Abstract
The purpose of this project is to measure the elasticity of the human and non-human primate lens capsule at the microscopic scale using Atomic Force Microscopy (AFM). Elasticity measurements were performed using AFM on the excised anterior lens capsule from 9 cynomolgus monkey (5.9–8.0 years), 8 hamadryas baboon (2.8–10.1 years), and 18 human lenses (33–79 years). Anterior capsule specimens were obtained by performing a 5mm continuous curvilinear capsulorhexis and collecting the resulting disk of capsular tissue. To remove the lens epithelial cells the specimen was soaked in 0.1% trypsin and 0.02% EDTA for five minutes, washed, and placed on a Petri dish and immersed in DMEM. Elasticity measurements of the capsule were performed with a laboratory-built AFM system custom designed for force measurements of ophthalmic tissues. The capsular specimens were probed with an AFM cantilever tip to produce force-indentation curves for each specimen. Young’s modulus was calculated from the force-indentation curves using the model of Sneddon for a conical indenter. Young’s modulus of elasticity was 20.1–131kPa for the human lens capsule, 9.19–117kPa for the cynomolgus lens capsule, and 13.1–62.4kPa for the baboon lens capsule. Young’s modulus increased significantly with age in humans (p=0.03). The age range of the monkey and baboon samples was not sufficient to justify an analysis of age dependence. The capsule elasticity of young humans (<45 years) was not statistically different from that of the monkey and baboon. In humans, there is an increase in lens capsule stiffness at the microscale that could be responsible for an increase in lens capsule bulk stiffness.
Keywords: Atomic Force Microscopy, lens capsule, mechanical properties
1. Introduction
The crystalline lens is completely enclosed by the lens capsule, a viscoelastic membrane composed primarily of collagen arranged in a beehive structure (Courtois, 1987; Marshall et al, 1992) as well as fibrillin, laminin, and heparin sulfate proteoglycan. Since the capsule maintains the shape of the lens, it plays an important role in accommodation. It is the molding action of the capsule that elicits the change in shape of the lens during accommodation (Fincham, 1937; Kessler 1964, 1966; Glasser and Kaufman, 2003). Lens and capsule-based theories of presbyopia suggest that a main factor in the loss of accommodation is the decreased elasticity of both the lens and capsule with age (Werner et al, 2002). The loss of elasticity results in the inability of the capsule to mold the lens material, which is necessary for the lens shape changes during accommodation. The mechanical properties of the capsule, and their changes with age, are vital to a more complete understanding of accommodation and the capsule’s role in the onset of presbyopia (Krag et al, 1997).
The strength and elasticity of the lens capsule have been previously investigated using pressure loading (Fisher, 1969; Danielsen, 2004; Pedrigi et al, 2007), uniaxial stress-strain analysis (Yang et al, 1998), and by stretching capsular rings (Krag and Andreasse, 1998) or openings (Morgan et al, 1996; Wood and Schelonka, 1999; Andreo et al, 1999; Assia et al, 1991; Parel et al, 2006). Absolute values of the modulus of elasticity have been determined by Danielsen et al (2004) and Pedrigi et al (2007) using pressure loading and Krag et al (1997) using uniaxial stretching of capsular rings. These studies concur that Young’s modulus of elasticity of the lens capsule, on the macroscopic scale, increases with age by a factor of approximately 10, from 0.3 to 2.5MPa. The cause for this increase in bulk stiffness of the lens capsule is still unknown. Previous studies have shown that the anterior lens capsule becomes thicker with age (Krag et al, 1997), which could be responsible for the increase in bulk stiffness. However, changes in the microscale mechanical properties of the lens capsule could also be a factor in the increase in bulk stiffness. The bulk mechanical properties of tissues are affected by both the mechanical properties of the individual components on the microscale and the organization of the components on the macroscale (Rho et al, 1998; Intrigila et al, 2007; Bull, 1971). Therefore, the purpose of the current study was to use Atomic Force Microscopy (AFM) to measure the elasticity of the excised human and non-human primate lens capsule at the microscale to determine if there are changes with age.
2. Materials and Methods
2.1 Atomic Force Microscope
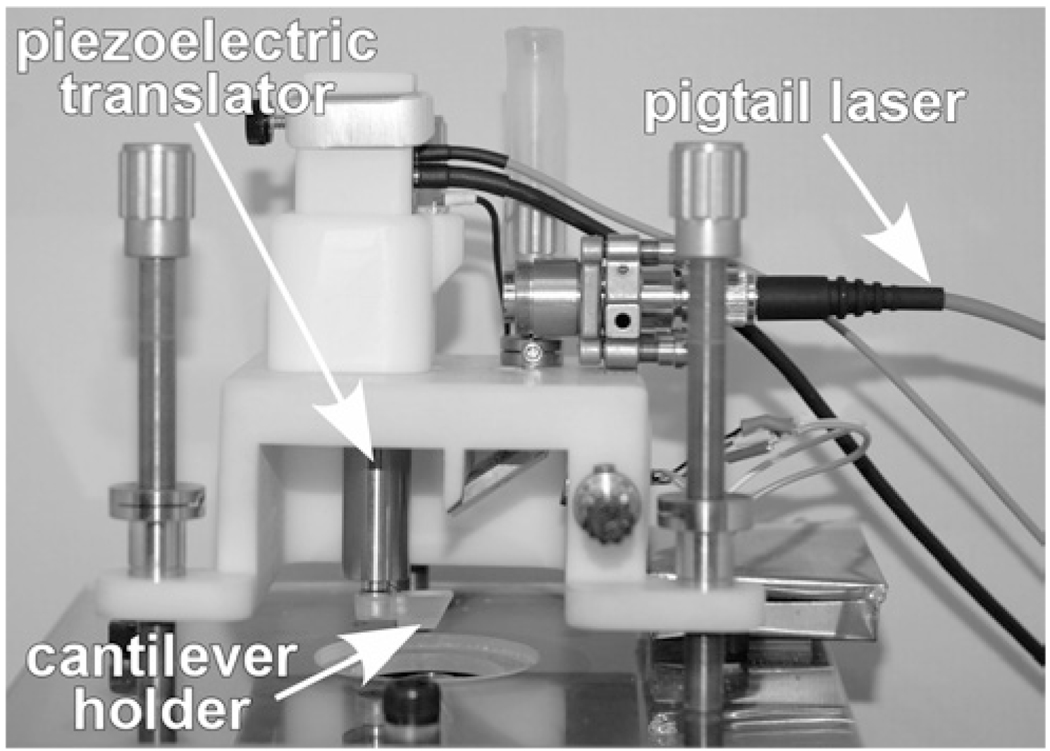
The AFM system used is a laboratory-made modification of the AFM design used for imaging (Figure 1; Ziebarth et al, 2007; Wojcikiewicz et al, 2003) that can be used to measure sample elasticity through the use of nanoindentation. The AFM cantilever tip (10nm tip radius, 40° full cone angle, CSC series, Mikromasch, San Jo se, CA) is lowered onto the sample using a piezoelectric mechanism (Physik Instrumente, Germany). In response, the cantilever undergoes a combination of indentation and deflection (bending) dependent on the softness of the sample: the harder the sample, the more the cantilever deflects and the less it indents the sample. The bending of the cantilever is proportional to the force that the AFM probe tip exerts on the sample. The recorded cantilever deflection-indentation curves can be used to derive the force-indentation curves for the sample after factoring out the deflection of the cantilever on a hard surface. According to the Sneddon contact mechanical model (Sneddon, 1965), the force-indentation relation is a function of Young’s modulus of elasticity:
where F [N] is the measured force (N), E [N/m2] is Young’s modulus (Pa), ν is Poisson’s ratio (ν=0.47 for the lens capsule; Fisher, 1969), α is the angle between the conical tip and the sample (70°), and D is the measured indentation. The custom AFM system and analysis procedure was calibrated with agarose gels of concentrations of 2.5% and 5.0%. The modulus results obtained were 283kPa and 845kPa compared to the published values of 254kPa and 929kPa for 2.5% and 5.0%, respectively (Normand et al, 2000).
Figure 1.
The AFM system for lens capsule elasticity measurements is a custom-built modification of the AFM design used for imaging. The cantilever is moved vertically using a piezoelectric translator. The Petri dish containing the lens capsule is placed below the cantilever. When in contact with the sample, the cantilever is bent causing the beam of the laser diode to be deflected. A photodiode monitors these deflections.
2.2 Experimental protocol
Experiments were conducted on 18 lens capsules from 14 humans (donor age range: 33–79 years), on 9 lens capsules from 9 healthy cynomolgus monkeys (Macaca fascicularis, age range: 5.9–8 years), and on 8 lens capsules from 7 healthy baboons (Papio hamadryas, age range: 2.8–10 years). The human and monkey globes arrived in sealed vials placed in styrofoam containers filled with ice. Upon arrival in the laboratory, the eyes were stored in the refrigerator at 4°C before they were used. Experiments were performed on human eyes less than twelve days postmortem (4.6±3.2 days), on monkey eyes less than two days postmortem (0.7±0.7 days) and on baboon eyes less than two days postmortem (1.1±0.4 days). The animal eyes were obtained after enucleation following approved institutional animal care guidelines. All animal experiments adhered to the ARVO Statement for the use of animals in research. All human eyes were obtained and used in compliance with the guidelines of the Declaration of Helsinki for research involving the use of human tissue.
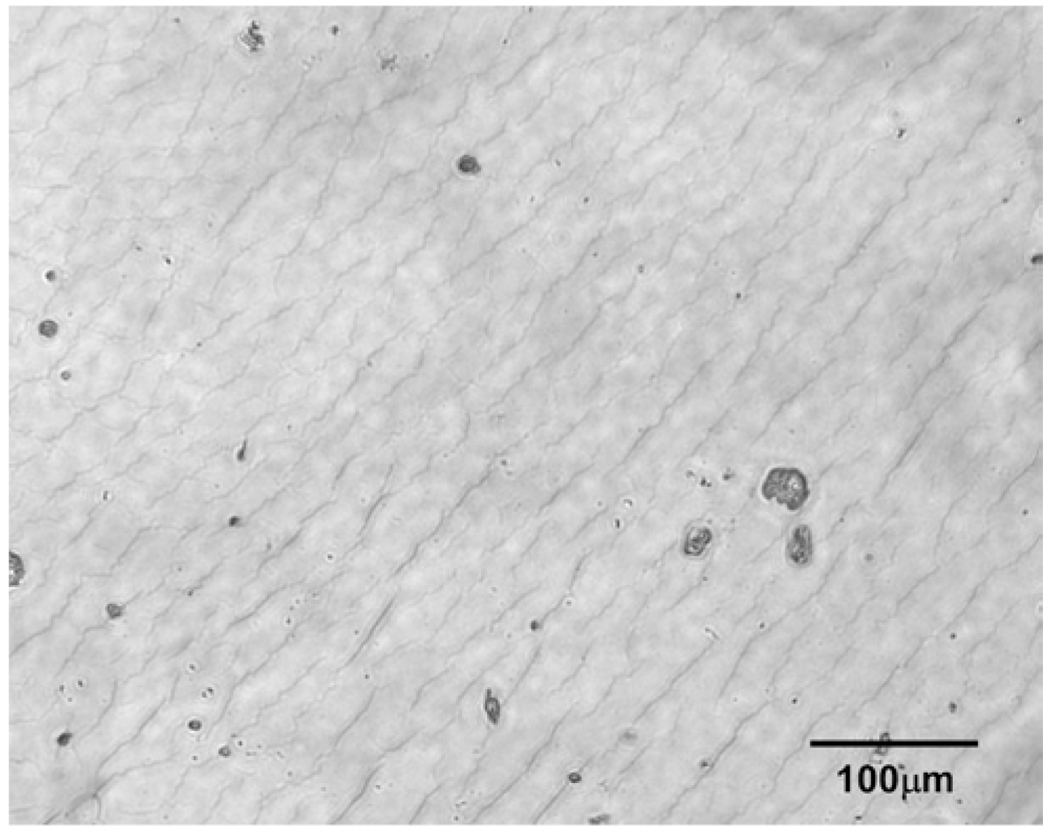
The anterior lens capsule was prepared by removing a portion approximately 5mm in diameter from the anterior of the whole lens. This was accomplished by using the continuous curvilinear capsulorhexis (CCC) technique. Between the anterior lens capsule and lens cortex, there is a layer of epithelial cells, which adhere to the lens capsule after it is removed. The presence of these soft cells between the lens capsule and the hard Petri dish substrate may impact the measurements obtained using AFM. Therefore, a protocol for removal of these cells was developed. After performing the anterior capsulorhexis, the capsule was placed in a Petri dish containing 0.1% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA) solution (Humphry et al, 1988). The anterior lens capsule was allowed to soak in this combined solution at room temperature for 5 minutes. After this procedure, the lens capsule was washed with cold DMEM/F-12 solution (D8437, Sigma, St. Louis, MO), and then placed on a dry Petri dish, with the interior side of the capsule placed towards the dish, and allowed to adhere for five minutes. DMEM/F-12 solution was placed on top of the lens capsule to maintain hydration during the experiments. Figure 2 shows a light micrograph of the lens capsule with this treatment to remove the lens epithelial cells.
Figure 2.
Image of a lens capsule with the treatment described in Section 5.3.1. No epithelial cells are visible.
After the lens capsule was prepared as described above, the Petri dish containing the sample was placed under the polymethylmethacrylate (PMMA) block containing the AFM cantilever. The sample was positioned so that the cantilever tip was over the central portion of the sample. The tip was lowered until it was just touching the surface of the anterior side of the capsule. This position was determined by the point when the reflected laser beam moves off the photodiode. The location of the cantilever on the sample was observed using the 10x microscope objective connected to the camera beneath the sample. This enabled the positioning of the cantilever above an area with no surface irregularities or adherent cells. The tip was then lowered using the piezoelectric control so that it was probing the tissue. The measurements were conducted using a cantilever approach and retraction speed of 1.5µm/s and a maximal indentation force of 20nN. This force corresponded to approximately 0.5–2µm indentation, depending on the stiffness of the sample. The voltage detected at the photodiode due to deflection of the cantilever was recorded as a function of piezoelectric displacement. These recordings were repeated at least 15 times per sample. All experiments were performed at room temperature.
2.3 Data analysis
The data analysis procedure for the AFM measurements has been described previously (Ziebarth et al, 2007). The photodiode voltage versus piezoelectric displacement recorded during the measurement scans was converted to force versus indentation after accounting for the cantilever spring constant and its behavior when probing a rigid substrate. The force versus indentation relationship was analyzed using the Sneddon model for a conical indenter (see Section 2.1). The determination of Young’s modulus was carried out using custom developed Matlab software. Each curve fit was verified visually. Since at least 15 measurements were performed for each sample, the average of these values was then used as the modulus for that sample. In the case of paired eyes, the moduli from left and right eyes of each donor were averaged to avoid skewing the statistics for age dependence.
3. Results
The coefficient of variation of repeated measurements of the same lens capsule (standard deviation divided by average) was 12% (range: 3–31%) for humans, 9% (range: 2–18%) for cynomolgus monkeys, and 11% (range: 2–18%) for baboons. There was no relationship between measured Young’s modulus and postmortem time.
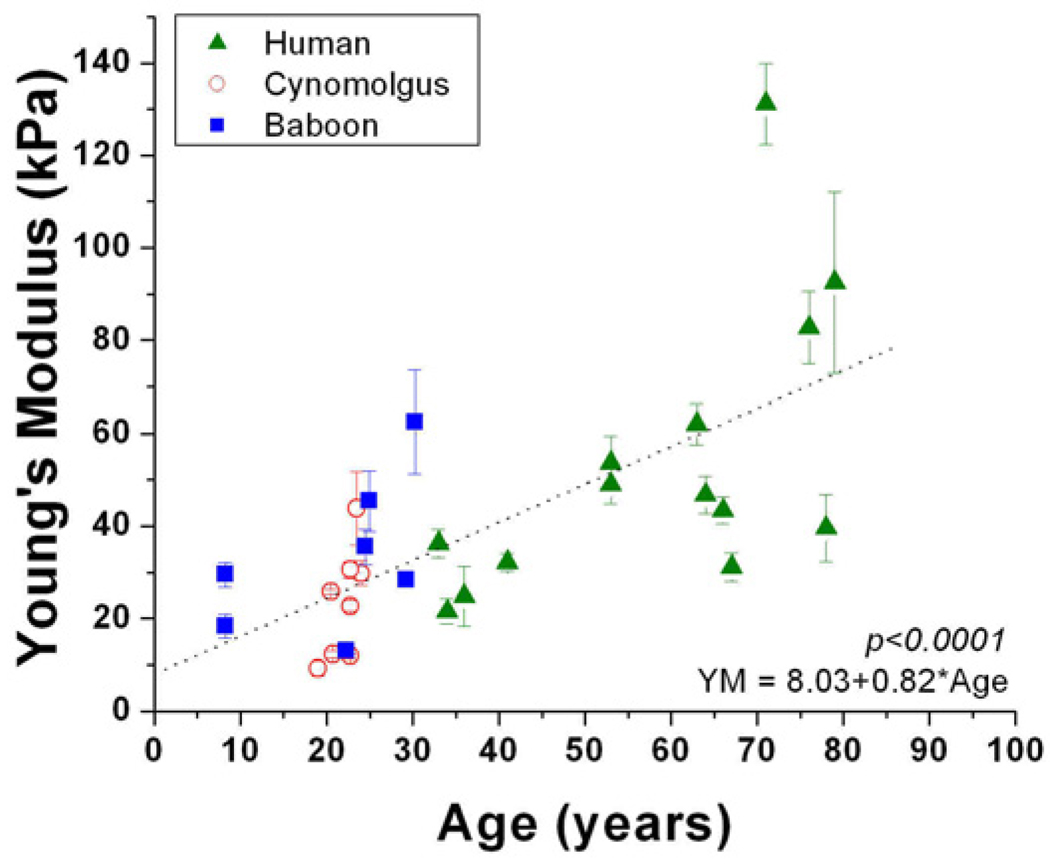
Young’s modulus of elasticity was 20.1–131kPa for the human lens capsule, 9.19–117kPa for the cynomolgus lens capsule, and 13.1–62.4kPa for the baboon lens capsule (Table 1). Although there were no evident experimental problems, the high data point for the cynomologus lens capsule (177kPa) was not included in the analysis because it was more than 10 standard deviations greater than the average. Young’s modulus increased significantly with age in humans (p=0.03; R2=0.4). The age range of the monkey and baboon samples was not sufficient to justify an analysis of age dependence. The human data was separated into three distinct age groups: young (<45 years), mid-age (45–70) and old (>70). The three human age groups were compared to the monkey and baboon data using a one-way analysis of variance followed by a post-hoc least significant difference analysis. This analysis confirmed that there were no differences between the two monkey species and the young humans. Therefore, the human and non-human primate data was grouped together using a scaling factor of 1 monkey year to 3 human years (Qiao-Grider et al, 2007; Bito et al, 1982; Figure 3). This analysis showed that young human capsule elasticity is comparable to non-human primate capsule elasticity.
Table 1.
Summary of experimental results. The humans were subdivided into three age groups based on clusters in the data. The separation enables easy comparison of young human data to the non-human primate data.
| Species | n | Postmortem Time (days) |
Age (years) | Young’s modulus of elasticity (kPa) |
|---|---|---|---|---|
| Cynomolgus Monkey |
9 | 0.7±0.7 (0.25–2.1) |
7.2±0.7 (5.9–8) |
33.7±33.1 (9.19–117) |
| Hamadryas Baboon |
8 | 1.0±0.5 (0.25–2) |
6.5±3.2 (2.8–10.1) |
33.3±16.7 (13.1–62.4) |
| Young Human (<45 years) |
4 | 4.4±2.0 (2–6) |
36±3.6 (33–41) |
28.7±6.7 (21.5–36.3) |
| Mid-Age Human (45–70 years) |
9 | 5.0±3.8 (1–12) |
61±6.4 (53–67) |
47.7±10.4 (31.1–62.0) |
| Old Human (>70 years) |
5 | 3.8±1.5 (2–5) |
76±3.6 (71–79) |
86.5±37.6 (39.6–131) |
Figure 3.
Young’s modulus as a function of age for human and non-human primate samples. The age scale for the non-human primates was 1 NHP year=3 human years26–27. Young’s modulus of elasticity of the combined data was analyzed as a function of age using linear regression analysis. The p-value of this regression analysis was used to evaluate statistical significance. Young human capsule elasticity is comparable to non-human primate capsule elasticity.
4. Discussion
In this study, a custom-built AFM for nanoindentation measurements was used to determine Young’s modulus of elasticity of individual collagen fibers of the human and non-human primate anterior lens capsule. Experiments were performed on fully hydrated samples by indenting the sample approximately 1µm with a cantilever tip 10nm in diameter while applying less than 20nN of force. The posterior lens capsule was not included in the current study because an appropriate preparation protocol would need to be developed. The posterior capsule is much thinner than the anterior, so it would be more difficult to remove using a capsulorhexis technique. In addition, adherent vitreous would need to be removed.
One difficulty in Atomic Force Microscopy measurements is to extract elasticity properties of the outer layer of the sample without probing the properties of the substrate. To avoid effects of the substrate, the indentation depth should be limited to less than 10% of the layer thickness. However, there will always be an elastic displacement of the substrate (Fischer-Cripps, 2002). The thickness of the anterior lens capsule is known to be between 10µm and 20µm (Krag et al, 1997). We had previously used AFM to measure the elasticity of whole, intact lenses (Ziebarth et al, 2007). In these previous experiments, the AFM cantilever tip was indenting the outer surface of the lens capsule less than 10%. However, the values obtained on whole lenses were much lower than the lens capsule alone presented in the current study (0.4–3.2kPa compared to 9.2–117kPa for cynomologus monkeys), demonstrating the effect of the soft substrate (lens epithelial cells and lens cortex). In the whole lens measurements, we hypothesize that the capsule is deformed in bending mode under the pressure of the AFM tip (Ziebarth et al, 2007), meaning that the force exerted by the AFM tip indents only the softest part of the lens – the epithelium and cortex. The elasticity values obtained in our previous study on cynomolgus monkey whole lenses were comparable to those found previously for the cortex of young human lenses (Ziebarth et al, 2007; Heys et al, 2004; Weeber et al, 2005). This suggests that AFM measurements of whole lenses provide information on the elasticity of the lens cortex. To measure the elasticity of the lens capsule alone, it must be removed from the lens to avoid substrate effects from the lens epithelial cells and lens cortex when performing AFM indentation testing.
Although only the approach scans were analyzed to determine the elastic modulus of the capsule, retract scans were recorded as well. The curves were nearly identical exhibiting very little hysteresis, suggesting a predominantly elastic response. Therefore, the speed of indentation should have little effect on the measurements obtained. The slow speed of 1.5µm/s was selected to limit possible sample damage during repeated scans.
Our results demonstrate a similarity between lens capsule elasticity of non-human primates and humans in the younger age range (see Figure 3 and Table 1). Previous research has illustrated that the non-human primate has an accommodative structure and mechanism similar to that of the human (Qiao-Grider et al, 2007; Bito et al, 1982; Glasser et al, 2006). Non-human primate lenses demonstrate an age-dependent flattening, decrease in refractive power (Borja et al, 2010), and decrease in accommodative amplitude similar to what has been observed in humans (Bito et al, 1982). Based on previous measurements, it has been determined that a scaling factor of 1 monkey year to 3 human years is appropriate to directly compare between species (Qiao-Grider et al, 2007; Bito et al, 1982). There are no studies that have compared the composition and structure of the human and non-human primate lens capsule, although the anterior and posterior thicknesses are similar (Ziebarth et al, 2005). Our current results suggest that the capsule mechanical properties are also comparable. Measurement of the monkey lens capsule elasticity and its changes with age may help elucidate changes that occur in the human.
The numerical elasticity values obtained using AFM for the collagen fibers were approximately 100 times smaller than those found by previous researchers for the whole lens capsule. This present study found elastic moduli between 9.19 and 131kPa, compared to 0.3 to 2.5MPa found previously (Krag et al, 1997; Fisher, 1969; Danielsen, 2004; Pedrigi et al, 2007). It was also found that Young’s modulus of elasticity of the human and non-human primate lens capsule increases significantly with age (becomes stiffer). Over a 100-year lifespan, the elastic modulus increases by a factor of 10. Previous studies have also found that the lens capsule on a macroscopic scale becomes stiffer with age (Krag et al, 1997; Danielsen, 2004; Pedrigi et al, 2007; Krag and Andreassen, 2003), but the increase is an order of magnitude greater than that of the current study. These differences could be partly due to differences in methodology. In previous measurements to produce the tensile modulus of elasticity, annular capsular specimens 100µm thick (outer diameter=3.2mm) were prepared from the anterior lens capsule and placed around two pins and stretched linearly (Krag et al, 1997). In additional measurements, the whole anterior lens capsule underwent circumferential pressure loading (Danielsen, 2004; Pedrigi et al, 2007). AFM measurements are performed in compression, and therefore provide the compressive modulus of elasticity. Due to anisotropy of the lens capsule, the difference in the direction of the measurement could have an impact on the values obtained for Young’s modulus.
The scale of the measurement technique could also explain the numerical differences between the current and previous studies. Previous experiments measured the bulk, macroscopic mechanical response of the capsule. Due to the scale of the AFM cantilever tip, the present measurements correspond to a localized value of the micro-elasticity of the lens capsule. It is known that tissue elasticity is affected by both mechanical properties of the individual components on the microscale and the organization of the components on the macroscale (Rho et al, 1998; Intrigila et al, 2007; Bull, 1971). The arrangement of the capsule collagen could be responsible for the unique elastic response of the whole lens capsule compared to its individual components. The interwoven beehive structure of the lens capsule collagen (Courtois, 1987; Marshall, 1992; Barnard et al, 1992) may endow the capsule with increased strength, making it more resistant to stretching forces.
Although collagen accounts for approximately 70% of the capsule’s content (Marshall, 1992), it also contains laminin, fibrillin, and heparan sulfate proteoglycan. Because of the scale of the AFM cantilever tip, measurements could have corresponded to one of these other molecules. Previous AFM measurements of fully hydrated, isolated collagen I fibrils found that elasticity is depended on fibril size: 6.1±0.8kPa for small fibrils (<50nm), 7–97MPa for medium fibrils (100–200nm), and 70–170MPa for large (280–426nm) fibrils (Chung et al, 2010; Yang et al, 2008). Young’s modulus of isolated fibrillin microfibrils is approximately 78–96MPa (Sherratt et al, 2003). The measurements in the current study correspond best to small collagen fibrils, which is reasonable since the collagen filaments in the lens capsule are approximately 30nm in diameter (Barnard et al, 1992).
The AFM measurements in the current study show relatively large between-sample variability for samples of similar ages. This variability is most likely due to anisotropy of the lens capsule, rather than errors with the measurements technique, since measurements on the same sample in the same location have a variation of approximately 10%. The capsule contains non-collagen components, so measurement in an area with fibrillin or laminin rather than just collagen would produce different elasticity values. In addition, as stated previously, collagen elasticity is inversely proportional to collagen fiber diameter. Therefore, measurement of capsule collagen fibers with varying diameters would also affect the between-sample variability.
In summary, Atomic Force Microscopy was used to measure the elasticity of the lens capsule on the microscale. In humans, there is an increase in lens capsule stiffness at the microscale that could be responsible for an increase in lens capsule bulk stiffness.
Acknowledgements
Grant support: NIH EY14225 (JMP); Vision Cooperative Research Centre, Sydney, New South Wales, Australia, supported by the Australian Federal Government through the Cooperative Research Centres Programme; American Federation for Aging Research (NMZ); Advanced Medical Optics, Inc.; Florida Lions Eye Bank; NIH center grant P30-EY014801; 5R01 GM086808 (VTM); NSF MRI 0722372 (VTM); University of Miami SAC Award (VTM); Research to Prevent Blindness; Henri and Flore Lesieur Foundation (JMP).
Norma Kenyon, PhD, and Dora Berman-Weinberg, PhD, of the Diabetic Research Institute and Linda Waterman, DVM and Daniel Rothen, DVM, of the Division of Veterinary Resources of the University of Miami gave scientific support. Izuru Nose, BSEE, and William Lee of the Ophthalmic Biophysics Center of Bascom Palmer Eye Institute gave technical support. Donor human eyes were provided by Rakhi Jain, PhD, of Advanced Medical Optics, Inc., the Florida Lions Eye Bank, Lions Eye Bank of Oregon, Lions Medical Eye Bank (Norfolk, VA), Lions Eye Institute for Transplantation and Research Inc. (Tampa, FL), Illinois Eye Bank, Alabama Eye Bank, Old Dominion Eye Foundation Inc. (Richmond, VA), North Carolina Eye Bank, Utah Lions Eye Bank, and the North West Lions Eye Bank (Seattle, WA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any proprietary or financial interest in any of the devices presented.
Presented in part at the Association for Research in Vision and Ophthalmology 2009 meeting.
References
- Andreo LK, Wilson ME, Apple DJ. Elastic properties and scanning electron microscopic appearance of manual continuous curvilinear capsulorhexis and vitrectorhexis in an animal model of pediatric cataract. Journal of Cataract and Refractive Surgery. 1999;25(4):534–539. doi: 10.1016/s0886-3350(99)80051-0. [DOI] [PubMed] [Google Scholar]
- Assia EI, Apple DJ, Barden A, Tsai JC, Castaneda VE, Hoggatt JS. An experimental study comparing various anterior capsulectomy techniques. Archives of Ophthalmology. 1991;109(5):642–647. doi: 10.1001/archopht.1991.01080050056028. [DOI] [PubMed] [Google Scholar]
- Barnard K, Burgess SA, Carter DA, Woolley DM. Three-Dimensional Structure of Type IV Collagen in the Mammalian Lens Capsule. Journal of Structural Biology. 1992;108:6–13. doi: 10.1016/1047-8477(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Bito LZ, DeRousseau CJ, Kaufman PL, Bito W. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Investigative Ophthalmology and Visual Science. 1982;23:23–31. [PubMed] [Google Scholar]
- Borja D, Manns F, Ho A, Ziebarth NM, Acosta AC, Arrieta-Quintero E, Augusteyn RC, Parel JM. Refractive power and biometric properties of the non-human primate isolated crystalline lens. Investigative Ophthalmology and Visual Science. 2010;51(4):2118–2125. doi: 10.1167/iovs.09-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull HB. An Introduction to Physical Biochemistry. 2nd Edition. Philadelphia: FA Davis Company; 1971. [Google Scholar]
- Chung KH, Bhadriraju K, Spurlin TA, Cook RF, Plant AL. Nanomechanical Properties of Thin Films of Type I Collagen Fibrils. Langmuir. 2010;26(5):3629–3636. doi: 10.1021/la903073v. [DOI] [PubMed] [Google Scholar]
- Courtois, Yves . The Capsule of the Crystalline Lens. In: Lawrence Stark, Gerard Obrecht., editors. Presbyopia: Recent research and reviews from the third international symposium. Professional Press Books/Fairchild Publications Division of Capital Cities Media, Inc.; 1987. [Google Scholar]
- Danielsen CC. Tensile mechanical and creep properties of Descemet’s membrane and lens capsule. Experimental Eye Research. 2004;79(3):343–350. doi: 10.1016/j.exer.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Fincham EF. The mechanism of accommodation. British Journal of Ophthalmology. 1937 Monograph Supplement VIII. [Google Scholar]
- Fisher RF. Elastic constants of the human lens capsule. Journal of Physiology. 1969;201:1–19. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Cripps AC. Nanoindentation. New York: Springer; 2002. [Google Scholar]
- Glasser A, Kaufman PL. Accommodation and presbyopia. In: Kaufman PL, Alm A, editors. Adler’s Physiology of the Eye. Clinical Application. 10th Edition. St Louis, MO: Mosby; 2003. pp. 197–233. [Google Scholar]
- Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Investigative Ophthalmology and Visual Science. 2006;47:1087–1095. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Molecular Vision. 2004;10:956–963. [PubMed] [Google Scholar]
- Humphry RC, Davies EG, Jacob TJC, Thompson GM. The Human Anterior Lens Capsule-an attempted chemical debridement of epithelial cells by ethylenediaminetetracetic acid (EDTA) and trypsin. British Journal of Ophthalmology. 1988;72:406–408. doi: 10.1136/bjo.72.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intrigila B, Melattib I, Tofanic A, Macchiarelli G. Computational models of myocardial endomysial collagen arrangement. Computer Methods and Programs in Biomedicine. 2007;86:232–244. doi: 10.1016/j.cmpb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Kessler J. Experiments in refilling the lens. Archives of Ophthalmology. 1964;71:412–417. doi: 10.1001/archopht.1964.00970010428021. [DOI] [PubMed] [Google Scholar]
- Kessler J. Refilling the rabbit lens. Further experiments. Archives of Ophthalmology. 1966;76(4):596–598. doi: 10.1001/archopht.1966.03850010598021. [DOI] [PubMed] [Google Scholar]
- Krag S, Andreassen TT. Effect of freezing on lens capsule mechanical behavior. Ophthalmic Research. 1998;30:280–285. doi: 10.1159/000055485. [DOI] [PubMed] [Google Scholar]
- Krag S, Andreassen TT. Mechanical properties of the human posterior lens capsule. Investigative Ophthalmology and Visual Science. 2003;44(2):691–696. doi: 10.1167/iovs.02-0096. [DOI] [PubMed] [Google Scholar]
- Krag S, Olsen T, Andreassen TT. Biomechanical characteristics of the human anterior lens capsule in relation to age. Investigative Ophthalmology and Visual Science. 1997;38:357–363. [PubMed] [Google Scholar]
- Marshall GE, Konstas AG, Bechrakis NE, Lee WR. An Immunoelectron Microscope Study of the Aged Human Lens Capsule. Experimental Eye Research. 1992;54:393–401. doi: 10.1016/0014-4835(92)90051-s. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Ellingham RB, Young RD, Trmal GJ. The mechanical properties of the human lens capsule following capsulorhexis or radiofrequency diathermy capsulotomy. Archives of Ophthalmology. 1996;114:1110–1115. doi: 10.1001/archopht.1996.01100140312010. [DOI] [PubMed] [Google Scholar]
- Normand V, Lootens DL, Amici E, Plucknett KP, Aymard P. New Insight into agarose gel mechanical properties. Biomacromolecules. 2000;1:730–738. doi: 10.1021/bm005583j. [DOI] [PubMed] [Google Scholar]
- Parel JM, Ziebarth N, Denham D, Fernandez V, Manns F, Lamar P, Rosen A, Ho A, Erickson P. Assessment of the strength of mini-capsulorhexes. Journal of Cataract and Refractive Surgery. 2006;32:1366–1373. doi: 10.1016/j.jcrs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Pedrigi RM, David G, Dziezyc J, Humphrey JD. Regional mechanical properties and stress analysis of the human anterior lens capsule. Vision Research. 2007;47:1781–1789. doi: 10.1016/j.visres.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee C, Ramamirtham R, Smith EL. Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Research. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Medical Engineering & Physics. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- Sherratt MJ, Baldock C, Haston JL, Holmes DF, Jones CJ, Shuttleworth CA, Wess TJ, Kielty CM. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J Mol Biol. 2003;332(1):183–193. doi: 10.1016/s0022-2836(03)00829-5. [DOI] [PubMed] [Google Scholar]
- Sneddon IN. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Engng Sci. 1965;3:47–57. [Google Scholar]
- Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RGL. Dynamic mechanical properties of human lenses. Experimental Eye Research. 2005;80:425–434. doi: 10.1016/j.exer.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Werner LP, Werner L, Pandey SK, Apple DJ. Physiology of Accommodation and Presbyopia. In: Amar Agarwal., editor. Presbyopia: A Surgical Textbook. Thorofare, NJ: Slack Incorporated; 2002. [Google Scholar]
- Wojcikiewicz EP, Zhang X, Chen A, Moy VT. Contributions of molecular binding events and cellular compliance to the modulation of leukocyte adhesion. Journal of Cell Science. 2003;166(23):2531–2539. doi: 10.1242/jcs.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MG, Schelonka LP. A porcine model predicts that a can-opener capsulotomy can be done safely in pediatric patients. Journal of American Association for Pediatric Ophthalmology and Strabismus. 1999;3(6):356–362. doi: 10.1016/s1091-8531(99)70045-5. [DOI] [PubMed] [Google Scholar]
- Yang X, Zou L, Binrong M, Dong D, Dai H, Lu X. Tensile strength of lens capsules in eye-bank eyes. Journal of Cataract and Refractive Surgery. 1998;24(4):543–546. doi: 10.1016/s0886-3350(98)80299-x. [DOI] [PubMed] [Google Scholar]
- Yang L, van der Werf KO, Fitie CFC, Bennink ML, Dijkstra PJ, Feijen J. Mechanical Properties of Native and Cross-linked Type I Collagen Fibrils. Biophysical Journal. 2008;94:2204–2211. doi: 10.1529/biophysj.107.111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebarth N, Manns F, Uhlhorn S, Venkatraman A, Parel JM. Non-contact optical measurement of lens capsule thickness in human, monkey, and rabbit postmortem eyes. Investigative Ophthalmology and Visual Science. 2005;46:1690–1697. doi: 10.1167/iovs.05-0039. [DOI] [PubMed] [Google Scholar]
- Ziebarth NM, Wojcikiewicz EP, Manns F, Moy VT, Parel J-M. Atomic Force Microscopy measurements of lens elasticity in monkey eyes. Molecular Vision. 2007;13:504–510. [PMC free article] [PubMed] [Google Scholar]