Abstract
Introduction
Although most smokers diagnosed with lung cancer report that they want to quit smoking, many do not succeed. Smokers who quit when lung cancer is diagnosed have improved treatment efficacy, quality of life, and survival. Effective smoking cessation interventions targeted to thoracic oncology patients are needed.
Design and Methods
This pilot study examined the feasibility and potential efficacy of a 12-week program that combined smoking cessation counseling with varenicline. 7-day point prevalence tobacco abstinence rates at the end of treatment were compared to a usual care control group. From 01/08-08/09, patients with a diagnosed or suspected thoracic malignancy were recruited at their initial visit to a thoracic surgeon or thoracic oncologist at Massachusetts General Hospital.
Results
Of 1130 patients screened, 187 (17%) were current smokers, and an additional 66 (6%) reported quitting within the past 6 months. 116 (67%) of smokers were eligible, and 49 (42%) of eligible smokers enrolled (control group n=17, intervention group n=32). Intervention participants completed a median of 9 counseling sessions; 50% of intervention participants completed the full varenicline course. At 12-week follow-up, biochemically-validated 7-day point prevalence tobacco abstinence rates were 34.4% in the intervention group vs. 14.3% in the control group (OR = 3.14, 95% CI = .59-16.62, p = .18).
Conclusion
Our findings support the feasibility and acceptability of this program. At the end of treatment, quit rates were higher in the control group. Further testing is indicated to establish efficacy of this treatment package in a randomized clinical trial.
Cigarette smoking is the major cause of lung cancer, and over 219,000 new cases of lung cancer are diagnosed each year in the United States.1 Approximately 20-30% of lung cancer patients are smoking at diagnosis.2, 3 The majority of patients who receive a lung cancer diagnosis report that they want to quit smoking, but many are unable to do so.4-6 This is unfortunate since quitting at the time of diagnosis can improve quality of life, improve chances for treatment efficacy, reduce treatment complications, reduce risk of recurrence and secondary tumors, and increase chances of long-term survival.2, 7-13
There has never been an RCT for smoking cessation targeted to lung cancer patients; there have been few randomized controlled smoking cessation trials for cancer patients in general, and these trials have not demonstrated a significant intervention effect.14 This lack of demonstrated effectiveness in tobacco treatment trials for cancer patients could be due to several factors including low power to detect differences due to small sample sizes, lack of integration into the cancer treatment setting, and delayed smoking cessation treatment initiation.15-19 Another potential explanation for the lack of success of smoking cessation programs for cancer patients is that most programs tested did not combine behavioral and pharmacologic treatment strategies, as the USPHS Treating Tobacco Use and Dependence Clinical Practice Guideline recommends.20, 21
Varenicline, a partial agonist of the alpha-4 beta-2 nicotinic acetylcholine receptor, received FDA approval in 2006 as a smoking cessation aid and was recommended in the 2008 USPHS guideline as a first-line pharmacologic treatment option.22 The efficacy of varenicline for smoking cessation in cancer patients has not been reported.
The objective of our study was to assess the feasibility and potential efficacy of a smoking cessation treatment program that combined behavioral counseling with varenicline and was targeted to patients upon their entry into thoracic clinics.
Methods
This study was approved by the Massachusetts General Hospital Institutional Review Board.
Study design and patient recruitment
This study used a non-randomized design, which began with a usual care control group enrollment period that was followed by an intervention group enrollment period. We recruited control group participants (January 2008-June 2008) and intervention group participants (June 2008-August 2009) from patients referred to thoracic surgery and oncology clinics at the Massachusetts General Hospital (MGH) in Boston, MA.
Eligibility
Inclusion criteria for both groups were the following: 1) suspected diagnosis of thoracic cancer; 2) smoked a cigarette in the past two weeks; 3) spoke English; 4) no metastatic disease at initial presentation; and 5) considered medically eligible by their surgeon or oncologist. In addition, a patient had to be willing to take varenicline in order to be eligible for the intervention group; if a patient who was otherwise eligible for the intervention group was taking nicotine replacement therapy or bupropion, he/she had to be willing switch to varenicline. Patients with severe psychiatric illness as documented in the medical record (e.g., severe major depressive disorder, active psychosis) were cleared by their psychiatrist or primary care physician before participating in the study. We excluded patients known to have metastatic thoracic cancer prior to enrollment; although quitting smoking can improve physical symptoms such as ease of breathing in these patients, we felt that they would require a different focus for a smoking cessation intervention.
Enrollment and Assessment Procedures
Smoking status of patients seen in participating clinics was identified by chart review and a clinic intake form. Eligible patients provided written informed consent and completed a baseline assessment. Two and 12 weeks after enrollment, participants completed a follow-up survey. Smoking status of reported nonsmokers was biochemically confirmed by obtaining a saliva sample to test for cotinine, a nicotine metabolite. Participants who were taking nicotine replacement therapy at the time of an assessment provided an expired air carbon monoxide sample. Participants were paid $20 for completing each of the assessments.
Intervention
Intervention participants were provided with a 12-week program consisting of varenicline (1mg bid, with initial titration up over week 1) and smoking cessation counseling targeted to the issues of thoracic cancer patients. We had proposed to offer 7 counseling sessions but were flexible in offering additional sessions when needed. The counseling was delivered by a certified Tobacco Treatment Counselor using motivational interviewing (MI) techniques.23 MI is a counseling style that seeks to enhance individuals' readiness to change behavior by emphasizing a smoker's choice, personal responsibility, and self-efficacy.
The initial counseling session took place during the baseline visit or by telephone within 48 hours of study enrollment and focused on the cancer-specific benefits of quitting smoking, forming a quit smoking plan, and instructions on study medication use and adherence. Follow-up counseling sessions were conducted by telephone, or in person when possible, with standardized counseling modules. All sessions were structured according to the 5 As brief counseling model (Ask, Advise, Assess, Assist, Arrange follow-up) and included cancer-specific and general smoking cessation and relapse prevention topics.22 Intervention content was selected based on the principal investigator's previous work,24, 25 published smoking cessation work with cancer patients, and lessons learned about the population during the control group observation period (e.g., high levels of environmental tobacco smoke). We targeted modifiable factors that previous research has shown associated with cancer patients' quitting and staying quit: emotional distress (lower anxiety and depression scores) and smoking and cancer beliefs (higher perceived risk of cancer recurrence, higher self-efficacy to quit, higher quit motivation).5, 17, 18, 20, 26-33
Measures
Sociodemographics
At baseline, age, gender, race/ethnicity, education, marital status, and employment were assessed with a questionnaire and medical record review.
Cancer and medical history
Previous cancer history and family history of lung disease, cancer, and heart disease were collected from the clinic screening form. Cancer status variables (diagnosis, stage, treatment modalities) were abstracted from the medical records.
Psychosocial Factors
Emotional support was measured at baseline and follow-up using 4 items from the emotional/informational scale of the Medical Outcomes Study social support survey, a reliable scale (alpha=.96) that has been widely used with cancer patients.34-37 Smoking-specific support was measured at baseline and follow-up using a single, Likert-type, response item. Depression and anxiety symptoms were measured at baseline and follow-up via the Hospital Anxiety and Depression Scale (HADS),38 a 14-item assessment of mood with depression and anxiety subscales that has been well tested in cancer patients.35, 38-40 Participants with scores >8 on the depression or anxiety subscales were considered to have elevated levels of depressed mood or anxiety. Participants rated current levels of pain and stress at baseline and follow-up on a 0-10 scale.41
Smoking Characteristics
Smoking history (years smoked, past cessation treatment) was assessed in the baseline questionnaire. Current nicotine dependence was measured at baseline using the 2-items from the Fagerström Test for Nicotine Dependence (FTND) 42-44 which have been found to account for the bulk of the predictive validity of the FTND: number of cigarettes per day and time to first cigarette after waking. Smoking environment (live with a smoker, home policy) was assessed at baseline and follow-up. Participants were asked to rate, on a 10-point scale, their attitudes about quitting smoking (the importance of quitting smoking, confidence in ability to quit) and knowledge about the benefits of quitting smoking following a cancer diagnosis.
Feasibility
Feasibility was assessed by rates of study eligibility, recruitment, retention, and intervention adherence (counseling and medication).
Smoking outcome
7-day point prevalence abstinence (“Have you smoked a cigarette, even a puff, in the past 7 days?”) was assessed at 2 and 12-week follow-up points. Self reported abstinence was confirmed only if a salivary cotinine level was < 15 ng/ml or an expired carbon monoxide measurement was <10 ppm. Some participants returned saliva samples with insufficient quantity for continine assay. For the primary analysis, participants who did not complete the survey or return analyzable saliva samples were considered to be smokers. A secondary analysis counted self-reported nonsmokers who returned saliva samples with insufficient quantity for assay as nonsmokers.
Data Analysis
All data analyses were conducted using SPSS 18.0. Using chi-square tests and one-way ANOVAs, we compared the sociodemographics and smoking histories of eligible participants who enrolled to those who declined to enroll to determine if study participants were similar to the MGH thoracic patient population. A similar analysis explored the comparability of the intervention and usual care control groups. The primary outcome measure, biochemically-confirmed 7-day point prevalence tobacco abstinence as defined above, was compared between groups using a univariate logistic regression. Analyses examined variables associated with 12- week smoking status with participants from both treatment groups. Logistic regressions were conducted with baseline characteristics of interest as the independent variable and then with changes in intervention targets predicting 12-week smoking status.
Results
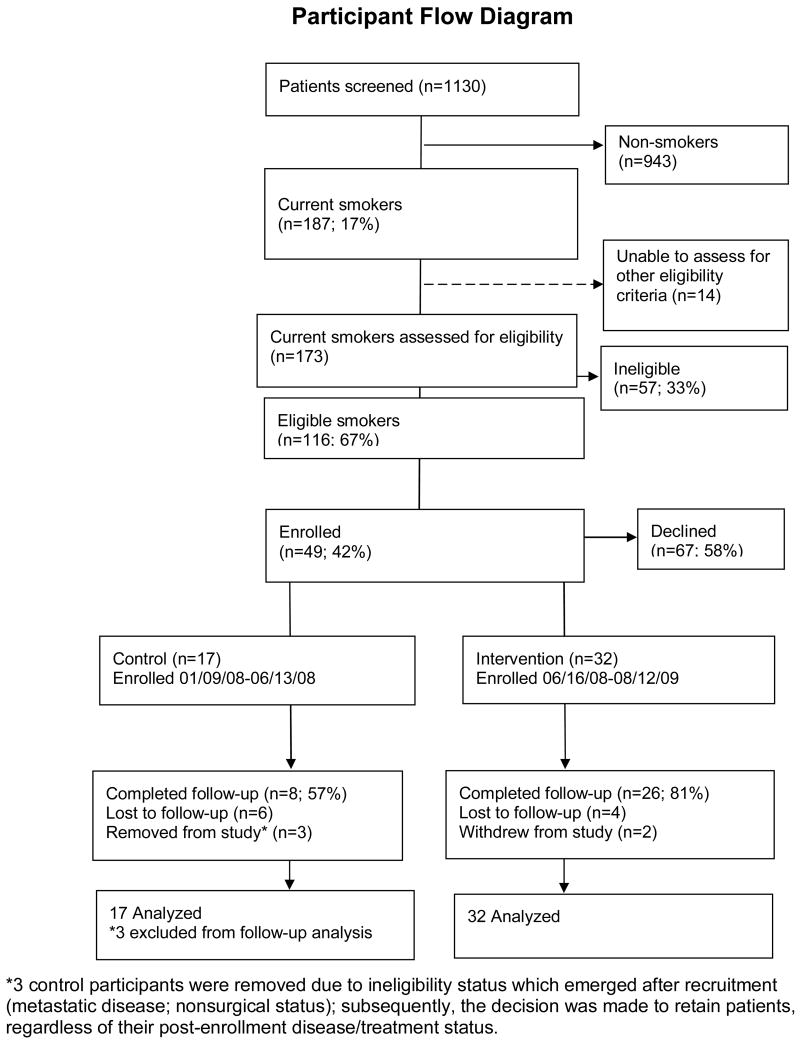
Recruitment (Figure 1)
Figure 1. Study recruitment and retention.
We screened 1130 patients in the MGH thoracic surgery and oncology clinics; 187 (17%) identified themselves as smokers, and an additional 66 (6%) reported quitting within the past six months. Of the current smokers in whom eligibility could be assessed, 116 (67%) were eligible, and 49 (42%) enrolled. The most common reason for ineligibility was having metastatic cancer. The main reason for declining participation was reluctance to take varenicline. Other reasons for refusal included preferring to quit on one's own, having no interest in quitting, feeling overwhelmed, or not having time to participate. Those who enrolled did not differ from those who refused in terms of sociodemographic characteristics, past medical history, or past use of nicotine replacement therapy or bupropion (all p >.05). However, those who enrolled were significantly more likely to report using or having used varenicline (34.8% vs. 12.8%; p =.02) and marginally more likely to have used smoking cessation counseling in the past (31.0% vs. 14.8%; p =.06). Enrollees had higher rates of anxiety (55.9% vs. 26.1%; p = .007).
Participants
Tables 1 and 2 display the characteristics of the 49 participants; 59.2% were female, 87.8% were white, and the mean age was 57.7 years. The intervention and control group participants did not differ significantly in sociodemographic factors, smoking history, or medical history. Most participants were long-term, highly dependent smokers with low levels of confidence in their ability to quit. Only 31.0% had ever used smoking cessation counseling, and 64.4% had ever used pharmacotherapy. Participants generally agreed that quitting would reduce the risk of treatment complications and the risk of future tumors.
Table 1. Sociodemographic, medical, and psychosocial characteristics of participants.
| Variable | All (N=49) |
Control (N=17) |
Intervention (N=32) |
p-value |
|---|---|---|---|---|
| SOCIODEMOGRAPHICS | ||||
|
| ||||
| Age M (SD) | 57.7 (12.4) | 58.0 (10.5) | 57.5 (13.4) | 0.90 |
| Female (%) | 59.2 | 52.9 | 62.5 | 0.56 |
| White, non Hispanic (%) | 87.8 | 94.1 | 84.4 | 0.65 |
| Education (%) | ||||
| <High School | 12.2 | 5.9 | 15.6 | 0.65 |
| High School/GED | 36.7 | 35.3 | 37.5 | |
| >High School | 51.0 | 58.8 | 46.9 | |
| Marital status (%) | ||||
| Married/living with a partner | 49.0 | 52.9 | 46.9 | 0.69 |
| Widowed/divorced/separated | 32.7 | 35.3 | 31.3 | |
| Never married | 18.4 | 11.8 | 21.9 | |
| Employed (%) | 49.0 | 58.8 | 43.8 | 0.38 |
| MEDICAL HISTORY | ||||
| Past history of cancer (%) | 18.9 | 23.5 | 15.0 | 0.68 |
| Thoracic cancer diagnosis (%) | 66.0 | 58.8 | 70.0 | 0.53 |
| Cancer Treatment (%) | ||||
| Surgery only | 57.6 | 72.7 | 50.0 | 0.45 |
| Chemotherapy +/or radiation | 30.3 | 18.2 | 36.4 | |
| Surgery + chemotherapy/radiation | 12.1 | 9.1 | 13.6 | |
| PSYCHOSOCIAL FACTORS | ||||
| Emotional support M (SD)* | 3.5 (1.3) | 3.8 (1.5) | 3.4 (1.2) | 0.26 |
| Smoking-specific support M (SD)* | 3.7 (0.67) | 3.8 (0.4) | 3.6 (0.8) | 0.40 |
| HADS Anxiety subscale ≥8 (%) | 63.3 | 52.9 | 68.8 | 0.34 |
| HADS Depression subscale ≥8 (%) | 34.7 | 35.3 | 34.4 | 1.00 |
| Stress rating M (SD)* | 6.4 (3.1) | 6.4 (3.2) | 6.4 (3.0) | 0.98 |
| Pain rating M (SD)* | 3.3 (3.3) | 3.8 (3.6) | 3.0 (3.1) | 0.44 |
KEY
Emotional support, 1-5 scale; 1= “none of the time,” 5= “all of the time”
Smoking-specific support, 1-4 scale; 1= “none,” 4= “a lot”
Pain and stress, 0-10 scale; 0= “no pain/stress,” 10= “worst pain/stress imaginable”
Hospital Anxiety and Depression Scale (HADS), >8 on the depression or anxiety subscales = elevated levels of depressed mood or anxiety
Table 2. Baseline smoking characteristics.
| Variable SMOKING CHARACTERISTICS |
All (N=49) |
Control (N=17) |
Intervention (N=32) |
p-value |
|---|---|---|---|---|
| Years smoked M (SD) | 37.8 (13.9) | 34.1 (13.7) | 39.7 (13.8) | 0.18 |
| Cigarettes per day M (SD) | 16.4 (11.6) | 15.4 (9.0) | 17.0 (12.8) | 0.65 |
| Smoke within 30 minutes of waking (%) | 76.6 | 73.3 | 78.1 | 0.72 |
| Past cessation treatment: % | ||||
| Any counseling | 31.0 | 41.7 | 26.7 | 0.46 |
| Any pharmacotherapy | 64.4 | 73.3 | 60.0 | 0.38 |
| Nicotine replacement therapy | 44.4 | 50.0 | 41.9 | 0.75 |
| Varenicline | 34.8 | 43.8 | 30.0 | 0.52 |
| Bupropion | 25.0 | 33.3 | 21.4 | 0.45 |
| Live with a smoker (%) | 34.7 | 35.3 | 34.4 | 0.95 |
| Allows smoking in home (%) | 87.8 | 94.1 | 84.4 | 0.33 |
| Importance of quitting M (SD)* | 8.9 (2.5) | 8.1 (3.1) | 9.3 (2.0) | 0.12 |
| Confidence in ability to quit M (SD)* | 5.9 (2.8) | 5.35 (3.0) | 6.25 (2.6) | 0.28 |
| Knowledge about smoking & cancer M (SD)* | ||||
| Quitting will reduce treatment complications | 8.9 (2.0) | 8.6 (2.6) | 9.0 (1.8) | 0.56 |
| Quitting will result in living longer | 9.3 (1.5) | 9.4 (1.4) | 9.2 (1.5) | 0.73 |
| Quitting will reduce likelihood of future tumors | 8.9 (2.0) | 8.7 (1.9) | 9.0 (2.1) | 0.62 |
KEY
Importance of quitting, 1-10 scale; 1= “not at all”, 10= “very important”
Confidence in ability to quit, 1-10 scale; 1= “not at all”, 10= “very confident”
Knowledge about smoking and cancer, 0-10 scale; 0= “not at all,” 10= “very much”
Intervention Adherence
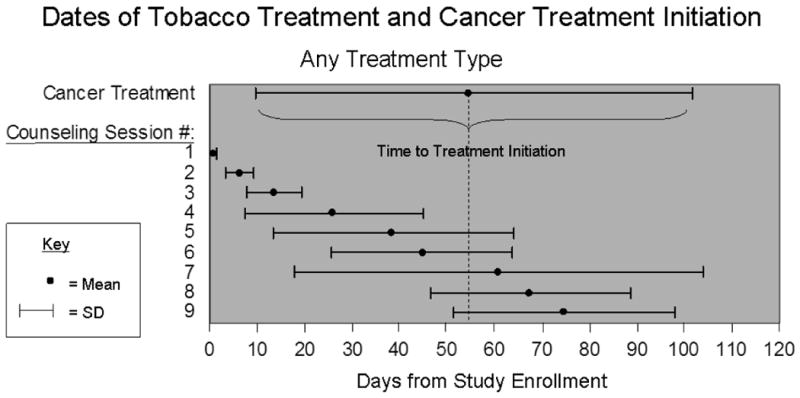
Participants in the intervention group completed a median of 9 counseling sessions and had a median of 88 minutes of total counseling contact time. The average initial contact was approximately 20 minutes and follow-up sessions were approximately 10 minutes. Participants received an average of 6 counseling sessions before initiating cancer treatment, which started a mean of 51 (SD=45.2) days after study enrollment (Figure 2). Half of participants who took varenicline completed the full treatment course, and 23.3% took the medication for 4-8 weeks. The most common side effect was nausea, reported by one-third of participants; 8 (26.7%) participants discontinued the medication due to side effects (7 abnormal dreams and/or nausea, 1 feelings of agitation/aggression).
Figure 2. Timeline of smoking cessation counseling sessions and cancer treatment.
Smoking cessation rates (Table 3)
Table 3. Smoking outcomes.
| ALL (n=46) |
INTERVENTON (n=32) |
CONTROL (n=14) |
OR (95% CI.) |
|
|---|---|---|---|---|
| 7-DAY POINT PREVALENCE TOBACCO ABSTINENCE | ||||
| 2 weeks | ||||
| Self-reported | 17/46 (37.0%) | 13/32 (40.6%) | 4/14 (28.6%) | 1.23 (.34-4.52) |
| + Cotinine-confirmed (version 1) | 16/46 (34.8%) | 12/32 (37.5%) | 4/14 (28.6%) | 1.50 (.38-5.86) |
| * Cotinine-confirmed (version 2) | 11/46 (23.9%) | 9/32 (28.1%) | 2/14 (14.3%) | 2.35 (.44-12.64) |
| 12 weeks | ||||
| Self-reported quit | 17/46 (37.0%) | 14/32 (43.8%) | 3/14 (21.4%) | 2.85 (.67-12.22) |
| Cotinine-confirmed (version 1) | 15/46 (32.6%) | 13/32 (40.6%) | 2/14 (14.3%) | 4.11 (.79-21.48) |
| * Cotinine-confirmed (version 2) | 13/46 (28.3%) | 11/32 (34.4%) | 2/14 (14.3%) | 3.14 (.59-16.62) |
Analysis with insufficient returned saliva samples counted as nonsmokers.
Analysis with insufficient returned saliva samples counted as smokers.
Self-reported nonsmokers complied with 97% of requests for saliva samples. Using the conservative method of considering participants with insufficient saliva samples as smokers (Table 3, version 2), cotinine-confirmed 7-day point prevalence abstinence rates were 28.1% in the intervention group vs. 14.3% in the control group (OR = 2.35, 95% CI = .44-12.64, p = .32) at 2 week follow up, and 34.4% in the intervention group vs. 14.3% in the control group (OR = 3.14, 95% CI = .59-16.62, p = .18) at 12 week follow-up. In a secondary analysis that considered participants with insufficient cotinine samples as nonsmokers (Table 3, version 1) cotinine-confirmed 7-day point prevalence abstinence rates were 37.5% in the intervention group vs. 28.6% in the control group (OR = 1.50, 95% CI = .38-5.86, p =.56) at 2 week follow-up, and 40.6% in the intervention group vs. 14.3% in the control group (OR = 4.11, 95% CI = .79-21.48, p =.09) at 12 week follow-up.
Characteristics associated with cotinine confirmed (version 2) 12 week abstinence across groups
Sociodemographic factors were not associated with abstinence at 12 weeks. Participants with lower baseline levels of depressive symptoms were more likely to be abstinent (OR = .81, 95% CI = .67-.98, p =.03). There were trends associating 12-week abstinence with smoking within 30 minutes of waking (OR= 3.86, 95% CI = .87-17.16, p =.08), believing that quitting smoking was important (OR = 1.28, 95% CI = .94-1.75, p =.11), and believing that continued smoking caused surgical complications (OR =1.56, 95% CI = .87-2.79, p=.14. Receiving a cancer diagnosis by the end of the study period (OR = 4.05, 95% CI = .77-21.26, p = .10) and having surgery (OR = 3.05, 95% CI = .78-1.96, p =.11) were marginally associated with 12 week abstinence. Time to treatment was not associated with 12 week abstinence (OR = .99, 95% CI = .98-1.01, p = .48). An increase in quit confidence from baseline to follow-up was associated with 12 week abstinence (OR = 1.74, 95% CI = 1.06-2.88, p =.03).
Discussion
The current study is, to our knowledge, the first controlled study of a combined behavioral and pharmacological tobacco treatment program targeting patients at their entry into thoracic surgery and thoracic oncology clinics. We found that the program was feasible and acceptable. Furthermore, the intervention produced higher biochemically-validated smoking cessation rates at the end of treatment, although the difference did not reach statistical significance due to the small sample size of this pilot study.
Our findings show that those who continue smoking following a lung cancer diagnosis are a challenging population with a long smoking history, high nicotine dependence, and low confidence to quit. This indicates that this particular population of smokers likely needs intensive tobacco treatment, preferably one that combines pharmacological support with extended counseling in order to achieve abstinence.
We were able to enroll almost half of eligible patients at their entry into the thoracic oncology setting, a rate similar to previous smoking cessation studies that enrolled patients following cancer treatment. 33 Enrolling as early as possible is critical because the closer to the time of diagnosis that smoking cessation treatment is delivered, the greater the health benefits, including reduced perioperative morbidity, and the higher the likelihood for continued abstinence.2, 4, 5, 32, 45 The main reasons for refusal, reluctance to take varenicline and wanting to quit unassisted, could represent quitting preferences, but these could also be proxies for not wanting to quit. Another factor to consider is that during the study enrollment period varenicline received a black box warning about psychiatric side effects, which may have caused some reluctance in smokers.46
We were able to engage patients in tobacco treatment during a vulnerable and critical period. The program length matched the USPHS recommended 90 minutes of contact time, but the number of contacts exceeded the USPHS minimum recommended number of sessions which are usually offered for telephone-delivered smoking cessation interventions.22, 24, 47 These patients seemed to need frequent, brief contacts and social support in order to promote tobacco abstinence.
Despite initial concerns that cancer patients might not tolerate varenicline due to side effects similar to cancer treatment side effects (e.g., nausea), participant adherence rates to varenicline were similar to NRT use in cancer patients and non-cancer patients. 48, 49 Similar to varenicline in the general population, nausea was the most common side effect, reported in one-third of participants.
This study had several limitations. The generalizability of the findings was limited by using only a single study site. The statistical power to detect differences was limited by the small sample size of this pilot study. Although the sample size cannot be increased in accordance to power calculations that would enable detection of a statistically significant difference, we believe the encouraging non-statistical trend provides a rationale for an adequately powered RCT. Its non-randomized design leaves open the potential for unmeasured confounding to effect results due to group dissimilarities.
Despite these limitations, our combined behavioral and varenicline intervention produced promising feasibility and potential efficacy results. In pursuit of a larger scale randomized trial to assess the efficacy of our counseling plus varenicline treatment, we recommend comparison of two treatment arms: an “intensive” counseling plus varenicline versus a “brief” counseling plus varenicline. A counseling only or varenicline only comparison group would not be in compliance with clinical practice guidelines. Additionally, a counseling only comparison would not build on this work which supported the tolerability of varenicline in this population, and, given the psychological and medical vulnerability of this population, it is not preferable to use a varenicline only comparison.
References
- 1.American Cancer Society. Cancer facts & figures 2009. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126(6):1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 3.Gritz ER. Rationale for treating tobacco dependence in the cancer setting. Conference presentation at: Treating Tobacco Dependence at the National Cancer Institute's Cancer Centers; 12/07/2009; Bethesda, MD. [Google Scholar]
- 4.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110(5):1199–202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 5.Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with stage I non-small-cell lung cancer. Cancer Cause Control. 1991;2:105–112. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 6.Sarna L. Smoking behaviors of women after diagnosis with lung cancer. J Nurse Scholarship. 1995;27(1):35–41. doi: 10.1111/j.1547-5069.1995.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 7.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP. Successes and failures of the teachable moment: Smoking cessation in cancer patients. Cancer. 2006;106(1):17–27. doi: 10.1002/cncr.21598. [DOI] [PubMed] [Google Scholar]
- 8.Garces YI, Schroeder DR, Nirelli LM, et al. Tobacco use outcomes among patients with head and neck carcinoma treated for nicotine dependence: A matched-pair analysis. Cancer. 2004;101(1):116–124. doi: 10.1002/cncr.20350. [DOI] [PubMed] [Google Scholar]
- 9.Dresler CM. Is it more important to quit smoking than which chemotherapy is used? Lung Cancer. 2003;39(2):119–124. doi: 10.1016/s0169-5002(02)00455-5. [DOI] [PubMed] [Google Scholar]
- 10.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med. 1993;119(5):383–390. doi: 10.7326/0003-4819-119-5-199309010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. lung cancer working cadre. J Natl Cancer Inst. 1997;89(23):1782–1788. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 12.Garces YI, Schroeder DR, Nirelli LM, et al. Second primary tumors following tobacco dependence treatments among head and neck cancer patients. Am J Clin Oncol. 2007;30(5):531–539. doi: 10.1097/COC.0b013e318059adfc. [DOI] [PubMed] [Google Scholar]
- 13.Klosky JL, Tyc VL, Hum A, et al. Establishing the predictive validity of intentions to smoke among preadolescents and adolescents surviving cancer. J Clin Oncol. 2010;28(3):431–436. doi: 10.1200/JCO.2008.21.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Moor JS, Elder K, Emmons KM. Smoking prevention and cessation interventions for cancer survivors. Semin Oncol Nurs. 2008;24(3):180–192. doi: 10.1016/j.soncn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Wewers ME, Jenkins L, Mignery T. A nurse-managed smoking cessation intervention during diagnostic testing for lung cancer. Oncol Nurs Forum. 1997;24(8):1419–1422. [PubMed] [Google Scholar]
- 16.Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients--influence of a smoking-related diagnosis: A pilot study. Heart Lung. 1994;23(2):151–6. [PubMed] [Google Scholar]
- 17.Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2(3):261–270. [PubMed] [Google Scholar]
- 18.Croghan GA, Croghan IT, Frost MH, et al. Smoking cessation interventions and post-operative outcomes in esophageal and lung cancer patients. Conference presentation at: 2005 Annual Meeting, Society for Research on Nicotine and Tobacco; 03/20/2005-03/23/2005; Prague, Czechoslovakia. [Google Scholar]
- 19.Griebel B, Wewers ME, Baker CA. The effectiveness of a nurse-managed minimal smoking-cessation intervention among hospitalized patients with cancer. Oncol Nurs Forum. 1998;25(5):897–902. [PubMed] [Google Scholar]
- 20.Gritz ER, Schacherer C, Koehly L, Nielsen IR, Abemayor E. Smoking withdrawal and relapse in head and neck cancer patients. Head Neck. 1999;21(5):420–427. doi: 10.1002/(sici)1097-0347(199908)21:5<420::aid-hed7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Lerman C, Patterson F, Berrettini W. Treating tobacco dependence: State of the science and new directions. J Clin Oncol. 2005;23(2):311–323. doi: 10.1200/JCO.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 22.Fiore MC, Jaen C, Baker T, et al. Treating Tobacco use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 23.Miller W, Rollnick S. Motivational Interviewing. New York: The Guilford Press; 1991. [Google Scholar]
- 24.Park ER, Puleo E, Butterfield RM, et al. A process evaluation of a telephone-based peer-delivered smoking cessation intervention for adult survivors of childhood cancer: The partnership for health study. Prev Med. 2006;42(6):435–42. doi: 10.1016/j.ypmed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Emmons KM, Butterfield RM, Puleo E, et al. Smoking among participants in the childhood cancer survivors cohort: The partnership for health study. J Clin Oncol. 2003;21(2):189–196. doi: 10.1200/JCO.2003.06.130. [DOI] [PubMed] [Google Scholar]
- 26.Park ER. Feasibility and short-term efficacy of a controlled pilot study to integrate a smoking cessation intervention into thoracic clinic settings. Conference presentation at: 2010 Annual Meeting, Society for Research on Nicotine and Tobacco; 02/24/2010-02/27/2010; Bethesda, MD. [Google Scholar]
- 27.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 28.Vander Ark W, DiNardo LJ, Oliver DS. Factors affecting smoking cessation in patients with head and neck cancer. Laryngoscope. 1997;107(7):888–892. doi: 10.1097/00005537-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Pinto BM, Trunzo JJ. Health behaviors during and after a cancer diagnosis. Cancer. 2005;104(11 Suppl):2614–23. doi: 10.1002/cncr.21248. [DOI] [PubMed] [Google Scholar]
- 30.Humphris GM, Rogers SN. The association of cigarette smoking and anxiety, depression and fears of recurrence in patients following treatment of oral and oropharyngeal malignancy. Eur J Cancer Care. 2004;13(4):328–335. doi: 10.1111/j.1365-2354.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- 31.Hay JL, Ostroff J, Burkhalter J, Li Y, Quiles Z, Moadel A. Changes in cancer-related risk perception and smoking across time in newly-diagnosed cancer patients. J Behav Med. 2007;30(2):131–142. doi: 10.1007/s10865-007-9094-7. [DOI] [PubMed] [Google Scholar]
- 32.Schnoll RA, Zhang B, Rue M, et al. Brief physician-initiated quit-smoking strategies for clinical oncology settings: A trial coordinated by the eastern cooperative oncology group. J Clin Oncol. 2003;21(2):355–65. doi: 10.1200/JCO.2003.04.122. [DOI] [PubMed] [Google Scholar]
- 33.Schnoll RA, Rothman RL, Wielt DB, et al. A randomized pilot study of cognitive-behavioral therapy versus basic health education for smoking cessation among cancer patients. Ann Behav Med. 2005;30(1):1–11. doi: 10.1207/s15324796abm3001_1. [DOI] [PubMed] [Google Scholar]
- 34.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 35.Kornblith AB, Dowell JM, Herndon JE, 2nd, et al. Telephone monitoring of distress in patients aged 65 years or older with advanced stage cancer: A cancer and leukemia group B study. Cancer. 2006;107(11):2706–2714. doi: 10.1002/cncr.22296. [DOI] [PubMed] [Google Scholar]
- 36.Naughton MJ, Herndon JE, 2nd, Shumaker SA, et al. The health-related quality of life and survival of small-cell lung cancer patients: Results of a companion study to CALGB 9033. Qual Life Res. 2002;11(3):235–248. doi: 10.1023/a:1015257121369. [DOI] [PubMed] [Google Scholar]
- 37.Queenan JA, Feldman-Stewart D, Brundage M, Groome PA. Social support and quality of life of prostate cancer patients after radiotherapy treatment. Eur J Cancer Care (Engl) 2009 doi: 10.1111/j.1365-2354.2008.01029.x. [DOI] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 39.Pandey M, Devi N, Thomas BC, Kumar SV, Krishnan R, Ramdas K. Distress overlaps with anxiety and depression in patients with head and neck cancer. Psychooncology. 2007;16(6):582–586. doi: 10.1002/pon.1123. [DOI] [PubMed] [Google Scholar]
- 40.Sellick SM, Edwardson AD. Screening new cancer patients for psychological distress using the hospital anxiety and depression scale. Psychooncology. 2007;16(6):534–542. doi: 10.1002/pon.1085. [DOI] [PubMed] [Google Scholar]
- 41.Daniel M, Keefe FJ, Lyna P, et al. Persistent smoking after a diagnosis of lung cancer is associated with higher reported pain levels. J Pain. 2009;10(3):323–328. doi: 10.1016/j.jpain.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. The fagerstrom test for nicotine dependence. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 43.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 44.Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9 4:S555–70. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox LS, Patten CA, Ebbert JO, et al. Tobacco use outcomes among patients with lung cancer treated for nicotine dependence. J Clin Oncol. 2002;20(16):3461–3469. doi: 10.1200/JCO.2002.10.085. [DOI] [PubMed] [Google Scholar]
- 46.FDA. Varenicline (marketed as chantix) information. [Accessed August 30, 2010];US Food and Drug Administration website. Availailable at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106540.htm. Updated August 20, 2010.
- 47.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16 1:i3–8. doi: 10.1136/tc.2006.019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schnoll RA, Martinez E, Tatum KL, et al. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes Control. 2010 doi: 10.1007/s10552-010-9507-8. [DOI] [PubMed] [Google Scholar]
- 49.Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. Am J Prev Med. 2008;34(3):212–215. doi: 10.1016/j.amepre.2007.11.010. [DOI] [PubMed] [Google Scholar]