area postrema (AP) in control mice (A, D, G) and after water avoidance stress (B, E, H). The inserts in B, E and H show higher magnification of neurons with Fos immunoreactivity co-localizing with nesfatin-1 immunoreactivity in the rNTS (insert B), mNTS (insert E) and cNTS (insert H). The scale bar in A is 100 µm representing the scale for all the panels and 10 µm in the inserts. Unilateral cell count/section in the rNTS (C), mNTS (F) and cNTS (I) as mean ± SEM of 4 mice/group. *p < 0.05, **p < 0.01 compared with respective control. Other abbreviations: 4V: fourth brain ventricle; CC: central canal; DMN: dorsal motor nucleus of the vagus nerve.
WAS for 1 h significantly increased the number of Fos-ir neurons/section monitored 1 h after the end of stress compared to non-stressed controls in the PVN (127.7 ± 17.9 vs. 8.5 ± 4.1; p < 0.001; Fig. 5B, C), EW (28.3 ± 3.8 vs. 12.5 ± 5.8; p < 0.05; Fig. 5E, F), LC (38.9 ± 3.1 vs. 2.6 ± 1.3; p < 0.001; Fig. 5H, I), rRPa (25.3 ± 3.3 vs. 2.9 ± 1.3; p < 0.001; Fig. 6B, C), VLM (15.8 ± 2.0 vs. 2.3 ± 1.6; p < 0.01; Fig. 6E, F), rostral (r) NTS (22.1 ± 3.5 vs. 5.4 ± 2.5; p < 0.01; Fig. 7B, C), middle (m) NTS at the level of the AP (26.7 ± 5.8 vs. 8.4 ± 3.4; p < 0.05; Fig. 7E, F) and caudal (c) NTS (22.1 ± 3.5 vs. 5.4 ± 2.5; p < 0.01; Fig. 7H, I) while the number of nesfatin-1-positive neurons was similar as in the control group monitored 1 h after the stress (Figs. 5–7). However, WAS significantly increased the number of double Fos/nesfatin-1-ir labeled cells in the PVN (45.1 ± 6.6 vs. 2.8 ± 1.4, p < 0.001; Fig. 5C), LC (19.5 ± 3.8 vs. 0.6 ± 0.2, p < 0.01; Fig. 5I), rRPa (13.7 ± 1.6 vs. 2.0 ± 0.8, p < 0.001; Fig. 6C), VLM (9.1 ± 0.7 vs. 1.4 ± 1.2, p < 0.01; Fig. 6F), mNTS (10.5 ± 1.7 vs. 4.3 ± 1.3, p < 0.05; Fig. 7F) and cNTS (9.3 ± 1.4 vs. 3.5 ± 1.6, p < 0.05; Fig. 7I) compared to controls but not in the EW (p > 0.05, Fig. 5F) and rNTS (p > 0.05, Fig. 7C). Double Fos/nesfatin-1 labeling showed that of the Fos-positive cells, 57.6% in the VLM (Fig. 6F), 54.2% in the rRPa (Fig. 6C), 50.2% in the LC (Fig. 5I), 39.3% in the mNTS (Fig. 7F), 35.3% in the PVN (Fig. 5C) and 33.4% in the cNTS (Fig. 7I) were NUCB2/nesfatin-1-ir.
2.5. Intracerebroventricular injection of nesfatin-1 decreases gastric emptying in mice
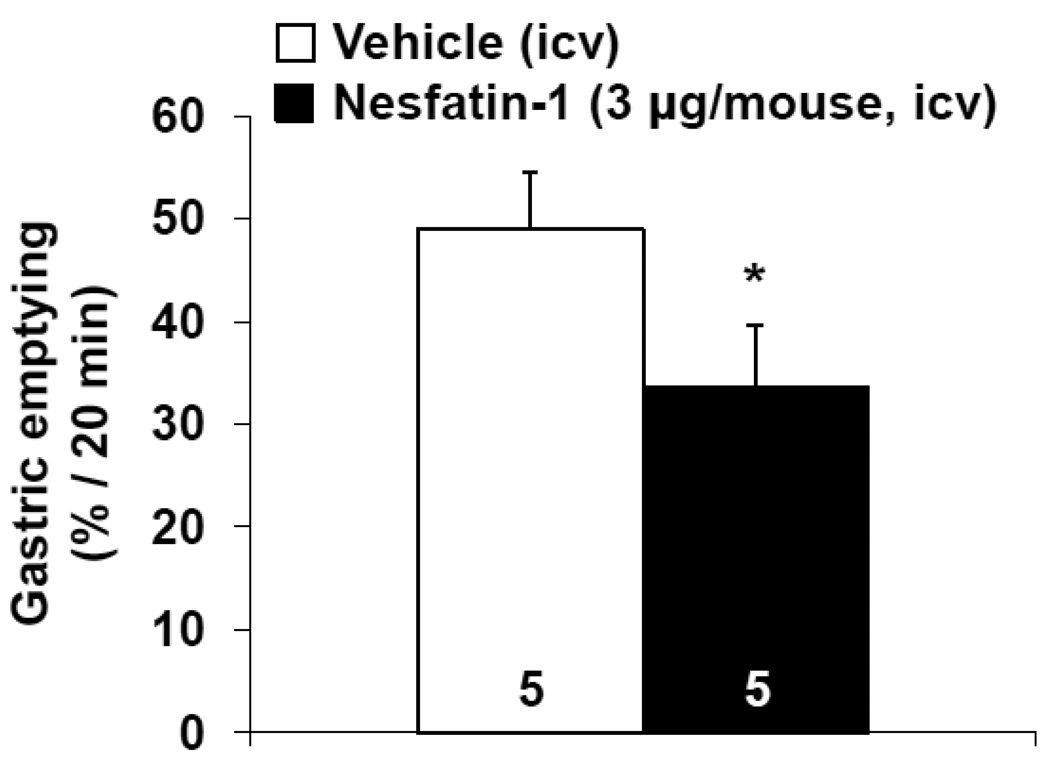
Mice, deprived of food for 8 h and icv injected with vehicle (n=5) under short inhalation anesthesia before the dark phase had 49.0 ± 5.5% of the non-nutrient solution emptied from their stomach within 20 min. Nesfatin-1 (3 µg/mouse, icv, n=5) significantly reduced gastric emptying to 33.7 ± 5.9% (p < 0.05, Fig. 8) as assessed during the150–170 min period after icv injection which corresponds to the peak response of the dark phase food intake suppression (Goebel et al., 2011; Maejima et al., 2009; Stengel et al., 2009b).
Fig. 8.
Intracerebroventricular injection of nesfatin-1 inhibits dark phase gastric emptying of a non-nutrient solution in mice. Mice were fasted for 8 h with free access to water, injected icv with vehicle or nesfatin-1 at the beginning of the dark phase and water was removed. Mice received an orogastric gavage of a non-nutrient solution 150 min later. Gastric emptying was monitored 20 min thereafter. *p < 0.05 vs. vehicle. Each column represents the mean ± SEM of number of mice indicated at the bottom.
3. Discussion
Immunohistochemical brain localization of NUCB2/nesfatin-1 has been extensively described in the rat autonomic nuclei of the hypothalamus and brainstem (Brailoiu et al., 2007; Foo et al., 2008; Fort et al., 2008; Goebel et al., 2009a; Inhoff et al., 2010; Oh-I et al., 2006) while there is a paucity of studies in mice. So far, NUCB2/nesfatin-1-ir neurons in mice were reported in feeding regulatory brain centers including the PVN, SON, Arc, DMH, DVC and EW (Goebel et al., 2011; Okere et al., 2010). In the present study, we first ascertain the specificity of the commercially available rat nesfatin-1 antiserum to stain NUCB2/nesfatin-1 in mice brain. Using immune dot blotting, we established that this antibody equally detects rat and mouse nesfatin-1 polypeptide as well as rat and mouse hypothalamic NUCB2/nesfatin-1 protein. In addition, when this antibody was pre-absorbed with rat and mouse nesfatin-1 polypeptide, immunostaining was completely abolished in mouse brain sections. Besides nesfatin-1, the antibody used also recognizes full length NUCB2, but does not cross-react with other polypeptides including nesfatin-2 and nesfatin-3 (Brailoiu et al., 2007). Therefore, the present expression pattern in mice is likely to reflect NUCB2/nesfatin-1 immunoreactivity.
The use of this antibody allowed us to unravel extensive distribution of NUCB2/nesfatin-1 in mice. Those immunoreactive neurons are located in specific forebrain and hindbrain nuclei, which have not been identified so far in mice in addition of brain sites previously reported to be NUCB2/nesfatin-1 positive (Goebel et al., 2011; Okere et al., 2010) that we confirmed in the present study. In particular, immunolabeling was detected in the olfactory nuclei, piriform and insular cortex, endopiriform nucleus, lateral septum, ventromedial preoptic nucleus, Pe, LH, central amygdaloid nucleus, PF, Hi, LC, raphe nuclei, LPBE, VLM, cerebellum, NTS, DMN and 12N. This pattern of brain nesfatin-1 expression is very similar to extensive and consistent reports in the rat brain showing NUCB2/nesfatin-1 in the same brain areas (Brailoiu et al., 2007; Foo et al., 2008; Fort et al., 2008; Goebel et al., 2009a; Inhoff et al., 2010; Oh-I et al., 2006). Of note, there was a group of intensely labeled NUCB2/nesfatin-1 immunoreactive neurons located in the dorsomedial area of the hypothalamus similar to neurons containing melanocortin-concentrating hormone in the rat hypothalamus (no mouse data available) (Fort et al., 2008; Hahn, 2010; Kerman et al., 2007). We named this area the intermediate dorsomedial area of the hypothalamus (IDMH) as it is neither encompassed within the posterior part of the anterior hypothalamic area nor the juxtadorsomedial region of the LH as defined previously (Franklin and Paxinos, 1997; Hahn, 2010). Although NUCB2/nesfatin-1 is widespread in mice brain, selectivity was reflected by the complete lack of immunostaining in specific areas such as the lamina IV of the cortex, thalamus and medial and lateral geniculate bodies.
At the cellular level, in previous brain mapping studies in rats, NUCB2/nesfatin-1 was reported to be confined to cell bodies and primary dendrites while immunolabeling was absent in axon terminals (Foo et al., 2008; Goebel et al., 2009a). Likewise, in mice, nesfatin-1 immunoreactivity was also localized mainly in cell bodies of neurons. However, we found that primary dendrites of neurons were immunostained in several areas namely the Pe, DMH, LH, EW, LC, Rob, VLM and DVC while only cell bodies were stained in others such as the olfactory nuclei, cortex, basal ganglia, amygdaloid nuclei, Hi, PF, LPBE and Purkinje cells.
Based on the pattern of nesfatin-1 immunoreactivity in mice it is expected that the polypeptide may have similar broader central biological actions other than the well established anorexigenic effects reported in rats (Yosten and Samson, 2010) and recently in mice (Goebel et al., 2011). Consistent studies showed that icv injection of nesfatin-1 reduced dark phase feeding with the strongest reduction occurring between 2 and 3 h post injection in both, rats and mice (Goebel et al., 2011; Maejima et al., 2009; Stengel et al., 2009b) while less prominently inhibiting the re-feeding response after a fast during the light phase (Atsuchi et al., 2010; Yosten and Samson, 2010). Based on the high expression of NUCB2/nesfatin-1 neurons in hypothalamic and brainstem nuclei regulating gastric function through autonomic pathways (Cunningham and Sawchenko, 1988; Flanagan et al., 1992; Landgraf et al., 1990; Rinaman, 1998; Rogers and Hermann, 1987) such as the PVN, NTS and DMN, we assessed whether exogenous nesfatin-1 would influence gastric motor function in mice under conditions previously reported to induce food intake reduction (2–3 h post icv injection in the dark phase). Nesfatin-1 icv injected reduced gastric emptying of a liquid non-nutrient solution by 31% compared to vehicle injection as assessed during the 150–170 min period post injection in the dark phase. Such a delay in gastric emptying may have a bearing with the long-lasting decreased dark phase food intake after icv injection of nesfatin-1 in mice (Goebel et al., 2011). This is supported by evidence that increased gastric volume induced by food/fluid ingestion influences the degree of gastric fullness and reduces subsequent food intake (Phillips and Powley, 1996). The underlying mechanisms of delayed gastric emptying may involve the reduction of propulsive motor activity as recently reported after icv injection of nesfatin-1 in mice (Atsuchi et al, 2010). However, the inhibitory effect on gastric antral motility was significant only at 1 nmol in mice injected icv in the light phase (Atsuchi et al., 2010) and not observed at lower doses as used in the present study. In rats, icv injection of nesfatin-1 suppressed gastric emptying of a non-nutrient solution during the dark phase (Stengel et al., 2009b) indicative of similar central effects of nesfatin-1 in mice and rats not only to influence food intake but also visceral function. Important steps forward in the assessment of the physiological role of nesfatin-1 in the brain regulation of gastric motor function will come from the characterization of the yet to be identified nesfatin-1 receptor and associated development of antagonists. So far existing evidence on the pharmacological property of the putative nesfatin-1 receptor or binding site suggests that nesfatin-1 interacts with a G protein-coupled receptor, leading to an increase of Ca2+(i), linked to protein kinase A activation in cultured rat hypothalamic neurons (Brailoiu et al., 2007)..
To establish whether in mice nesfatin-1-ir neurons in brain autonomic centers would be responsive to an acute stressor as has been reported in rats (Bonnet et al., 2009; Goebel et al., 2009b; Stengel et al., 2010d; Xu et al., 2010; Yoshida et al., 2010), we exposed mice to 1 h of WAS. Such a stressor has been characterized in rodents to induce stress-related visceral responses. In particular, chronic exposure to WAS induced colonic epithelial barrier dysfunction (Cameron and Perdue, 2005), reactivation of dextran-sulfate-sodium-induced colitis (Melgar et al., 2008) and visceral hypersensitivity (Larauche et al., 2010) in mice. However, brain sites activated by such a stressor in mice are still unknown. Here, we showed that 1 h exposure to WAS in mice induces Fos-ir neurons in the PVN, LC, EW, VLM, rRPa and NTS. Such a pattern of Fos expression in the mouse brain is similar to that reported in rats exposed to WAS with Fos-immunoreactivity localized in the BNST, lateral septum, PVN, LC, dorsal raphe nucleus, VLM, NTS and lumbo-sacral spinal cord (Bonaz and Taché, 1994; Million et al., 2000). Other studies established that several stressors including restraint stress (O'Mahony et al., 2010), foot shock (Liu et al., 2009), hypertonic saline injection (Pirnik et al., 2004), ether (Korosi et al., 2005) or swim stress (Stone et al., 2007) induced Fos in stress-responsive neuronal populations in specific brain nuclei in mice namely the cingulate cortex, amygdala, PVN, Arc, paraventricular thalamic nucleus, Hi, EW, LC, dorsal raphe nucleus or VLM (Korosi et al., 2005; Liu et al., 2009; O'Mahony et al., 2010; Stone et al., 2007).
The phenotype of neurons that are activated after acute stress in mice has been little investigated. Pirnik et al. reported Fos activation after injection of hypertonic saline in oxytocin-, vasopressin- and tyrosine hydroxylase positive neurons of the hypothalamus in mice consistent with pathways sensitive to osmotic changes (Pirnik et al., 2004). In the present study, we show that WAS induces Fos expression in NUCB2/nesfatin-1-ir neurons of various brain nuclei and of the activated neurons 35% in the PVN, 50% in the LC, 54% in the rRPa, 58% in the VLM, 33% in the cNTS and 39% in the mNTS were nesfatin-1-ir. Collectively, these data identified for the first time that WAS induces Fos expression in stress-susceptible regions of the mouse brain. In addition, we identified NUCB2/nesfatin-1-ir neurons as a novel phenotype involved in the brain response to WAS including the majority of specific brain nuclei which are neuroanatomical substrates activated by this stressor. We recently reported in rats that restraint stress and abdominal surgery, a mixed psychological/physical and mainly physical stressor, respectively, induced Fos expression in nesfatin-1 containing neurons of the rat SON, PVN, LC, EW, rRPa, VLM and NTS (Goebel et al., 2009b; Stengel et al., 2010d). These data are indicative that the same nesfatin-1-ir autonomic brain nuclei were activated after WAS in mice except for the SON where we did not find Fos induction while it has been shown to contain abundant nesfatin-1 immunoreactivity in mice and rats (Brailoiu et al., 2007; Foo et al., 2008; Goebel et al., 2009a; Oh-I et al., 2006). WAS induced only few Fos-positive cells in the SON in rats (Bonaz and Taché, 1994) which may be related to the non-nociceptive nature of WAS. Nevertheless, the similarity of recruitment of nesfatin-1 neurons by distinct stressors in rats and mice remains striking.
Although WAS resulted in significantly higher Fos expression in the EW and rNTS compared to non-stressed animals, these brain regions did not have significantly higher numbers of double-labeled neurons. On the other hand, the cNTS and mNTS showed significant double-labeling suggesting selective activation of nesfatin-1 neurons in these regions. Visceral sensory afferents are organized in an overlapping topographic manner within the NTS subnuclei with terminal fields from the abdominal viscera being represented in the middle and caudal NTS (Altschuler et al., 1989; Altschuler et al., 1991; Shapiro and Miselis, 1985). Since WAS stimulates colonic motor function in mice (Larauche et al., 2010), this could explain the significant activation of nesfatin-1 neurons of the caudal and middle NTS but not the rostral part. Nesfatin-1 neurons in the EW were shown before to be activated in mice after restraint stress exposure for 2 h (Okere et al., 2010) and chronic stress consisting of a series of unpredictable variable mild stressors for 14 days (Xu et al., 2010). Here, we found Fos expression after 1 h WAS in 64% of nesfatin-1 neurons located in the EW but the number did not reach statistical significance which may be related to distinct nesfatin-1 neuronal populations within the EW that are being recruited by different stressors and/or the duration and type of stressors used.
Further characterization of nesfatin-1 involvement in brain circuitries activated by stress will come from studies targeting nesfatin-1signaling pharmacologically or by molecular approaches which so far has not been achieved..
Taken together, we validated the anti-rat nesfatin-1 antibody for immunostaining in mouse neuronal tissue and mapped nesfatin-1 immunoreactivity in the mouse brain. NUCB2/nesfatin-1 neurons are prominently localized in autonomic brain nuclei responsive to stress as shown by NUCB2/nesfatin-1 double labeling in mice exposed to acute WAS. These data provide neuro-anatomical support for an important role of nesfatin-1 in the efferent component of brain neuronal circuits responsive to stress in rodents including alterations of gastrointestinal functions as shown here by the central action of nesfatin-1 to reduce gastric transit.
4. Experimental procedures
4.1. Animals
Adult male C57BL/6 mice (Harlan, San Diego, CA, body weight: 28–35 g) were group housed (four animals/cage) under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 06:00 a.m./06:00 p.m.), humidity, and temperature (22 ± 2 °C). Animals were fed with a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water ad libitum. For all experiments involving single housing mice were accustomed to conditions and separated for 6 h during the light phase on three consecutive days before the experiment. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committees at Veterans Affairs Greater Los Angeles Healthcare System (animal protocol # 9906-820).
4.2. Immunohistochemistry
4.2.1. Characterization of the nesfatin-1 antibody
The anti-nesfatin-1 antibody was raised in rabbit against rat nesfatin-1 corresponding to rat NUCB2 amino acid residues 1–82 (Phoenix Pharmaceuticals Inc.. Burlingame, CA). This antibody has been shown to stain a Western Blot band corresponding to rat NUCB2, the precursor of rat nesfatin-1 which also contains the epitope (manufacturer’s technical information). We recently demonstrated in rats that nesfatin-1 immunoreactivity corresponded to NUCB2 mRNA expression as shown e.g. in the nucleus accumbens, cerebellar cortex, and PVN (Goebel et al., 2009a). Since rat and mouse nesfatin-1 only differ in two amino acids (Oh-I et al., 2006), we used this antiserum to establish specificity to stain mouse neuronal tissue. Pre-absorption of the antibody (1 ml, 1:10,000) with synthetic rat nesfatin-1 (10 µg, Phoenix Pharmaceuticals Inc.) or mouse nesfatin-1 (10 µg, Abgent Technologies, San Diego, CA) was performed to further assess specificity. After 24-h incubation the solution was centrifuged and the supernatant used for nesfatin-1 immunostaining as described below in selective brain regions.
Dot immunoblotting was processed to display the binding ability of the antibody against rat nesfatin-1 to mouse nesfatin-1. Hypothalamic protein (6.0 µg, 3.0 µg, 1.5 µg and 0.5 µg in 2 µl double distilled H2O) from rat and mouse (n=1 for each species), mouse nesfatin-1 (0.5 µg/2 µl double distilled H2O; Abgent Technologies), rat nesfatin-1 (0.5 µg/2 µl double distilled H2O; Phoenix Pharmaceuticals Inc.) and bovine serum albumin (BSA, 0.5 µg/2 µl double distilled H2O) were slowly pipetted on a nitrocellulose membrane (Bio-Rad, Hercules, CA) at the center of a pre-drawn grid. The membrane was dried and then washed twice with tris-buffered saline (TBS; 10 mM Tris, 150 mM NaCl, and 0.05% Tween, vol/vol) and incubated in TBS containing 5% (wt/vol) nonfat milk (Carnation, Nestlé, Glendale, CA). After 30 min, the membranes were incubated in the nesfatin-1 antibody solution (Phoenix Pharmaceuticals Inc.) diluted 1:1000 in TBS. After 1 h, membranes were washed five times with TBS and incubated with the secondary antibody solution (anti-rabbit IgG conjugated to alkaline phosphatase; Promega, Madison, WI) diluted 1:2000 in TBS. After 1 h, membranes were washed three times before color development in alkaline phosphatase buffer [100 mM Tris, 100 mM NaCl, and 5 mM MgCl2 (pH 9.5)] containing 0.3% nitroblue tetrazolium solution (vol/ vol) and 0.15% 5-bromo-4-chloro-3-indolyl-l-phosphate solution (vol/vol) according to the manufacturer’s instructions (Promega) for 5–10 min.
4.2.2. Tissue processing for nesfatin-1 single labeling and Fos and nesfatin-1 double immunohistochemistry
Transcardial perfusion was performed in anesthetized mice as described before (Goebel et al., 2011; Stengel et al., 2009a). Briefly, the thoracic cavity was opened and a cannula inserted into the ascending aorta via the left heart ventricle. Perfusion consisted of a quick flush with sodium chloride (0.9% NaCl) and 40–50 ml of 4% paraformaldehyde, 14% saturated picric acid in 0.1 M phosphate buffer (pH 7.2). Brains were removed and post-fixed overnight in the same fixative, rinsed and cryoprotected in 10% sucrose for 24 h and snap-frozen in dry ice-cooled 2-methylbutane. Mouse brains were processed in two batches to ensure consistency. The first batch contained brains of mice euthanized during the light or dark phase. These brains were only subjected to nesfatin-1 immunoreactivity. Coronal sections (25 µm) in 3 sets of the whole brain were cut using a cryostat (Microm International GmbH, Walldorf, Germany). Free-floating sections (every third brain section) were stained using the avidin–biotin–peroxidase complex (ABC, Vector, Vermont, CA) method as previously described (Goebel et al., 2009a). Endogenous peroxidase was inactivated by 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 30 min. Sections were incubated for 36 h at 4 °C in rabbit polyclonal anti-nesfatin-1 antibody (1:10,000; raised against rat nesfatin-1 N-terminus (1–82); Catalog No. H-003–22, Phoenix Pharmaceuticals Inc.), then in biotinylated goat anti-rabbit IgG (1:1000; Jackson Immuno Research, West Grove, PA) for 1 h at room temperature (RT), followed by ABC (Vector) for 1 h at RT. Sections were developed with 3,3’-diaminobenzidine tetrahydrochloride hydrate (DAB) and hydrogen peroxide (Sigma Chemical Co, St. Louis, MO) for 10 min and color development was frequently checked by light microscope. Each step of incubation was followed by a 3 × 5 min washing step in PBS. Sections were mounted on Fisher Super Frost Plus slides, air-dried for 24 h and completely dehydrated through a gradient of ethanol and xylene before coverslipping. Anatomical correlations were made according to landmarks given in the mouse brain stereotaxic atlas of Franklin and Paxinos (Franklin and Paxinos, 1997).
The second batch contained brains of mice subjected to WAS or left undisturbed. This batch was subjected to consecutive double immunohistochemical labeling for Fos and NUCB2/nesfatin-1. Free-floating brain sections (every third brain section) were incubated in rabbit anti-c-Fos serum (1:10,000, Catalog No. PC#38, Oncogene, Cambridge, MA) as primary antibody followed by biotinylated goat anti-rabbit Fab fragment (1:1000, Catalog No. 111-067-003, Jackson ImmunoResearch Laboratories Inc.) and ABC method (Vector) as previously described (Goebel et al., 2010). Staining was visualized with DAB and nickel ammonium sulfate. Sections were thereafter incubated in rabbit anti-rat nesfatin-1 antibody (1:10,000) followed by biotinylated goat anti-rabbit antibody (1:1000) and ABC method and developed with DAB. Cells with dark blue nuclear staining were Fos-ir and cells with strong brown cytoplasmic staining were NUCB2/nesfatin-1-ir.
4.2.3. Cell counting
Fos and nesfatin-1-ir cells were observed by light microscopy (Axioscop II, Carl Zeiss, Germany). The intensity of NUCB2/nesfatin-1 staining was classified into two categories, namely intense or moderate relative to the established intense staining of the LC. The density of NUCB2/nesfatin-1-ir cells in each nucleus or area was determined in a field of 100 µm × 100 µm using a 10x objective with a grid in the ocular of the microscope in at least five sections, and assigned according to a scale of + to ++++ in which “+” corresponds to approximately 1–4 cells, “++” 5–10, “+++” 10–20 and “++++” more than 20 immunoreactive cells (Goebel et al., 2009a). For quantitative assessment, unilateral cell count was chosen based on the observation that no hemispheric differences were observed in our previous studies (Goebel et al., 2009b; Goebel et al., 2010; Stengel et al., 2010d; Wang et al., 1998; Wang et al., 2002; Wang et al., 2009). The number of ir cells was counted unilaterally in the right brain hemisphere in several sections of selected brain nuclei that contained NUCB2/nesfatin-1 immunoreactivity. Nucleus coordinates were identified with the Franklin and Paxinos mouse brain atlas (Franklin and Paxinos, 1997) and are given in mm from bregma: PVN, 5 sections: −0.58 to −0.94; EW, 7 sections: −3.28 to −3.88; LC, 4 sections: −5.34 to −5.68; rRPa, 6 sections: −6.12 to −6.64; VLM, 10 sections: −6.72 to −7.48; rNTS, 3 sections: −6.96 to −7.2; mNTS at the level of the AP, 5 sections: −7.2 to −7.5 and cNTS, 3 sections: −7.5 to −7.76. Images were acquired by a digital camera (Hamamatsu, Bridgewater, NJ) using the image acquisition system SimplePCI (Hamamatsu Corporation, Sewickley, PA). The average number of single or double labeled Fos-ir and NUCB2/nesfatin-1-ir cells/section for each animal was calculated. Since no consecutive sections were used for the detection of the same neuronal marker, no corrections for double counting were applied. The investigator was blinded to the treatment.
4.3. Procedures
4.3.1. Intracerebroventricular injection of nesfatin-1
Injections into the lateral brain ventricle were performed under short isoflurane anesthesia (2–3 min, 4.5% vapor concentration in oxygen; VSS, Rockmart, GA) as detailed in our previous studies (Goebel et al., 2011; Stengel et al., 2010a; Stengel et al., 2010c). The site of injection was localized at the apex of the equal triangle between the eyes and the back of the head. The site was cleaned with Povidone-Iodine 10% (Aplicare Inc., Meriden, CT) and the skull punctured manually at the point of least resistance with a 30-gauge needle equipped with a polyethylene tube leaving 4–4.5 mm of the needle tip exposed and attached to a Hamilton syringe. On average, mice completely recovered from anesthesia within 5 min. The accuracy of the injections was confirmed as in our previous studies by injecting cresyl violet dye icv under similar conditions (Martinez et al., 2004).
4.3.2. Gastric Emptying
Gastric emptying of a non-nutrient viscous meal was determined as previously described in mice (Luckey et al., 2003; Stengel et al., 2009b). Briefly, a semi-liquid, non-nutrient test meal (0.3 ml) consisting of 1.5% methylcellulose (Sigma Chemical Co.) and phenol red (50 mg/100 ml; Sigma Chemical Co.) was administered via orogastric gavage to conscious mice. After 20 min, mice were killed by cervical dislocation and the stomach quickly removed. The specimens were placed in 14 ml of 0.1 N NaOH and homogenized (Polytron; Brinkman Instruments, Inc., Westbury, NY) for approximately 30 s. The homogenate was allowed to stand for 1 h, and 5 ml of the supernatant was added to 0.5 ml of 20% trichloroacetic acid (wt/vol; Sigma Chemical Co.). The solution was centrifuged at 4 °C for 20 min at 3000 rpm, and 3 ml of the supernatant was added to 4 ml of 0.5N NaOH. The absorbance of the samples was determined at a wavelength of 560 nm (Shimadzu 260 spectrophotometer, Kyoto, Japan). Two non-treated mice were killed immediately following intragastric administration of the test meal and served as standards (reference stomachs, 0% gastric emptying). The percentage of gastric emptying was calculated using the following equation: % emptying = (1 - absorbance of test sample/absorbance of standard) × 100.
4.4. Experimental Protocols
4.4.1. Assessment of brain NUCB2/nesfatin-1 immunoreactivity during the light and dark phase in mice
To assess nesfatin-1 immunoreactivity in the brain in the light and dark phase, one group of naïve group-housed mice (n=4) with free access to food and water was deeply anesthetized with sodium pentobarbital (100 mg/kg, intraperitoneally, Nembutal, Abbott Lab.) at 8:00 a.m. during the early light phase and the second group at 08:00 p.m. during the early dark phase. Mice were transcardially perfused and brains processed for NUCB2/nesfatin-1 immunoreactivity.
4.4.2. Water avoidance stress in mice for assessment of neuronal brain activation in the light phase
One group of mice was placed individually at 09:00 a.m. for 1 h on a rectangular platform (3 cm length × 3 cm width × 6 cm height) located inside a container (31 cm length × 31 cm width × 21 cm height) filled with warm water up to 1 cm below the top of the platform as in our previous studies (Larauche et al., 2010). Animals did not have access to food or water during the 1 h WAS. Thereafter, mice were returned to group-housing (4/cage) in their familiar home cage with access to food and water for 1 h. The control group consisted of undisturbed mice housed in groups with access to food and water. Two h after the start of the procedure, mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, ip, Nembutal, Abbott Lab.), transcardially perfused and brains processed for Fos and nesfatin-1 double immunohistochemistry.
4.4.3. Assessment of gastric emptying after icv injection of nesfatin-1 in mice in the dark phase
Mice previously accustomed to single housing were fasted for 8 h during the light phase in individual cages with ad libitum access to water. Immediately before the beginning of the dark phase, mice were injected icv with nesfatin-1 (3 µg/mouse or 300 pmol/mouse in 5 µl double distilled H2O) or vehicle (5 µl double distilled H2O, n=5 per group) and water bottles were removed. The nesfatin-1 dose (3 µg/mouse ~ 300 pmol/mouse) was based on our previous study showing a robust anorexigenic effect in mice with a peak response at 150 min post injection (Goebel et al., 2011; Maejima et al., 2009; Stengel et al., 2009b). At 150 min after icv injection, mice were gavaged with 0.3 ml of a non-nutrient viscous solution and 20 min thereafter, animals were euthanized by cervical dislocation to assess gastric emptying.
4.5. Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) followed by all pair wise multiple comparison procedures (Tukey post hoc test). Differences were considered significant when p < 0.05. Data are expressed as mean ± SEM.
Acknowledgements
This work was supported by the VA Research Career Scientist Award, Department of Veterans Affairs, NIHDK 33061 (Y.T.) and Center grant DK-41301 (Animal Core, Y.T.). We are grateful to Ms. Honghui Liang for her excellent technical support and Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Ferenci DA, Lynn RB, Miselis RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol. 1991;304:261–274. doi: 10.1002/cne.903040209. [DOI] [PubMed] [Google Scholar]
- Atsuchi K, Asakawa A, Ushikai M, Ataka K, Tsai M, Koyama K, Sato Y, Kato I, Fujimiya M, Inui A. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport. 2010;21:1008–1011. doi: 10.1097/WNR.0b013e32833f7b96. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Taché Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD. Central nesfatin-1 expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–5094. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther. 2005;314:214–220. doi: 10.1124/jpet.105.085373. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Flanagan LM, Olson BR, Sved AF, Verbalis JG, Stricker EM. Gastric motility in conscious rats given oxytocin and an oxytocin antagonist centrally. Brain Res. 1992;578:256–260. doi: 10.1016/0006-8993(92)90255-8. [DOI] [PubMed] [Google Scholar]
- Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, Mori M, Luppi PH. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–181. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press, Inc; 1997. Vol. [Google Scholar]
- Goebel M, Stengel A, Wang L, Lambrecht NW, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009a;452:241–246. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Taché Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009b;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Coskun T, Alsina-Fernandez J, Rivier J, Taché Y. Pattern of Fos expression in the brain induced by selective activation of somatostatin receptor 2 in rats. Brain Res. 2010;1351:150–164. doi: 10.1016/j.brainres.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Taché Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides. 2011;32:36–43. doi: 10.1016/j.peptides.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD. Comparison of melanin-concentrating hormone and hypocretin/orexin peptide expression patterns in a current parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2010;468:12–17. doi: 10.1016/j.neulet.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inhoff T, Stengel A, Peter L, Goebel M, Taché Y, Bannert N, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides. 2010;31:257–262. doi: 10.1016/j.peptides.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- Konczol K, Bodnar I, Zelena D, Pinter O, Papp RS, Palkovits M, Nagy GM, Toth ZE. Nesfatin-1/NUCB2 may participate in the activation of the hypothalamic-pituitary-adrenal axis in rats. Neurochem Int. 2010;57:189–197. doi: 10.1016/j.neuint.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Korosi A, Schotanus S, Olivier B, Roubos EW, Kozicz T. Chronic ether stress-induced response of urocortin 1 neurons in the Edinger-Westphal nucleus in the mouse. Brain Res. 2005;1046:172–179. doi: 10.1016/j.brainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Malkinson T, Horn T, Veale WL, Lederis K, Pittman QJ. Release of vasopressin and oxytocin by paraventricular stimulation in rats. Am J Physiol. 1990;258:R155–R159. doi: 10.1152/ajpregu.1990.258.1.R155. [DOI] [PubMed] [Google Scholar]
- Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13:343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MH, Miketa A, Martinez V. Lack of interaction between psychological stress and DSS-induced colitis affecting colonic sensitivity during colorectal distension in mice. Stress. 2009;12:434–444. doi: 10.1080/10253890802626603. [DOI] [PubMed] [Google Scholar]
- Liu X, Tang X, Sanford LD. Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol Behav. 2009;97:321–326. doi: 10.1016/j.physbeh.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey A, Wang L, Jamieson PM, Basa NR, Million M, Czimmer J, Vale W, Taché Y. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–365. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgar S, Engstrom K, Jagervall A, Martinez V. Psychological stress reactivates dextran sulfate sodium-induced chronic colitis in mice. Stress. 2008;11:348–362. doi: 10.1080/10253890701820166. [DOI] [PubMed] [Google Scholar]
- Million M, Wang L, Martinez V, Taché Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000;877:345–353. doi: 10.1016/s0006-8993(00)02719-0. [DOI] [PubMed] [Google Scholar]
- Mönnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology. 1993;104:716–723. doi: 10.1016/0016-5085(93)91006-4. [DOI] [PubMed] [Google Scholar]
- O'Mahony CM, Sweeney FF, Daly E, Dinan TG, Cryan JF. Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav Brain Res. 2010;213:148–154. doi: 10.1016/j.bbr.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317C:92–99. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996;271:R766–R769. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- Pirnik Z, Mravec B, Kiss A. Fos protein expression in mouse hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei upon osmotic stimulus: colocalization with vasopressin, oxytocin, and tyrosine hydroxylase. Neurochem Int. 2004;45:597–607. doi: 10.1016/j.neuint.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol. 1998;399:101–109. doi: 10.1002/(sici)1096-9861(19980914)399:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985;238:473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, Taché Y, Wang L. CRF over-expressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009a;150:153–160. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Lambrecht NW, Taché Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009b;150:4911–4919. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog ODT8-SST induces a somatostatin2 receptor-mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010a;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Taché Y. Nesfatin-1: a novel inhibitory regulator of food intake and body weight. Obes Rev. 2010b;12:261–271. doi: 10.1111/j.1467-789X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, Taché Y. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010c;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides. 2010d;31:263–270. doi: 10.1016/j.peptides.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Taché Y. Nesfatin-1--role as possible new potent regulator of food intake. Regul Pept. 2010;163:18–23. doi: 10.1016/j.regpep.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of behavioral depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1196–1207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Taché Y, Yang H, Kaneko H. Caudal raphe-dorsal vagal complex peptidergic projections: role in gastric vagal control. Peptides. 1995;16:431–435. doi: 10.1016/0196-9781(94)00212-o. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Barrachina MD, Taché Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–166. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Larauche M, Taché Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res. 2009;1247:79–91. doi: 10.1016/j.brainres.2008.09.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz LT. Sex-specific effects of fasting on Urocortin 1, Cocaine- and Amphetamine-Regulated Transcript peptide and Nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2010;170:478–488. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Maejima Y, Sedbazar U, Ando A, Kurita H, Damdindorj B, Takano E, Gantulga D, Iwasaki Y, Kurashina T, Onaka T, Dezaki K, Nakata M, Mori M, Yada T. Stressor-responsive central nesfatin-1 activates corticotropin-releasing hormone, noradrenaline and serotonin neurons and evokes hypothalamic-pituitary-adrenal axis. Aging (Albany NY) 2010;2:775–784. doi: 10.18632/aging.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–R336. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–R1647. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]