Abstract
In this study, we explored the mechanisms by which the angiotensin converting enzyme inhibitor (ACEI), enalapril, and the Ang II receptor blocker (ARB), losartan suppress oxidative stress and NF-κB activation-induced inflammatory responses in aged rat kidney. The experimentations were carried out utilizing aged (24-month-old) Brown Norway x Fischer 344 (F1) male rats which were randomized into 3 groups and administered enalapril (40 mg/kg), losartan (30 mg/kg) or placebo for 6 months (daily p.o.). The level of reactive species (RS), peroxynitrite (ONOO−), GSH/GSSG and lipid peroxidation were measured. The activity of the pro-inflammatory transcription factor NF-κB, and gene expression of proteins in upstream signaling cascades were measured by electro-mobility shift assay (EMSA) and Western blotting. Enalapril and losartan differentially attenuated redox imbalance and the redox-sensitive transcription factor, NF-κB pathway. Furthermore, stimulation of the NF-κB activation pathway by phosphorylation of p65 was attenuated by both compounds. Moreover, mediation of phosphorylation of p65 by phosphorylation of IκB kinase αβ (IKKαβ) and mitogen- and stress-activated protein kinase-1 (MSK1), were also inhibited by enalapril and losartan. Finally, both compounds also lowered expression of NF-κB-dependent inflammatory genes, such as cyclooxygenase-2 (COX-2),) and inducible NO synthase (iNOS). Only losartan lowered levels of 5-lipoxygenase (5-LOX). These findings indicate that enalapril and losartan differentially suppress inflammatory responses via inhibition of oxidative stress-induced NF-κB activation in aged rat kidney.
INTRODUCTION
Angiotensin II (Ang II) is the primary hemodynamic effector molecule of the renin–angiotensin system; but has also been demonstrated to play a primary role in the modulation of cellular redox status and inflammatory response. Indeed, Ang II generates reactive species (RS) during the inflammatory response via multiple signaling pathways [Cheng et al., 2005, Sachse & Wolf 2007, Kang et al., 2008] involving activation of the Ang II type 1 receptor (AT1) [Carey et al., 2007]. Several cell culture studies suggest that Ang II itself induces O2− production which is rapidly converted to H2O2, a pro-inflammatory mediator [Zafari et al., 1998]. In addition, direct administration of Ang II downregulates activity of endogenous anti-oxidant enzymes such as superoxide dismutase (SOD) and catalase, and induces imbalance in redox signaling in the kidney [Griendling et al., 1994].
Ang II signaling increases with aging [da Silva et al., 2005, Inserra et al., 1995], whereas suppression of Ang II signaling attenuates the development of age-related vascular diseases [Kosugi et al., 2006]. Angiotensin converting enzyme inhibitors (ACEI) block synthesis of Ang II, and angiotensin receptor blockers (ARB) block the interaction of Ang II with the angiotensin type 1 (AT1) receptor (Fig. 1). Both strategies are used clinically for the treatment of chronic renal disease to improve kidney hypertension and vascular function by decreasing glomerulosclerosis and atherosclerosis [Ciechanowicz, 1999; Cunha et al., 2005; Li et al., 2005; Ordaz et al., 2010]. In this context, developing preclinical models of late-life intervention strategies for combating declining organ function has enormous significance [de Grey, 2007; Rae et al., 2010]. With the continued “graying” of the world-wide population, the number of individuals at risk of developing renal abnormalities continues to increase and the sky-rocketing social, emotional and economic cost [Olshansky et al., 2009] of caring for such individuals mandates the need for testing the effectiveness of health-promoting interventions within this cohort.
Figure 1. Chemical structure of angiotensin II antagonists.
Angiotensin converting enzyme inhibitor, enalapril (A) and angiotensin II receptor blocker, losartan (B)
Indeed, we have previously shown that aged Fischer 344 X Brown Norway (F344/BN) rats, while relatively protected from glomerulosclerosis, do demonstrate increased glomerular ischemia/atrophy, tubular atrophy and interstial fibrosis with age [Moningka et al. 2010]. Furthermore, when these animals were treated late in life (between 24 and 30 months of age) with the ARB, losartan, this tubule-interstitial injury was prevented, relative to animals treated with the ACEI, enalapril or age-matched controls; although enalapril treated animals consistently showed lowered levels of injury relative to controls [Moningka et al., 2010]. Therefore, it is possible that losartan, is a more effective modulatior of ANG II mediated cell signaling processes. This is entirely plausible given that losartan blocks the action of ANG II by interfering with its interaction with its receptor; whereas enalapril only modulates levels of ANG II by blocking its synthesis from ANG I. Therefore, in the present study, we attempted to further these findings by exploring in more depth, changes in inflammatory and redox status in the aged kidney after late-life treatment with the ACEI, enalapril, and the ARB, losartan using the same treated animals from the cohort described above [Moningka et al, 2010; Carter et al, in press]. More specifically, we sought to test the hypothesis that oxidative stress observed in age rat kidney is regulated by the inflammatory transcription factor, nuclear factor-κB (NF-κB), and may be attenuated by blocking the action of ANGII.
MATERIALS & METHODS
Materials
All chemical reagents were obtained from Sigma (St. Louis, MO, USA), except where noted. Dichlorodihydrofluorescein diacetate (DCF-DA), dihydrorhodamine 123 (DHR-123) and radionucleotide [γ32P]-ATP were obtained from Amersham Life Science (Buckinghamshire, UK). West-zol™ Plus was purchased from iNtRON Biotechnology (Seongnam, Korea). Antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Cell Signaling Technology (New England Bio Labs, Hertfordshire, UK). Polyvinylidene difluoride (PVDF) membranes were obtained from Millipore Corporation (Bedford, MA, USA). All other materials were of the highest available grade.
Animals
Experiments were conducted according to the Guiding Principles in the Care and Use of Laboratory Animals, and the procedures were approved by the University of Florida's Institute on Animal Care and Use Committee. F344/BN male rats (n = 30, 10/each treatment group) were obtained from the National Institute on Aging colony at Harlan Industries (Indianapolis, IN, USA) at 24 months of age. Testing began at 24 months of age and continued for 6 months. Rats were housed in individual cages (24 × 9 × 12 inches) maintained on a 12-hour light/12-hour dark cycle with food (Purina standard lab chow; Nestle Purina, St. Louis, MO, USA) and water available ad libitum, and assessed monthly for signs of overt health problems using a standardized form.
Enalapril and losartan administration
Rats were randomized to daily administration of 40 mg/kg of enalapril, 30 mg/kg of losartan, or saline solution as placebo control compounded into bacon flavored tablets (BioServ #F05072) and were followed for 6 months (to age 30 months). Rats were sacrificed by decapitation and the kidneys were quickly removed. The tissue was immediately frozen in liquid nitrogen and stored at −80 °C. We used the kidney because of its vulnerability to age-related oxidative stress and inflammatory responsiveness. In addition, all of the components of the renin-angiotensin system are synthesized locally within the kidney [Vaziri et al., 2007].
Tissue preparation of the cytosolic and nuclear fraction
All solutions, tubes, and centrifuges were maintained at 0–4 °C. Kidney tissue (300 mg) was homogenized with 2 ml of homogenate buffer A (10 mM HEPES, pH 7.8, 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1 mM EDTA, 0.1 mM PMSF, 1 μM pepstatin, and 1 mM p-aminobenzamidine) with a tissue homogenizer for 20 sec. Homogenates were kept on ice for 15 min, 125 μl of 10% Nonidet p40 (NP40) solution was added and mixed for 15 sec, and the mixture was then centrifuged at 12,000 rpm for 2 min; the supernatant containing thcytosol proteins was then collected. The pelleted nuclei were washed once with 400 μl of buffer A plus 25 μl of 10% NP40, centrifuged, suspended in 50 μl of buffer C (50 mM HEPES, pH 7.8, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM PMSF and 10% (vol/vol) glycerol), mixed for 20 min, and centrifuged at 12,000 rpm for 30 min. The supernatant containing the nuclear proteins was stored at −80 °C.
Quantification of redox status
(1) Total RS level
RS generation was measured by a 2',7'-dichlorofluorescin diacetate (DCF-DA) assay. A fluorometric assay was used to determine levels of RS, such as ·O2−, ·OH, and H2O2. Nonfluorescent DCF-DA was oxidized to the highly fluorescent 2',7'-dichlorofluorescin (DCF) in the presence of esterases and RS, including lipid peroxides. DCF-DA (25 μM) was added to the homogenates to create 250 μl final volume. Changes in fluorescence intensity were measured every 5 min for 30 min on a fluorescence plate reader, TECAN (GENius, Tecan Instruments, Salzburg, Austria) with excitation and emission wavelengths set at 485 and 535 nm, respectively.
(2) Total ONOO− level
ONOO− generation was measured by monitoring the oxidation of dihydrogenrhodamine (DHR) 123. Briefly, 10 μl of homogenates were added to the rhodamine solution (50 mM sodium phosphate buffer, 90 mM sodium chloride, 5 mM diethylenetriaminepentaacetate [DTPA], and DHR 123). Changes in fluorescence intensity were measured every 5 min for 30 min on a fluorescence plate reader with excitation and emission wavelengths set at 485 and 530 nm.
(3) GSH/GSSG
For the assay to measure glutathione levels, 1 mM EDTA-50 mM phosphate buffer was added to the supernatant of trichloric acid (TCA)-treated homogenates, followed by o-phthaldehyde, and the mixture was incubated for 25 min at room temperature. To assay GSSG level, N-ethylmaleimide was added to the supernatant of TCA-treated homogenates. After 30 min at room temperature, 0.5 N NaOH and o-phthaldehyde were added to sample and then incubated for 25 min. Both GSH and GSSG levels were measured at excitation and emission wavelengths set at 360 and 460 nm, respectively.
(4) Lipid peroxidation
Malondialdehyde (MDA)/4-hydroxyalkenals (HAE) concentrations were determined through use of a Bioxytech LPO-586 Assay Kit (OXIS Health Products, Foster, CA, USA). The kit uses a chromatogenic reagent that reacts with the lipid peroxidation products, malondialdehyde and 4-hydroxynonenales, yielding a stable chromophore with maximum absorbance at 586 nm.
Western blot
Protein concentration was determined by the bicinchoninic (BCA) method using bovine serum albumin (BSA) as a standard. Equal amounts of protein were separated on 10–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. The gels were subsequently transferred onto a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA) by electroblotting for 2 h at 60–75V. The membranes were blocked in a 5% nonfat milk solution in Tris-buffered saline (TBS) with 0.5% Tween-20, and incubated with primary antibodies as indicated. Pre-stained blue protein markers (Bio-Rad) were used for molecular-weight determination.
EMSA
The electrophoretic mobility shift assay (EMSA) method was used to characterize the binding activities of NF-κB transcription factors in the nuclear extracts. The NF-κB oligonucleotide sequence was 5′-GAGAGGCAAGGGGATTCCCTTAGTTAGGA-3′. The protein-DNA binding mixture containing 20 μg of nuclear protein extract was incubated for 20 min at 4 °C in binding medium containing 5% glycerol, 1 mM MgCl2, 50 mM NaCl, 0.5 mM EDTA, 2 mM DTT, 1% NP-40, 10 mM Tris (pH 7.5), and 1 μg poly(dI-dC) to block nonspecific binding. Radiolabeled transcription factor consensus oligonucleotide (20,000 cpm of γ-32P) was added, and the complete mixture was incubated for an additional 20 min at room temperature. DNA-binding complexes were resolved by 7% native PAGE with 0.5 × TBE (50 mM Tris, 45 mM boric acid, and 0.5 mM EDTA) for 90 min at 200 V. The gel was dried and exposed to Fuji X-ray film 1 – 2 days at −80 °C.
Statistical analysis
ANOVA was used to analyze differences among all groups. Differences in the means of individual groups were assessed by the Fischer's protected least significant difference post hoc test. Values of P < 0.05 were considered statistically significant and all data are reported as mean ± standard error of the mean (SEM).
RESULTS
Modulation of redox status by enalapril and losartan
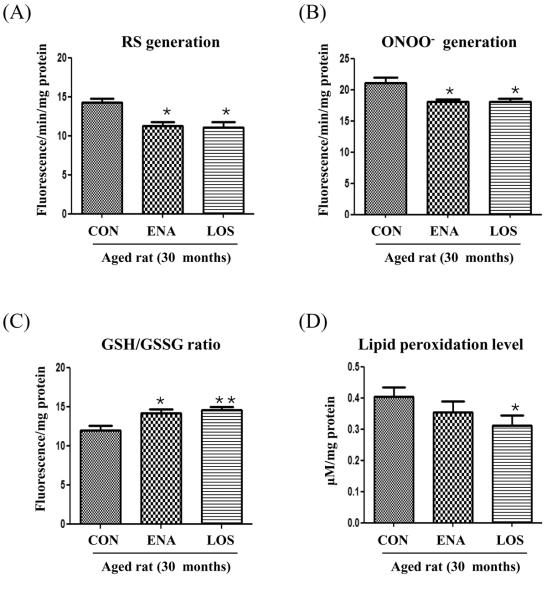
We first investigated the effect of enalapril and losartan on oxidative stress in aged rat kidneys. As shown in Fig. 2A and B, RS generation and ONOO− generation were lower in the enalapril and losartan treatment groups compared with the control. The level of reduced glutathione (GSH), a well known biological antioxidant, was expressed as the ratio of GSH to oxidized glutathione (GSH/GSSG). The GSH/GSSG ratio was increased by enalapril and losartan treatment compared with control (Fig. 2C), When comparing lipid preoxidation products (Fig. 2D), the levels of malondialdehyde and hydroxyalkenes were lowered by only losartan treatment. These results indicated that losartan may be a more effective modulator of redox imbalance, relative to enalapril, in aged rat kidneys.
Figure 2. Effect of enalapril and losartan on redox imbalance in the aged rat.
Kidney homogenates were prepared to determine relative levels of oxidative stress. RS levels were determined by DCF-DA with a fluorescent probe (A). ONOO− was measured by DHR-123 with a fluorescent probe (B). GSH/GSSH was measured by o-phthaldehyde (C). MDA+HAE was determined using a Bioxytech LPO-586 Assay Kit (D). Each value is expressed as mean ± S.E.M. of three determinations and normalized mg protein. *P<0.01, **P<0.001 vs. normal aged rat by ANOVA. RS, reactive species; ONOO–, peroxynitrite; MDA, malondialdehyde; HAE, hydroxyalkene; GSH, glutathione; GSSG, glutathione disulfide ENA, enalapril; LOS, losartan.
Regulation of MAPKs by enalapril and losartan
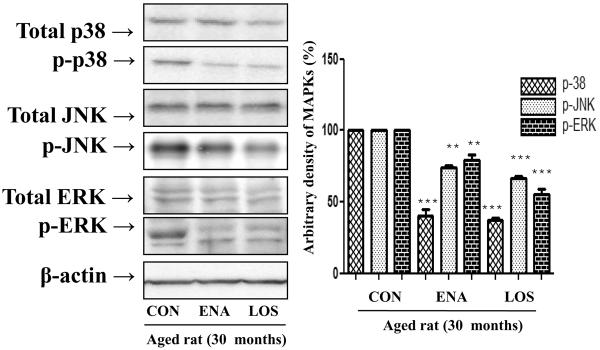
The MAPKs signaling pathway activates NF-κB, which causes age-related oxidative stress, hence we evaluated whether enalapril and losartan inhibit phosphorylation of MAPKs in the aged rat kidney because. In addition, we examined the effect of enalapril and losartan on extracellular signal regulated kinase (ERK), c-Jun terminal kinase (JNK), and p38MAPK proteins as detected by Western blotting using p-p38-, p-ERK- and p-JNK-specific monoclonal antibodies and total p38-, ERK- and JNK-specific monoclonal antibodies. As shown in Fig. 3, the results showed that enalapril and losartan treatment inhibit the phosphorylation of ERK, JNK, and p38 compared with control. These results indicated that enalapril and losartan treatment positively regulate MAPK phosphorylation via inhibition of RS generation..
Figure 3. Effect of enalapril and losartan on MAPKs activation in the aged rat.
Cytosolic proteins were prepared to determine the phosphorylation levels of MAPKs. p-ERK, p-p38, and p-JNK levels were detected by Western blotting using anti p-p38-, anti p-ERK- and anti p-JNK-specific monoclonal antibodies in the cytosol. One representative blot of each protein is shown from 3 experiments that yielded similar results. Levels were normalized to total ERK, p38, JNK and β-actin. Values are the relative optical intensity of each band normalized as a percentage of the untreated control. **P<0.01, ***P<0.001 vs. normal aged rat by ANOVA. MAPKs, mitogen-activated protein kinases; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; ENA, enalapril; LOS, losartan.
Suppression of NF-κB activation by enalapril and losartan
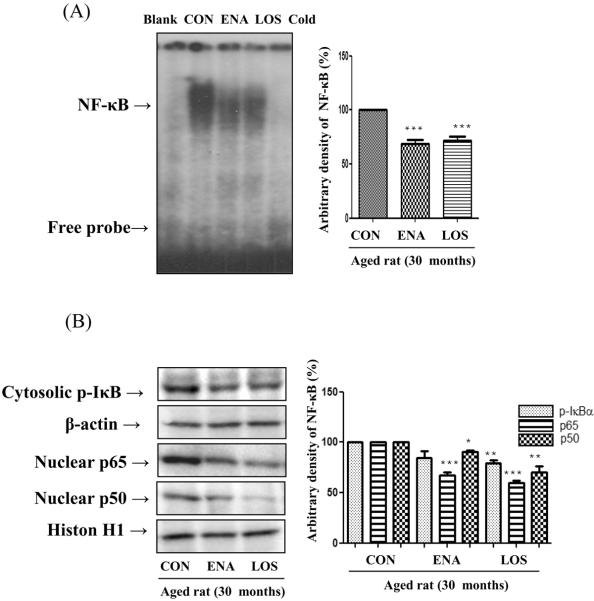
Transcription factor NF-κB is a well-known pro-inflammatory marker in aged rat. To evaluate whether enalapril and losartan alter NF-κB activation in the aged rat kidney, we examined NF-κB DNA binding activity and IκB phosphorylation. First, we investigated whether losartan and enalapril inhibit DNA binding activity as detected by EMSA. Indeed, activity of NF-κB was suppressed by enalapril and losartan treatment compared with control (Fig. 4A). Moreover, the levels of phosphorylated IkBα in the cytoplasmic extract were also lowered by losartan, but unaffected by enalapril. To determine treatment effects on nuclear activation of NF-κB, we examined nuclear protein levels by Western blotting using anti p65-and anti p50-specific polyclonal antibodies. Fig. 4B shows that age-related nuclear translocation of NF-κB was significantly lowered by enalapril and losartan treatment compared with control. Thus, these results indicate that while both treatments maybe equally effective in blocking the nucelar effects of NF-κB, losartan treatment may be a more efficient in blocking the pro-inflammatory response through suppression enhanced suppresion of IκB phosphorylation.
Figure 4. Effect of enalapril and losartan on NF-κB activation in the aged rat.
Nuclear proteins were prepared to determine the transcriptional activity of NF-κB. NF-κB binding activity was detected by EMSA in nuclear proteins (A). Phosphorylation of IκB and nuclear translocation of p65/p50 subunit were detected by Western blotting using anti p-IκB-, anti p65- and anti p50-specific polyclonal antibodies. (B). Levels were normalized to β-actin in the cytosolic fraction and histone H1 in the nuclear fraction. One representative blot is shown from 3 experiments that yielded similar results. * P<0.05, **P<0.01, ***P<0.001 vs. normal aged rat. BL, probe without nuclear protein sample; Cold, competition assay using 100-fold excess of unlabeled NF-κB oligonucleotide; ENA, enalapril; LOS, losartan.
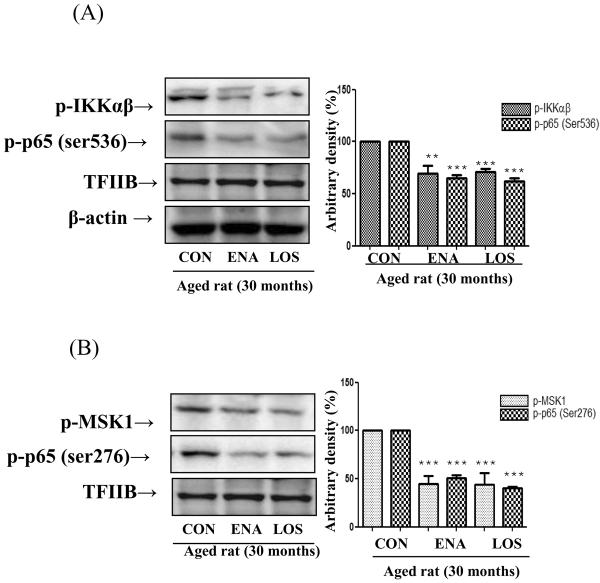
Suppression of p65 phosphorylation by enalapril and losartan
Transcriptional activity of NF-κB is stimulated upon serine residue phosphorylation of p65 by various kinases. Thus, we investigated whether enalapril and losartan suppress NF-κB activation through p65 phosphorylation. In addition, previous studies have demonstrated that Ser 536 residue phosphorylation of p65 is triggered by IKKαβ. Therefore, we examined whether losartan and enalapril inhibit Ser 536 phosphorylation of p65 and its-related IKKαβ phosphorylation as detected by Western blotting using anti p-p65- (Ser 536) and anti p-IKKαβ-specific polyclonal antibodies. As shown in Fig. 5A, enalapril and losartan treatment suppressed Ser 536 residue p65 phosphorylation compared with controls through inhibiting IKKαβ phosphorylation. We also explored whether enalapril and losartan treatment inhibit NF-κB through Ser 276 phosphorylation of p65. It has been known that Ser 276 phosphorylation of p65 is associated ERK and MSK1 signaling. To determine Ser 276 phosphorylation of p65 and its-related MSK-1, we examined nuclear protein levels by Western blotting using anti p-p65- (Ser 276) and anti p-MSK1-specific polyclonal antibodies. Figure 5B shows that Ser 276 phosphorylation of p65 significant was significantly lowered by enalapril and losartan through inhibiting of MSK-1 and ERK phosphorylation. These results indicate that enalapril and losartan treatment modulate NF-κB activation through age-related p65 phosphorylation.
Figure 5. Effect of enalapril and losartan on phosphorylation of p65 in the aged rat.
Cytosolic and nuclear proteins were prepared to determine the phosphorylation levels of p65. Ser 536 phosphorylation of p65 and IKKαβ phosphorylation were detected by Western blotting using anti p-p65- (Ser 536) and anti p-IKKαβ-specific polyclonal antibodies (A). Ser 276 phosphorylation of p65 and MSK-1 phosphorylation were detected by Western blotting using anti p-p65- (Ser 276) and anti p-MSK-1-specific polyclonal antibodies (B). Levels were normalized to β-actin in the cytosolic fraction and histone H1 in the nuclear fraction. One representative blot is shown from 3 experiments that yielded similar results. **P<0.01, ***P<0.001 vs. normal aged rat. IKKαβ, IκB kinaseαβ; MSK-1. mitogen- and stress-activated protein kinase-1; ENA, enalapril; LOS, losartan.
Inhibition of NF-κB-related gene expression by enalapril and losartan
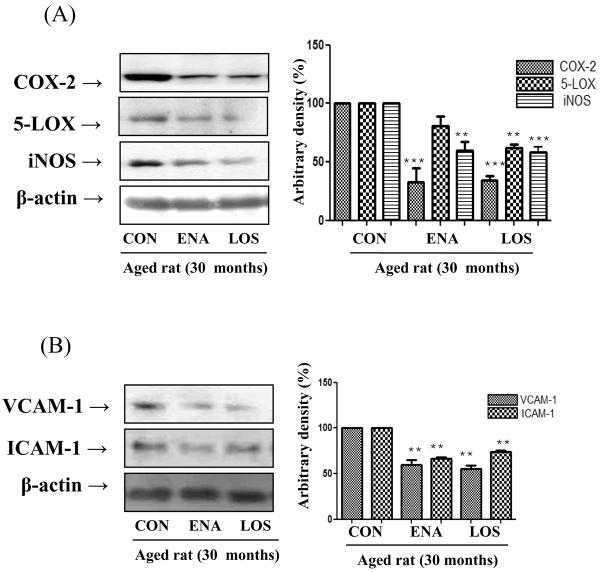
We next examined whether enalapril and losartan inhibit the induction of NF-κB-responsive proteins, such as COX-2, 5-LOX, and iNOS. These genes are predominantly regulated by NF-κB to produce RS and inflammatory responses. These genes have been known to have an NF-κB binding site in their promoter regions, and are controlled through NF-κB regulation. The expression level of COX-2, 5-LOX, and iNOS were detected by Western blotting using anti COX-2-, anti 5-LOX-, and anti iNOS-specific polyclonal antibodies (Fig. 6A). Enalapril and losartan both were equally effective in lowering levels of COX-2 and iNOS; however only losartan significantly lowered levels of 5-LOX.
Figure 6. Effect of enalapril and losartan on NF-κB-dependent pro-inflammatory genes and adhesion molecules expression in the aged rats.
Cytosolic proteins were prepared to determine the expression levels of NF-κB-dependent pro-inflammatory gene and adhesion molecules. Western blotting was performed to detect renal COX-2, iNOS, and 5-LOX levels in cytoplasmic extracts from aged rats (A). NF-κB-dependent Adhesion molecules levels were detected by Western blot using anti VCAM-1 and anti ICAM-1-specific polyclonal antibodies. Levels were normalized to β-actin. One representative blot is shown from 3 experiments that yielded similar results. **P<0.01, ***P<0.001 vs. normal aged rat by ANOVA. COX-2; cyclooxygenase 2, 5-LOX; 5-lipoxygenase, iNOS; inducible NO synthase; VCAM-1; vascular cell adhesion molecule 1, ICAM-1; Inter-Cellular Adhesion Molecule 1, ENA, enalapril; LOS, losartan
Finally, we explored whether enalapril and losartan treatment lower expression level of VCAM-1 and ICAM-1. NF-κB regulates vascular adhesion molecules such as VCAM-1 and ICAM-1. Indeed, these adhesion molecules were lowered by enalapril and losartan treatment compared with placebo (Fig. 6B), indicating that these compounds blocked age-related pro-inflammatory gene expression by inhibiting NF-κB activation.
DISCUSSION
We have previously shown that late-life administration of the ARB, losartan, prevents age-related tubule-interstitial injury in F344/BN rats, relative to animals treated with the ACEI, enalapril or age-matched controls [Moningka et al., 2010]. This suggests that losartan, may be a more effective modulator of ANG II mediated cell signaling. Therefore, in the present study, we attempted to further these findings by exploring in more depth, changes in inflammatory and redox status in the aged kidney after late-life treatment with, enalapril, and, losartan.
Beyond its hemodynamic role, Ang II is considered a pro-inflammatory mediator that participates in inflammatory response such as angiogenesis, vascular remodeling and vascular inflammation [Saleh et al., 2009, Cheng et al., 2005, Sachse & Wolf 2007, Kang et al., 2008]. Ang II increases with age [Thompson et al., 2000], and blocking of Ang II reduces age-related cardiovascular dysfunction [Inserra et al., 2009]. The vascular protective effects exhibited by ACEIs and ARBs are not limited to pathological conditions, such as diabetes, hypertension, heart failure or heart attack. However, this reduction alone is not sufficient to explain its therapeutic benefits. It is known that inhibition of Ang II activity using ACEIs and ARBs improve age-related vascular inflammation via inhibiting NF-κB activation [Ishida et al., 2009]. In addition, ACEIs and ARBs can also attenuate certain degenerative changes associated with aging [Wei et al., 2008], and it has been suggested that Ang II plays an important role in the aging process. Therefore, in the current study we explored whether enalapril and losartan modulate age-related Ang II-induced redox imbalance in aged rat kidneys and to test the hypothesis that increased oxidative stress via ANG II is regulated by the transcription factor nuclear factor-κB (NF-κB).
Ang II and AT1 binding induces the activation of nuclear factor-κB (NF-κB), a redox-sensitive transcription factor. NF-κB activation is a hallmark of cellular inflammatory response [Chung et al, 2002]. Activation of NF-κB signaling by Ang II affects the transcription of cytokines, chemokines and growth factors. NF-κB can be activated by pro-inflammatory genes and RS, and it regulates the expression of inflammation-related adhesion molecules [Inserra et al., 1995, Kosugi et al., 2006]. The classic NF-κB pathway [Hoffman et al., 2002] is triggered by phosphorylation and degradation of the inhibitory κBα (IκBα), but Ang II can activate NF-κB via phosphorylation of p65 without phosphorylation of IκBα. Transcriptional activity of NF-κB is stimulated upon serine residue phosphorylation of p65 by various kinases. Ang II has been observed to activate NF-κB through Ser 536 phosphorylation of p65, which is mediated by IκB kinaseαβ (IKKαβ) phosphorylation [Rey & Pagano 2002, Feinman & Siegel 2004]. On the other hand, the Ser 276 phosphorylation pathway is mediated by mitogen- and stress-activated protein kinase-1 (MSK1) in the nucleus [Viatour et al., 2005, Zhong et al., 2002]. MSK-1 is also phosphorylated by extracellular response kinase (ERK) and p38 mitogen-activated protein kinase (MAPKs) phosphorylation.
Indeed, Ang II increases RS production and affects redox status, leading to pro-inflammatory responses via NF-κB activation [Laursen et al., 1997]. Therefore, we first investigated whether enalapril and losartan improve age-related redox imbalance in the aged rat kidney. We demonstrate that enalapril and losartan block the generation of RS, ONOO−, improved GSH/GSSG levels; however only losartan improved lipid peroxidation (Fig. 2). This is contrast to a recent paper in which young KK-Ay/Ta Jcl diabetic mice given either enalapril, losartan, or the combination showed decreased lipid peroxidation in kidney accompanied by improved glomerular function [Yamazaki et al., 2009]. However, in the kidney of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, enalapril had no impact on levels of lipid peroxidation [Sugimoto et al., 2002], To be sure, species differences may be one possible explanation for these disparate results; however in the context of the current study, it should be noted that while older F344/BN rats are insulin resistant with age, they are clearly not diabetic nor do they present with hypertension. Therefore, the effects of enalapril and losartan on lipid peroxidation may be wholly dependent on their differential mechanisms of action.
Recent reviews published from our laboratory on the interrelation between oxidative stress and inflammation during aging emphasize the important role NF-κB plays in the pro-inflammatory state of aged organisms [Sung et al., 2004]. Thus we next tested the hypothesis that enalapril and losartan lower inflammation via modulation of NF-κB activation. Our results show that enalapril and losartan inhibited age-related NF-κB activation by suppressing NF-κB DNA binding activity, p65/p50 translocation to the nucleus, and MAPK signaling. However, only losartan attenuated IκB phosphorylation. These findings suggest that losartan may be more efficient than enalapril in modulating NF-κB induced inflammation.
Furthermore, enalapril and losartan inhibited NF-κB activation through modulation of p65 phosphorylation. Optimal induction of NF-κB target genes also requires phosphorylation of NF-κB proteins, such as p65, within their transactivation domain by a variety of kinases in response to distinct stimuli. The impact of ANG II signaling on these pathways is unknown, yet in the current study we demonstrate that upregulation of age-related Ser 536 phosphorylation of p65 was decreased by enalapril and losartan through inhibition of IKKαβ phosphorylation. In addition, enalapril and losartan also inhibited age-related Ser 276 phosphorylation of p65 through reducing phosphorylation of MSK-1 in the nucleus.
Another novel finding of the current study is that NF-κB regulation of pro-inflammatory molecules, including COX-2, iNOS, is also lowered with enalapril and losartan treatment, however only losartan impacted the expression of 5-LOX . In addition age-related adhesion molecule expression is believed to initiate an inflammatory response associated with atherosclerosis and Ang II may actively contribute to the atherosclerotic process by upregulating VCAM-1 and ICAM-1 within the vascular wall in aging process [Basso et al., 2005, Anderson et al., 1996]. Our results show that enalapril and losartan treatment lower VCAM-1 and ICAM-1 expression.
In conclusion, we showed that enalapril and losartan differentially modulate age-related pro-inflammatory response and oxidative stress in aged rat kidney. Long-term treatment with enalapril decreases body weight gain and increases life span in rats [Carter et al., 2005; Santos et al., 2009] and protects multiple organs from oxidative damage [Cassis et al., 2010]. Taken together, these data strongly indicate that enalapril and losartan may therefore have therapeutic potential as anti-inflammatory agents for aging.
ACKNOWLEGEMENT
This work was supported by an RO1 research application, AG024526-01, entitled: “ACE inhibition and physical performance in aged rat” and by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (20090093538). We thank the Aging Tissue Bank for providing the research materials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1996:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso N, Paglia N, Stella I, de Cavanagh EM, Ferder L, Arnaiz M, Inserra F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Pept. 2005:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Carey RM. Angiotensin receptors and aging. Hypertension. 2007:33–4. doi: 10.1161/HYPERTENSIONAHA.106.086587. [DOI] [PubMed] [Google Scholar]
- Cassis P, Conti S, Remuzzi G, Benigni A. Angiotensin receptors as determinants of life span. Pflugers Archiv European Journal of Physiology. 2010;459:325–332. doi: 10.1007/s00424-009-0725-4. [DOI] [PubMed] [Google Scholar]
- Carter CS, Giovannini S, Seo DO, Dupree J, Morgan D, Chung HY, Lees H, Daniels M, Hubbard GB, Lee S, Ikeno Y, Foster TC, Buford TW, Marzetti E. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 x Brown Norway rats. Age (Dordr.),ahead of print. 2011 doi: 10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Cesari M, Ambrosius WT, Hu N, Diz D, Oden S, Sonntag WE, Pahor M. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–23. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005:194–205. [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc. Res. Tech. 2002;59(2002):264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2008;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanowicz A. Molecular mechanisms of nephro-protective action of enalapril in experimental chronic renal failure. Ann Acad Med Stetin. 1999:1–93. [PubMed] [Google Scholar]
- Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, Rutledge JC, Vergona R, Sullivan ME, Wang YX. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis. 2005:9–17. doi: 10.1016/j.atherosclerosis.2004.08.023. [DOI] [PubMed] [Google Scholar]
- da Silva Lemos M, Nardoni Gonçalves Braga A, Roberto da Silva J, Augusto Souza Dos Santos R. Altered cardiovascular responses to chronic angiotensin II infusion in aged rats. Regul Pept. 2005:67–73. doi: 10.1016/j.regpep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- de Grey AD. The case for prioritizing research on late-onset life-extension interventions in mammals. Rejuvenation Res. 2007;10:257–9. doi: 10.1089/rej.2007.0547. [DOI] [PubMed] [Google Scholar]
- Feinman R, Siegel D, Berenson J. Regulation of NF-kB in multiple myeloma: therapeutic implications. Clinical advances in hematology & oncology: H&O. 2004;2:162. [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw ZD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Inserra F, Basso N, Ferder M, Userpater M, Stella I, Paglia N, Inserra P, Tenembaum D, Ferder L. Changes seen in the aging kidney and the effect of blocking the renin-angiotensin system. Ther Adv Cardiovasc Dis. 2009:341–6. doi: 10.1177/1753944709339195. [DOI] [PubMed] [Google Scholar]
- Inserra F, Romano L, Ercole L, de Cavanagh EM, Ferder L. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension. 1995:437–42. doi: 10.1161/01.hyp.25.3.437. [DOI] [PubMed] [Google Scholar]
- Ishida S. Lifestyle-related diseases and anti-aging ophthalmology: suppression of retinal and choroidal pathologies by inhibiting renin-angiotensin system and inflammation. Nippon Ganka Gakkai Zasshi. 2009:403–22. [PubMed] [Google Scholar]
- Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-kappaB., Francis J. Cardiovasc Res. 2008:671–8. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi R, Shioi T, Watanabe-Maeda K, Yoshida Y, Takahashi K, Machida Y, Izumi T. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ J. 2006:482–8. doi: 10.1253/circj.70.482. [DOI] [PubMed] [Google Scholar]
- Laursen J, Rajagopalan S, Galis Z, Tarpey M, Freeman B, Harrison D. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- Li C, Sun BK, Lim SW, Song JC, Kang SW, Kim YS, Kang DH, Cha JH, Kim J, Yang CW. Results of a randomized, double-blinded, placebo-controlled c;onocal trail Combined effects of losartan and pravastatin on interstitial inflammation and fibrosis in chronic cyclosporine-induced nephropathy. Transplantation. 2005:1522–9. doi: 10.1097/01.tp.0000155305.49439.4c. [DOI] [PubMed] [Google Scholar]
- Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mech Ageing Dev. 2010 doi: 10.1016/j.mad.2010.10.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87:842–62. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz-Medina SM, González-Plascencia J, Martín del Campo F, Rojas-Campos E, Montañez-Fernández JL, Espinoza-Gómez F, Cueto-Manzano AM. Is systemic inflammation of hemodialysis patients improved with the use of enalapril? ASAIO J. 2010:37–41. doi: 10.1097/MAT.0b013e3181c1d830. [DOI] [PubMed] [Google Scholar]
- Rae MJ, Butler RN, Campisi J, de Grey AD, Finch CE, Gough M, Martin GM, Vijg J, Perrott KM, Logan BJ. The demographic and biomedical case for late-life interventions in aging. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000822. 40cm21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey FE, Pagano PJ. The reactive adventitia: fibroblast oxidase in vascular function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002:1962–1971. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- Sachse A, Wolf GJ. Angiotensin II-induced reactive oxygen species and the kidney. Am Soc Nephrol. 2007:2439–46. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- Saleh S, Ain-Shoka AA, El-Demerdash E, Khalef MM. Protective Effects of the Angiotensin II Receptor Blocker Losartan on Cisplatin-Induced Kidney Injury. Chemotherapy. 2009:399–406. doi: 10.1159/000262453. [DOI] [PubMed] [Google Scholar]
- Santos E, de Picoli Souza K, da Silva E, Batista E, Martins P, D'Almeida V, Pesquero J. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochemical pharmacology. 2009 doi: 10.1016/j.bcp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Tsuruoka S, Fujimura A. Effect of enalapril on diabetic nephropathy in OLETF rats: the role of an anti-oxidative action in its protective properties. Clin Exp Pharmacol Physiol. 2001;28:826–30. doi: 10.1046/j.1440-1681.2001.03530.x. [DOI] [PubMed] [Google Scholar]
- Sung B, Park SJ, Yu BY, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- Thompson MM, Oyama TT, Kelly FJ, Kennefick TM, Anderson S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol. 2000:R1787–R1794. doi: 10.1152/ajpregu.2000.279.5.R1787. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther. 2007:85–93. doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville M, Bours V, Chariot A. Phosphorylation of NF-[kappa] B and I [kappa] B proteins: implications in cancer and inflammation. Trends in biochemical sciences. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008:E345–51. doi: 10.1152/ajpendo.00456.2007. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Tanimoto M, Gohda T, Ohara I, Hagiwara S, Murakoshi M, Matsumoto M, Kaneko S, Aoki T, Toyoda H, Ishikawa Y, Funabiki K, Horikoshi S, Tomino Y. Combination effects of enalapril and losartan on lipid peroxidation in the kidneys of KK-Ay/Ta mice. Nephron Exp Nephrol. 2009;113:e66–76. doi: 10.1159/000228714. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor DR, Griendling KK. Novel role of NADH/NADPH oxidase-derived hydrogen peroxide in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 1998:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- Zhong H, May M, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-[kappa] B determines its association with CBP/p300 or HDAC-1. Molecular cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll R, Ghosh S. Phosphorylation of NF-kappaB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Molecular cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]