Abstract
Many countries are facing social and economic problems due to increased elderly demographics. With these demands, it is now critical to understand the fundamental regulatory mechanism for aging and longevity in mammals. Our studies on the mammalian NAD-dependent deacetylase SIRT1 and nicotinamide phosphoribosyltransferase (NAMPT)-mediated systemic NAD biosynthesis led us to propose a comprehensive model for the systemic regulatory network connecting metabolism and aging, termed the “NAD World.” In this article, I will discuss the importance of SIRT1 and NAMPT-mediated NAD biosynthesis in the NAD World and the system dynamics of this hierarchical network for the connection between metabolism and aging.
Keywords: aging, longevity, SIRT1, NAMPT, NAD biosynthesis, NAD World
Introduction
A number of European countries such as Germany, Italy, Spain, Sweden, Greece, and Austria, and Japan are on the verge of serious social and economic problems due to historically unprecedented increases in their elderly demographics [1,2]. This population shift has been implicated to cause labor shortage and significantly suppress economic growth in these countries. It will also put a pressure on governmental budgets on health and long-term care. Indeed, the Organisation for Economic Co-operation and Development (OECD) has projected that average health and long-term care spending across OECD countries would be almost doubled from ~7% of GDP in 2005 to ~13% in 2050 if no policy action is taken [3]. Interestingly, in this OECD's projection, it is assumed that longevity gains could be translated into additional years of good health, defined as “healthy ageing.” However, how we can practically achieve this goal still remains unclear. Given that aging per se is one of the serious risk factors for many chronic, costly diseases such as cancer, Alzheimer's disease, and type 2 diabetes, it is critical to better understand the fundamental mechanisms of aging and longevity and develop effective, affordable, anti-aging interventions to prevent those expensive diseases of aging.
Studies using experimental model organisms such as yeast, worms, flies, and mice have already identified a number of evolutionarily conserved regulators and signaling pathways for the control of aging and longevity, including insulin/insulin-like growth factor-I (IGF-I) signaling [4], mammalian target of rapamycin (mTOR) signaling [5,6], AMP-activated protein kinase (AMPK) signaling [7,8], and NAD-dependent sirtuins [9,10]. Because all these regulators and signaling pathways are known to play important roles in metabolic regulation, an intimate connection between metabolic and aging regulatory mechanisms has been implicated, stimulating researchers to dissect temporal and spatial dynamics of complex regulatory networks in each model organism. Whereas interplays between known regulators and signaling pathways are being investigated extensively [9], drawing a blueprint of such aging regulatory networks still remains a big challenge. To further dissect systemic regulatory networks of aging, there are several fundamental questions that need to be addressed: 1) Which organs and tissues have dominant roles in the systemic regulation of mammalian aging and longevity? 2) How do these “control centers” communicate with other organs and tissues to manage the process of aging and eventually longevity in mammals? 3) What are the molecular regulators and signaling pathways that coordinate such a regulatory network at a systemic level?
In this article, I will attempt to provide insight into these fundamental questions, focusing on the roles of the mammalian NAD-dependent deacetylase SIRT1 and nicotinamide phosphoribosyltransferase (NAMPT)-mediated systemic NAD biosynthesis in the regulation of metabolism and aging. SIRT1 functions as a key mediator that orchestrates metabolic responses to changes in nutritional availability in multiple tissues, whereas NAMPT-mediated NAD biosynthesis functions as a pace maker that regulates circadian oscillatory NAD production and fine-tunes SIRT1 activity at a systemic level [9-13]. Through such an intricate functional interplay, these two components comprise a systemic regulatory network that maintains the robustness of our physiological system in response to a variety of nutritional and environmental stimuli, which we have termed the “NAD World” [13-15]. I will introduce the concept of the NAD World and discuss its implication for the connection between metabolism and aging.
The mammalian NAD-dependent protein deacetylase SIRT1
SIR2 (silent information regulator 2) family proteins, now called “sirtuins,” are evolutionarily conserved from bacteria to humans and characterized by the signature structural motif called the core domain that is necessary for their NAD-dependent deacetylase or ADP-ribosyltransferase activities [10]. In mammals, there are seven sirtuins, SIRT1 through SIRT7, with highly divergent cellular localizations and functions [10]. Numerous studies in the past 10 years have firmly established that mammalian sirtuins, particularly SIRT1, regulate metabolic responses to changes in nutritional availability in multiple tissues [9,10,16-18].
Accumulating bodies of evidence have suggested that SIRT1 plays an important role in retarding age-associated pathophysiological changes and preventing from diseases of aging, such as type 2 diabetes, Alzheimer's disease, and cancer. Whole-body SIRT1-overexpressing transgenic mice show significant protection from the adverse effects of high-fat diet or aging on glucose metabolism [9,10]. SIRT1-activating compounds are also able to improve glucose homeostasis and insulin sensitivity in diet-induced and genetic type 2 diabetes animal models [9,10]. It has recently been demonstrated that SIRT1 prevents two critical pathological aspects of Alzheimer's disease: Aβ amyloid deposition and tauopathy. SIRT1 decreases the production of Aβ amyloid by deacetylating the retinoic acid receptor β and thereby up-regulating ADAM10, a major component of α-secretase [19]. SIRT1 also promotes degradation of phosphorylated tau by deacetylating it and prevents tau-mediated neurodegeneration [20]. Furthermore, SIRT1 regulates memory and synaptic plasticity, providing insight into potential intervention against age-associated cognitive disorders [21,22]. It has also been reported that SIRT1 transgenic mice show a lower incidence of spontaneous carcinomas and sarcomas and a reduced susceptibility to high-fat diet/carcinogen-induced liver tumors, compared to wild-type control mice [23]. These findings provide strong support for the importance of SIRT1 in the prevention of major age-associated diseases.
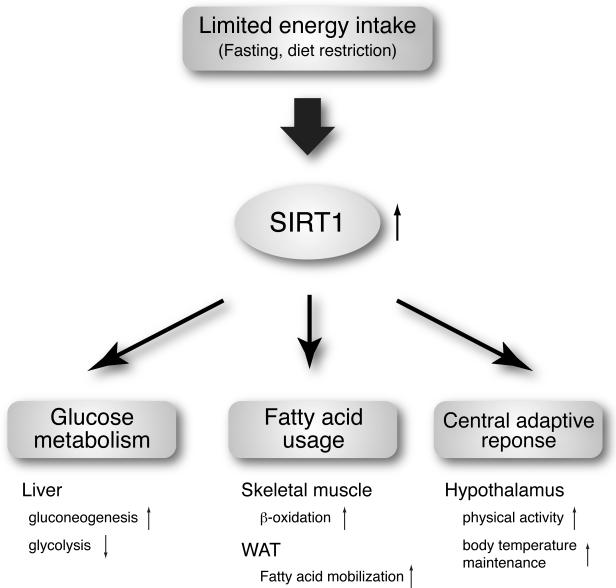
How can SIRT1 connect metabolic regulation to aging regulation? A key to understanding this important connection is the fact that SIRT1 is required to regulate adaptive responses to acute and chronic energy limitations, such as fasting and dietary restriction (Figure 1). For example, during fasting, peripheral tissues try to preserve blood glucose levels to maintain the functions of glucose-dependent tissues, particularly the brain. In the liver, SIRT1 represses glycolytic genes and up-regulates gluconeogenic genes by deacetylating PGC-1α, FOXO1, and STAT3, resulting in increased glucose production [24-27]. In white adipose tissue (WAT), SIRT1 mediates fatty acid mobilization by repressing PPARγ [28]. In skeletal muscle, the coordinated activation of AMPK and SIRT1 induces deacetylation of PGC-1α and FOXO1, switching the main energy source from glucose to fatty acids and enhancing their β-oxidation in mitochondria [29,30]. When energy limitation persists, the body triggers its adaptive mechanisms that enable individual animals to survive through nutritionally scarce conditions. In the hypothalamus, diet restriction significantly increases SIRT1 protein levels and thereby induces neural activation in the dorsomedial and lateral hypothalamic nuclei (DMH and LH, respectively), resulting in the increase in physical activity and counteracting the decrease in body temperature through the enhancement of the orexin type 2 receptor expression [31]. In pancreatic β cells, diet restriction enhances their postprandial insulin secretion [32, and our unpublished findings], which is phenocopied in pancreatic β cell-specific SIRT1-overexpressing (BESTO) mice [33]. In WAT, adiponectin production, which is regulated by SIRT1, increases in response to diet restriction, enhancing peripheral insulin sensitivity [34,35]. All these SIRT1-mediated adaptive responses to energy limitation are critical to keep our physiological system as robust as possible, by increasing blood glucose levels, utilizing fatty acid as an alternative energy source, and triggering neurobehavioral regulatory pathways, even under such nutritionally challenging conditions. During evolution, this exact function of SIRT1 and its orthologs might have become beneficial for organisms to keep their physiological system robust over a long period of time, culminating in the mechanism that affects the process of aging and eventually determines longevity within the limit of each organism's ecosystem. Based on this speculation, the organs and tissues in which SIRT1 function can be easily compromised by nutritional and environmental perturbations would be the frailty points for both metabolic and aging regulations in our physiological system. How can we identify such tissues and organs? Studies on mammalian NAD biosynthesis have provided an important clue to identify these potential frailty points, which will be discussed further below.
Figure 1.
The role of SIRT1 in mediating adaptive responses to limited energy intake. SIRT1 mediates adaptive responses to acute and chronic energy limitation, such as fasting and diet restriction, and maintains the robustness of our physiological system by increasing blood glucose levels, utilizing fatty acid as an alternative energy source, and triggering neurobehavioral regulatory pathways in multiple tissues indicated.
NAMPT-mediated systemic NAD biosynthesis
Because sirtuins absolutely require NAD for their enzymatic activities, how NAD is synthesized and supplied to sirtuins is important to understand the system dynamics of the NAD World. Interestingly, the functional connection between NAD biosynthesis and sirtuins is very ancient [36]. Genes encoding two key NAD biosynthetic enzymes (Nampt and Nmnat, described below) and a sirtuin have been identified in the T4-like, broad host range vibriophage KVP40, implicating that this connection is fundamental [37]. Indeed, these particular enzymes are highly conserved in mammals.
NAD is synthesized from three major precursors – tryptophan, nicotinic acid, and nicotinamide [38,39]. Lower eukaryotes and invertebrates, such as yeast, worms, and flies, use nicotinic acid, a form of vitamin B3, as a major NAD precursor, whereas mammals predominantly use nicotinamide, another form of vitamin B3, for NAD biosynthesis. In mammals, NAMPT initiates the major NAD biosynthesis pathway by converting nicotinamide and 5’-phosphoribosyl-1-pyrophosphate (5’-PRPP) to nicotinamide mononucleotide (NMN), which is the rate-limiting step in this NAD biosynthesis [11,12]. The second enzyme, nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT), completes NAD biosynthesis by transferring adenine from ATP to NMN. There are three distinct isoforms for NMNAT, NMNAT1-3, which are localized in nucleus, cytoplasm, and mitochondria, respectively, suggesting that NAD biosynthesis mediated by NAMPT and NMNAT might be compartmentalized in each subcellular compartment [40,41].
The enzymological features and the crystal structures of NAMPT have been studied extensively [36,42-44]. NAMPT belongs to a dimeric class of type II phosphoribosyltransferases. Interestingly, in mammals, NAMPT has both intra- and extracellular forms, defined as iNAMPT and eNAMPT, respectively [45]. eNAMPT (a.k.a. PBEF/visfatin) is actively secreted through an unidentified, non-classical secretory pathway from mouse and human matured adipocytes [45]. This secretion process appears to be regulated by protein modification in response to nutritional changes (our unpublished observation). Furthermore, eNAMPT secreted from matured adipocytes is approximately twice as active as iNAMPT [45], implying that the secretion and the activity of eNAMPT might be tightly regulated in adipocytes. It has been reported that hepatocytes, macrophages, and leukocytes also produce eNAMPT [46-48]. One recent study has demonstrated an interesting correlation between serum eNAMPT levels and circulating leukocyte counts, speculating that leukocytes might be a major source of circulating eNAMPT [47]. Given that NAMPT-mediated NAD biosynthesis is critical for neutrophilic granulocyte differentiation in humans [49], it is equally possible that increased eNAMPT levels promote leukocyte differentiation, resulting in increases in circulating leukocyte counts. Additionally, it should be noted that brown adipose tissue and adipocytes also express very high levels of NAMPT mRNA and protein and are capable of secreting eNAMPT in response to nutritional changes [45, and our unpublished findings]. Therefore, which cell type is indeed the major source of circulating eNAMPT and which cell type mainly contributes to the regulation of circulating eNAMPT levels remain unclear at this moment. Whatever its major source is, significant amounts of eNAMPT exist in mouse and human blood circulation, and circulating eNAMPT mainly takes the enzymatically active dimer form in normal human serum [45,50]. Based on these findings, it has been proposed that eNAMPT contributes to extracellular biosynthesis of NMN that is distributed to all tissues and organs to promote NAD biosynthesis at a systemic level [12,15]. Indeed, major metabolic tissues can rapidly incorporate NMN from blood circulation and utilize it for NAD biosynthesis (our unpublished results).
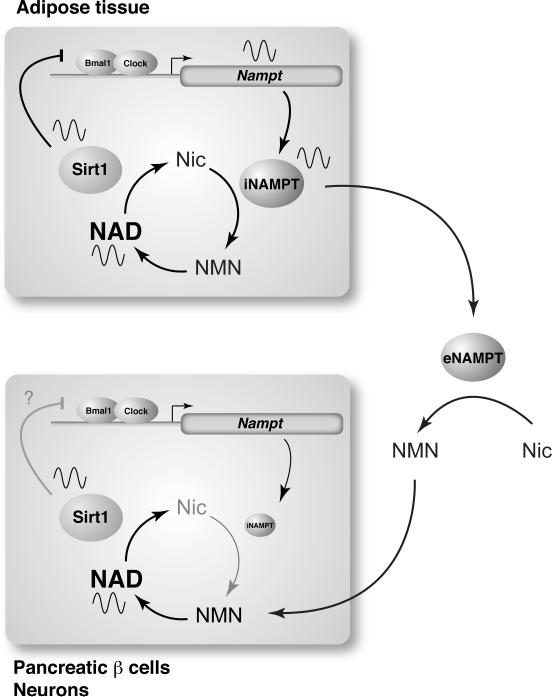
Recent studies have clearly demonstrated the tight functional connection between NAMPT-mediated NAD biosynthesis and sirtuins, particularly SIRT1, in a number of different cell types, including pancreatic β cells, vascular smooth muscle cells, skeletal myoblasts, cardiac myocytes, granulocytes, and others [11,13]. In these cell types, the NAMPT-SIRT1 pathway regulates metabolic response, cellular differentiation and life span, cell death, and other important biological events. Among these findings, the most important to understand the system dynamics of the NAD World is the discovery that NAMPT-mediated NAD biosynthesis and SIRT1 comprise a novel feedback loop in which NAD functions as a “metabolic oscillator” and regulates the core circadian clock machinery through SIRT1 and CLOCK/BMAL1, a key clock regulatory complex (Figure 2) [51,52]. In this feedback loop, levels of NAMPT and NAD display circadian oscillations that are regulated by CLOCK/BMAL1 in peripheral tissues, such as the liver and WAT. This NAD oscillation leads to periodic activation of SIRT1, which represses CLOCK/BMAL1-mediated transcription of clock target genes [53], including Nampt itself (Figure 2) [51,52]. Therefore, NAMPT-NAD and SIRT1-CLOCK/BMAL1 create an interlocked transcriptional-enzymatic feedback loop. Given that the core molecular clock machinery is one of the most powerful modifiers of metabolism [54] and also that the importance of physiological rhythmicity has been implicated for the regulation of metabolism and aging in the idea of “the aging clock” [55], this discovery has provided the first evidence for the significance of the NAMPT-SIRT1 pathway in the potential connection between physiological rhythmicity, metabolism, and aging in the NAD World [13]. Further investigation is required to examine whether this particular rhythmicity driven by the NAMPT-SIRT1 pathway plays an important role in the regulation of aging and possibly longevity in mammals.
Figure 2.
A circadian oscillatory feedback loop regulated by NAMPT, SIRT1, and CLOCK/BMAL1 and a possible functional interplay between adipose tissue and two frailty tissues in the NAD World, pancreatic β cells and neurons. NAMPT and NAD levels display circadian oscillations that are regulated by CLOCK/BMAL1 in peripheral tissues, such as the liver and WAT. This NAD oscillation periodically activates SIRT1, which represses CLOCK/BMAL1-mediated transcription of clock target genes, including Nampt itself, completing an interlocked transcriptional-enzymatic feedback loop involving NAMPT-NAD and SIRT1-CLOCK/BMAL1. In pancreatic β cells and central neurons, intracellular NAMPT (iNAMPT) levels are so low [45] that they may not be able to drive the NAMPT-NAD-SIRT1-dependent circadian feedback loop. However, their NAD oscillation might be generated by incorporating NMN that is likely synthesized from nicotinamide (Nic) by extracellular NAMPT (eNAMPT) that could be periodically secreted from adipose tissue.
Frailty points in the NAD World and the hierarchical view of mammalian aging
Every system has certain frailty points that play an important role in determining the robustness of the system. In the concept of the NAD World, critical frailty points are the tissues and organs that have very low levels of iNAMPT and therefore must rely on circulating NMN to maintain sufficient NAD biosynthesis to function. Pancreatic β cells and neurons, both of which have very low levels of iNAMPT (Figure 2) [45, our unpublished observation], are likely the most important frailty tissues whose functional defects have serious influences on many other tissues and affect the robustness of our physiological system. Indeed, a decrease in NAMPT-mediated systemic NAD biosynthesis causes impaired glucose tolerance in Nampt heterozygous mice, due to significant reduction in glucose-stimulated insulin secretion (GSIS) in pancreatic β cells [45]. These defects can be completely ameliorated by administering NMN, providing strong support to the notion that pancreatic β cells are one of the significant frailty points that are susceptible to alterations in NAMPT-mediated systemic NAD biosynthesis [15,45]. The susceptibility of pancreatic β cells to reduced NAMPT-mediated systemic NAD biosynthesis has also been demonstrated during aging, causing an age-associated decrease in SIRT1 activity and GSIS in vivo [56]. Remarkably, NMN administration can also restore GSIS in aged mice, providing further support to the frailty of pancreatic β cells in the NAD World [56].
Similarly, the brain (neurons) is likely another critical frailty point in the NAD World. Given that SIRT1 regulates memory and synaptic plasticity in the hippocampus [21,22] and neurobehavioral adaptation in the hypothalamus [31,57], it is conceivable that age-associated reduction in systemic NAD biosynthesis could reduce SIRT1 activity in these regions and cause neurological problems, including dementia and neurobehavioral complications called “the anorexia of aging” [58], in the elderly. If this is the case, NMN administration might also be able to restore normal NAD biosynthesis and ameliorate these neurological problems. Further investigation is currently underway for the pathophysiological significance of systemic NAD biosynthesis and the application of NMN for the regulation of brain function.
The consideration of critical frailty points in the NAD World also provides insight into the hierarchical regulatory system of mammalian aging. When systemic NAD biosynthesis declines due to nutritional and environmental perturbations over time, these frailty points would be the first to respond to this decline and start having functional defects due to insufficient NAD biosynthesis and thereby reduced SIRT1 activity. Functional defects in pancreatic β cells and central neurons cause problems in other peripheral tissues through insulin secretion and central metabolic regulation, triggering the gradual deterioration of physiological robustness at a systemic level. This cascade of robustness breakdown triggered by a decrease in systemic NAD biosynthesis is an important underlying process during aging. In this regard, the concept of the NAD World defines aging as the process in which organismal robustness gradually shifts and eventually breaks down according to a functional hierarchy determined by the susceptibility to systemic NAD biosynthesis. Until recently, aging has been considered as a plethora of random pathophysiological alterations that cause global functional decline. However, the paradigm of the NAD World suggests that there must be a definable hierarchical system structure for the control of aging and longevity in mammals.
Dissecting the system dynamics of the NAD World
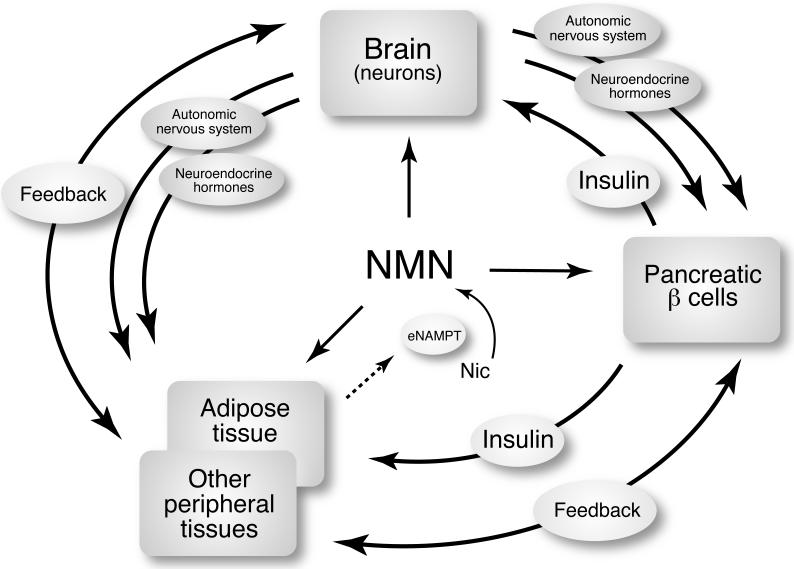
These features of the NAD World, as discussed above, provide important clues to address the three fundamental questions posed at the beginning of this article. Regarding which organs and tissues play dominant roles in the systemic regulation of mammalian aging, the susceptibility to systemic NAD biosynthesis defines such specific tissues as critical frailty points in the NAD World. Even though there must be other tissues that are very sensitive to changes in systemic NAD biosynthesis, it is intriguing that pancreatic β cells and central neurons share a similar weakness in this particular aspect. Both tissues have broad capabilities of regulating the functions of other tissues through humoral and neural communications, placing these two frailty points at the top of the aging-controlling hierarchy as potential “control centers” of aging (Figure 3). Consistent with this idea, it has been demonstrated that a specific subset of neurons, some of which have a capability of producing insulin-like peptides, plays a critical role in connecting metabolic regulation to aging regulation in worms and flies [59]. Thus, it is conceivable that the putative role of both cell types as “control centers” of aging is evolutionarily conserved for the connection between metabolic and aging regulatory mechanisms. In mammals, SIRT1 in pancreatic β cells and the hypothalamus plays essential roles in regulating their capabilities of communicating with other tissues through insulin secretion [33], neuroendocrine function [60], and possibly autonomic neural networks [31,57]. Therefore, it will be of great importance to test whether enhancing SIRT1 function in these frailty points might improve the systemic robustness of the NAD World over time and eventually promote longevity in mammals.
Figure 3.
The aging-controlling hierarchical structure and potential “control centers” of aging in the NAD World. Pancreatic β cells and central neurons, two major frailty points in the NAD World, determines the hierarchical regulatory cascade for mammalian aging and longevity by communicating with other tissues through insulin, neuroendocrine hormones, and autonomic nervous system. It is likely that other peripheral tissues also send feedback signals to these “control centers.” Adipose tissue might function as a critical modulator that coordinates NAD biosynthesis throughout the body and controls the stability of the NAD World by secreting eNAMPT to blood circulation. See text for details.
The idea that pancreatic β cells and central neurons function as potential “control centers” of aging immediately suggests that insulin, neuroendocrine hormones, and autonomic nervous system are likely important communication tools for these tissues to control the process of aging and eventually longevity in mammals (Figure 3). Indeed, studies on Ames and Snell dwarf mice, Ghrhr (growth hormone releasing hormone receptor) mutant mice, and the growth hormone receptor/binding protein (GHR/BP)-deficient mice have firmly established the importance of the neuroendocrine axis, the hypothalamic-pituitary axis in particular, for the regulation of mammalian aging and longevity [61]. The significance of the insulin-secreting capability of pancreatic β cells and the function of autonomic nervous system for the control of mammalian aging and longevity still remains unclear. Nonetheless, it has been reported that fat-specific insulin receptor knockout (FIRKO) mice show median and maximal lifespan extension [62], suggesting that the interplay between pancreatic β cells and adipose tissue is important for the regulation of mammalian aging and longevity. Additionally, transgenic mice overexpressing the uncoupling protein 2 in orexin/hypocretin neurons have been reported to show increased median lifespan [63]. Because orexin signaling is critical for the regulation of autonomic nervous system [64], it is likely that the communication between the hypothalamus and autonomic nervous system contributes to the regulation of aging and longevity. In this regard, it is of great interest to examine whether brain-specific SIRT1-overexpressing (BRASTO) transgenic mice, which exhibit increased activation of orexin type 2 receptor-positive neurons in the DMH and LH in response to acute and chronic energy limitation [31], can show significant lifespan extension compared to their control littermates.
Lastly, what molecular regulators and signaling pathways coordinate such a regulatory network at a systemic level? These regulators and signaling pathways must contribute to the stability of the NAD World. Given that adipose tissue actively secretes eNAMPT that has significantly higher enzymatic activity compared to iNAMPT [45], the regulation of the extent and the timing of eNAMPT secretion by adipose tissue might be a critical determinant for the proper coordination of NAD biosynthesis throughout the body (Figure 3). Particularly, the regulation of eNAMPT secretion is important for pancreatic β cells and central neurons, two major frailty points in the NAD World, to maintain their normal NAD biosynthesis (Figure 2). Furthermore, if adipose tissue is also capable of propagating its internal circadian rhythm of iNAMPT expression to the secretion of eNAMPT and thereby the extracellular biosynthesis of NMN, NAD biosynthesis and SIRT1 activity in those frailty tissues might be synchronized to display circadian oscillation even when their iNAMPT levels are not enough to drive their own NAD oscillation (Figure 2). This systemic synchronization of NAD biosynthesis might play a critical role in coordinating SIRT1-mediated metabolic responses in a circadian rhythm-dependent manner, contributing to the stability of the NAD World against fluctuating nutritional and environmental perturbations [13]. In this regard, adipose tissue might function as a critical modulator that determines the stability of the NAD World through the secretion of eNAMPT (Figure 3). We are currently analyzing the mechanism of eNAMPT secretion in adipose tissue and testing the idea regarding the physiological significance of eNAMPT in the NAD World.
Perspectives
The importance of the concept of the NAD World is to provide a foundation to analyze the system dynamics of the regulatory mechanism for aging and longevity in mammals. Additionally, this concept raises another important issue of possible trade-offs between robustness and frailty. Based on the theory of highly optimized tolerance (HOT) architecture [65,66], systems that have evolved to comprise a greater scale of complexity are optimized for specific perturbations but are also inevitably susceptible to unexpected perturbations. This trade-off between robustness and frailty is an inherent, unavoidable feature of all complex systems, including biological systems. Therefore, it is possible that certain efforts of enhancing the robustness of the system could further sensitize it to some specific perturbations [67]. This consideration is becoming more important, particularly when enthusiasm and demand to achieve healthy lifespan, namely “healthspan,” is getting higher in all countries whose societies are heavily aging. Therefore, it is critical and urgent to have better understanding the structure and the dynamics of the regulatory system for aging and longevity in mammals. Once we start understanding these aspects, then we might be able to design more effective anti-aging interventions that can optimize the balance between robustness and frailty throughout our body. Based on the concept of the NAD World, administering NMN at an appropriate timing or age might help achieve this healthy balance by enhancing and coordinating NAD biosynthesis and SIRT1 activity. Although more extensive research is necessary to further clarify this concept, dissecting the system control of the NAD World will provide the first step towards the brighter future of our aging world.
Acknowledgments
I apologize to those whose work is not cited due to space limitations. I thank members of the Imai lab for critical reading and comments on this manuscript. This work was supported in part by the National Institute on Aging (AG02150), the National Heart, Lung, and Blood Institute (HL097817), the Ellison Medical Foundation, the Longer Life Foundation, and the Diabetes Research and Training Center to S.I. and by institutional support from the Washington University Nutrition Obesity Research Center (P30DK056341) and the Washington University Diabetes Research and Training Center (DK20579). S.I. serves as a scientific advisory board member for Sirtris, a GSK company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carone G, Costello D. Finance and Development. Vol. 43. International Monetary Fund; 2006. Can Europe afford to grow old? [Google Scholar]
- 2.Hewitt PS. Global Action on Aging. New York: 2002. Depopulation and ageing in Europe and Japan: The hazardous transition to a labor shortage economy. [Google Scholar]
- 3.OECD . In Working Papers No. 477 (Economics Department) Organisation for Economic Co-operation and Development; Paris, France: 2006. Projecting OECD health and long-term care expenditures: What are the main drivers? [Google Scholar]
- 4.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 5.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell. Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim. Biophys. Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol. Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garten A, Petzold S, Korner A, Imai S, Kiess W. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 2009;20:130–138. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S. Nicotinamide phosphoribosyltransferase (Nampt): a link between NAD biology, metabolism, and diseases. Curr. Pharm. Des. 2009;15:20–28. doi: 10.2174/138161209787185814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai S. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim. Biophys. Acta. 2010;1804:1584–1590. doi: 10.1016/j.bbapap.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imai S. From heterochromatin islands to the NAD World: A hierarchical view of aging through the functions of mammalian Sirt1 and systemic NAD biosynthesis. Biochim. Biophys. Acta. 2009;1790:997–1004. doi: 10.1016/j.bbagen.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai S. The NAD World: a new systemic regulatory network for metabolism and aging - Sirt1, systemic NAD biosynthesis, and their importance. Cell. Biochem. Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J. Sirtuins: the ‘magnificent seven’, function, metabolism and longevity. Ann. Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 17.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Min SW, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michan S, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erion DM, et al. SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc. Natl. Acad. Sci. USA. 2009;106:11288–11293. doi: 10.1073/pnas.0812931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nie Y, Erion DM, Yuan Z, Dietrich M, Shulman GI, Horvath TL, Gao Q. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 27.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picard F, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/EBPalpha transcriptional complex. J. Biol. Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- 36.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 37.Miller ES, et al. Complete genome sequence of the broad-host-range vibriophage KVP40: comparative genomics of a T4-related bacteriophage. J. Bacteriol. 2003;185:5220–5233. doi: 10.1128/JB.185.17.5220-5233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revollo JR, Grimm AA, Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 40.Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci. 2009;14:410–431. doi: 10.2741/3252. [DOI] [PubMed] [Google Scholar]
- 41.Nikiforov A, Doelle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: From entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 2011 doi: 10.1074/jbc.M110.213298. Epub on April 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat. Struct. Mol. Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- 43.Kim MK, et al. Crystal Structure of Visfatin/Pre-B Cell Colony-enhancing Factor 1/Nicotinamide Phosphoribosyltransferase, Free and in Complex with the Anti-cancer Agent FK-866. J. Mol. Biol. 2006;362:66–77. doi: 10.1016/j.jmb.2006.06.082. [DOI] [PubMed] [Google Scholar]
- 44.Rongvaux A, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J. Immunol. 2008;181:4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 45.Revollo JR, et al. Nampt/PBEF/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 47.Friebe D, et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia. 2011 doi: 10.1007/s00125-010-2042-z. Epub on Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garten A, Petzold S, Barnikol-Oettler A, Korner A, Thasler WE, Kratzsch J, Kiess W, Gebhardt R. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is constitutively released from human hepatocytes. Biochem. Biophys. Res. Commun. 2009 doi: 10.1016/j.bbrc.2009.11.066. Epub on Nov 11. [DOI] [PubMed] [Google Scholar]
- 49.Skokowa J, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat. Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 50.Körner A, Garten A, Bluher M, Tauscher R, Kratzsch J, Kiess W. Molecular characteristics of serum visfatin and differential detection by immunoassays. J. Clin. Endocrinol. Metab. 2007;92:4783–4791. doi: 10.1210/jc.2007-1304. [DOI] [PubMed] [Google Scholar]
- 51.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramsey KM, et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everitt AV. The hypothalamic-pituitary control of ageing and age-related pathology. Exp. Gerontol. 1973;8:265–277. doi: 10.1016/0531-5565(73)90039-9. [DOI] [PubMed] [Google Scholar]
- 56.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in β cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramadori G, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donini LM, Savina C, Cannella C. Eating habits and appetite control in the elderly: the anorexia of aging. Int. Psychogeriatr. 2003;15:73–87. doi: 10.1017/s1041610203008779. [DOI] [PubMed] [Google Scholar]
- 59.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 60.Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartke A. Single-gene mutations and healthy ageing in mammals. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 63.Conti B, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front. Neuroendocrinol. 2003;24:141–150. doi: 10.1016/s0091-3022(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 65.Carlson JM, Doyle J. Highly optimized tolerance: Robustness and design in complex systems. Phys. Rev. Lett. 2000;84:2529–2532. doi: 10.1103/PhysRevLett.84.2529. [DOI] [PubMed] [Google Scholar]
- 66.Kitano H. Towards a theory of biological robustness. Mol Syst Biol. 2007;3:137. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imai S. SIRT1 and caloric restriction: an insight into possible trade-offs between robustness and frailty. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:350–356. doi: 10.1097/MCO.0b013e32832c932d. [DOI] [PMC free article] [PubMed] [Google Scholar]