Abstract
A biomimetic material that can assist bone tissue regeneration was proposed. A bone scaffold based on a hybrid hydrogel self-assembled from N-(2-hydroxypropyl)methacrylamide (HPMA) copolymers grafted with complementary β-sheet peptides was designed. Investigation of self-assembly by circular dichroism spectroscopy suggested that hydrogel formation was triggered through association of the complementary β-sheet motifs. Congo Red and thioflavin T binding, as well as transmission electron microscopy confirmed the formation of a fibril network. Besides mimicking the natural bone extracellular matrix and maintaining preosteoblast cells viability, this hydrogel, as shown by scanning electron microscopy and Fourier transform infrared spectroscopy, provided surfaces characterized by epitaxy that favored hydroxyapatite-like crystal nucleation and growth potentially beneficial for biointegration.
1. Introduction
In tissue engineering, the development of bone mimetic biocompatible materials with improved mechanical properties needs innovative synthetic approaches using natural bone as an inspiration [1,2]. Composite biomaterials based on polymer hydrogels and inorganic compounds are particularly appealing candidates for the design of bone mimetics. In these constructs, the hydrogel provides a 3-D structure similar to bone extracellular matrix (ECM) for cell colonization, migration, proliferation and differentiation [3-6], whereas the inorganic compound, usually a calcium phosphate mineral, enhances the mechanical strength and the osteoconductive properties of the scaffold [7,8]. Various bone-like composite materials were developed based on bone apatite, hydroxyapatite (HA, Ca10(PO4)6(OH)2), and hydrogels formed from poly(lactide-co-glycolide) [9], alginate [10], or silk [11] biopolymers. The presence of HA in the composites induced a significant increase in mechanical strength and enhanced the osteogenic potential when compared to single component hydrogel scaffolds; however, most of these materials lack a high level of nano and macro organization and the organic-inorganic integration seen in natural bone. Template-driven mineralization of HA inside the hydrogel that would result in a high-affinity integration of inorganic phase with the polymer-based scaffold is therefore needed. A series of studies showed that HA crystals could be grown on different hydrogel scaffolds using mineralizing solutions [12-15]. Poly(2-hydroxyethyl methacrylate) (poly(HEMA)) [12,13], poly(vinyl alcohol) (PVA)/poly(acrylic acid) (PAA) [14], and poly(ε-caprolactone) [16] are only a few of the artificial biomineralization systems tested so far.
Although not completely elucidated, it was suggested that mineralization in natural systems, as well as synthetic hydrogels, is initiated by the formation of poorly crystalline calcium apatites which then undergo phase transitions to form stable apatites with increased crystallinity [12,13,17,18]. The process is mediated by acidic proteins in the ECM, or anionic groups in the polymeric hydrogel, that serve as templates for binding inorganic cations and aligning them with the apatite crystal lattice [19]. Specifically, mineralization occurs on surfaces with repetitive patterns of negatively charged groups. In natural bone, the nanoscale collagen fibrils expose such surfaces that act as templates for the inorganic phase formation [20]. Other electrospun artificial nanofibers not only mimic the structure of ECM, but also promote bone regeneration as they provide the structural framework for the growth of calcium apatites [21]. More importantly, de novo-designed peptide-amphiphiles that self-assemble into nanofibrils are also able to promote HA mineralization; they can form a composite material that resembles bone at nano scale, in that the crystallographic c axis of HA is oriented along the long axis of the peptide fiber [22]. Further, peptide-amphiphile hydrogels modified with bioactive adhesion sequence Arg-Gly-Asp (RGD) proved to be optimal scaffolds for proliferation of odontogenic cells and regeneration of dentin [23] and enamel [24].
Acidic proteins/peptides in β-sheet conformation may be involved in the nucleation and growth of HA [25-30]. This assumption is supported by the structural correspondence between the dimensions of the hexagonal unit cell of crystalline HA (a=b=9.432 Α̊, c=6.881 Α̊) and those of the repeat units in self-assembled β-sheet structures (distance between any two β-strands=4.8 Α̊, about half of a or b axes of the apatite unit cell, whereas distance between every second amino acid along a β-strand=6.9 Α̊≈c) [31].
Inspired by previous studies, we designed a bone scaffold based on a hybrid hydrogel self-assembled from graft copolymers of poly(HPMA) and complementary β-sheet peptide grafts (Figure 1). Two complementary hybrid graft copolymers were prepared by coupling β-sheet and RGD peptides synthesized using solid-phase methodology onto thiazolidine-2-thione (TT) side chains of HPMA copolymers obtained by reversible addition-fragmentation chain transfer (RAFT) polymerization. Hydrogel formation appears to be triggered by the association of the complementary β-sheet motifs. The self-assembly of the copolymers into fibrils was characterized by circular dichroism (CD), Congo Red (CR) and thioflavin T (ThT) binding, and transmission electron microscopy (TEM). The morphology of the self-assembled hydrogel was investigated by scanning electron microscopy (SEM), whereas the gelation process was followed by rheology studies. In an effort to mimic Nature, the gel was biomineralized in simulated body fluid (SBF) [32], through the formation of bone-like apatite into the β-sheet fibrils template. The mineral grown was then analyzed with respect to its morphology and composition by electron microscopy and Fourier transform infrared (FTIR) spectroscopy. Finally, the potential of the hybrid copolymer hydrogel to perform optimally as a 3-D matrix for bone regeneration was evaluated in vitro using preosteoblast cells.
Figure 1.
Proposed model of hybrid hydrogel formation from self-assembled poly(HPMA)-g-β-sheet complementary copolymers.
We hypothesized that the proposed hybrid hydrogel, based on β-sheet structure, improves the performance of bone scaffolds by achieving a high level of control over the biomineralization process by promoting organized, oriented bone-like HA growth into the β-sheet fibril template.
This system potentially eliminates disadvantages encountered at scaffolds based on collagen, such as batch-to-batch variation, immunogenicity, complex molecular structure and poor mechanical strength, and also disadvantages of scaffolds based on β-sheet peptides-only, such as insolubility.
2. Experimental
2.1. Materials
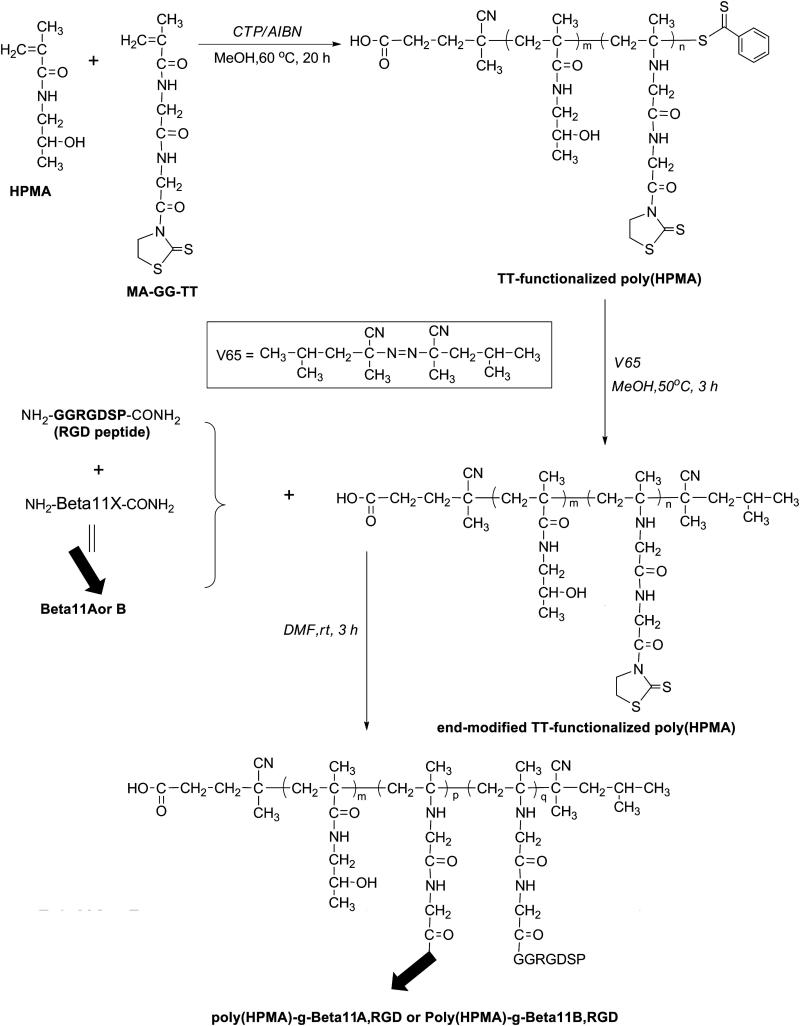
Fmoc-protected amino acids, rink amide 4-methyl-benzhydrylamine (MBHA) resin and 2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyl-uronium hexafluorophosphate (HATU, >98%) were purchased from AAPPTec (Louisville, KY). 1-Hydroxybenzotriazole (HOBt) was from AK Scientific (Mountain View, CA). N,N-dimethylformamide (DMF, 99.8%), N,N-diisopropylethylamine (DIPEA, 99%), piperidine (99.5+%, Biotech grade), triisopropylsilane (TIS, 99%), o-phthalaldehyde (OPA, 97%), 3-mercaptopropionic acid (MPA, 99+%), methanol, and thioflavin T (ThT) dye were from Sigma-Aldrich (St. Louis, MO). N,N-diisopropylcarbodiimide (DIC, >98%) was from Fluka (Milwaukee, WI). Congo Red (CR) dye was from Alfa Aesar (Ward Hill, MA). Trifluoroacetic acid (TFA, 99%) was purchased from Acros Organics (Morris Plains, NJ). Diethyl ether and dichloromethane (DCM) were from Mallinckrodt Baker (Phillipsburg, NJ). 2,2′-Azobisisobutyronitrile (AIBN) was from Soltech Ventures (Beverly, MA) and 2,2′-azobis(2,4-dimethyl valeronitrile) (V-65) was from Wako Chemicals (Richmond, VA). N-methacryloylated glycylglycine bearing thiazolidine-2-thione (TT) groups (MA-GG-TT) [33], 4-cyanopentanoic acid dithiobenzoate (CTP) [34], and N-(2-hydroxypropyl)methacrylamide (HPMA) [35] were synthesized as previously described.
2.2. Synthesis of complementary β-sheet peptides
Beta11A (Ac-Thr-Thr-Arg-Phe-Thr-Trp-Thr-Phe-Thr-Thr-Thr-amide) [36], Beta11B (Ac-Thr-Thr-Glu-Phe-Thr-Trp-Thr-Phe-Glu-Thr-Thr-amide), and RGD (NH2-Gly-Gly-Arg-Gly-Asp-Ser-Pro-amide) peptides were synthesized by manual Fmoc/tBu strategy and standard protocols for solid-phase peptide synthesis, using the rink amide MBHA resin (200 mg, 0.11 mmol per batch). After swelling of the resin beads in DCM (5 mL), and deprotection with 20% piperidine in DMF (2.5 mL), the first amino acid dissolved in DMF, was attached to the resin in the presence of HOBt and DIC. The rest of the amino acids (each 0.348 mmol) were dissolved in DMF/HOBt and attached to the resin beads after deprotection, one at a time, in the presence of HATU and DIPEA (each 0.348 mmol). The completion of each coupling and deprotection step was verified by Kaiser test. After coupling 11 amino acid residues, half of the resin-bound peptide batches of Beta11A and Beta11B were treated with a solution of acetic anhydride and DIPEA in DCM for acetylation of N-terminus, and then with TFA/TIS/H2O (95:2.5:2.5 vol.-%) for cleavage of the peptide from the resin beads and deprotection of the side chains. The products (Beta11A and Beta11B) were used for control experiments. The other half of the resin-bound peptide from each batch, as well as the RGD peptide, was directly cleaved from the resin using TFA/TIS/H2O (95:2.5:2.5 vol.-%) cocktail solution and used for synthesis of the complementary copolymers after precipitation with diethyl ether, filtration, and lyophilization. Peptides were identified by analytical reversed phase (RP)-HPLC (Agilent Technologies), equipped with Eclipse XDB-C8 column (4.6 × 150 mm, 5 μm particle size, 300 Α̊ pore size) and detectors at 220 and 280 nm, and by MALDI-TOF mass spectrometry (MS; Voyager-DE STR Biospectrometry Workstation, PerSeptive Biosystems, Framingham, MA). The molecular weights of the three synthesized peptides, Beta11A, Beta11B and RGD, were verified to be 1403, 1404, and 643 g·mol−1, respectively.
2.3. Synthesis of poly(HPMA) grafted with complementary β-sheet peptides and RGD motif
Complementary poly(HPMA)-g-Beta11A,RGD and poly(HPMA)-g-Beta11B,RGD copolymers were obtained in three synthetic steps as described below. In the first step, HPMA copolymer containing side-chain TT reactive groups was synthesized by RAFT copolymerization of HPMA with MA-GG-TT in methanol using CTP as the chain transfer agent and AIBN as the initiator. The copolymerization was conducted at 60 °C for 20 h with a ratio of [M]0/[CTA]0/[I]0=468:4:1. Briefly, in the first step, an ampoule containing HPMA (0.6 g, 4.2 mmol), MA-GG-TT (252 mg, 0.84 mmol), CTP (11.9 mg, 0.043 mmol), and AIBN (1.76 mg, 0.011 mmol) was attached to the Schlenk-line. After three vacuum-nitrogen cycles to remove the oxygen, 4 mL degassed methanol was added to the mixture. The ampoule was bubbled with nitrogen in ice-bath for 20 min before sealing. The polymer was obtained by precipitation with 1:1 (v/v) acetone/ether and purified by re-dissolving it in methanol and repeating the precipitation step. The powder with light-orange color was dried under vacuum. The yield was 52.4%. The copolymer (poly(HPMA)-TT) was analyzed by size exclusion chromatography (SEC) on an ÄKTA FPLC system (Amersham Pharmacia Biotech) equipped with miniDAWN TREOS and OptilabEX UV and RI detectors (Wyatt Technology, Santa Barbara, CA) using a Superose12 HR/10/30 column, previously calibrated with poly(HPMA) fractions, and phosphate buffer solution (PBS, pH 7.2) as mobile phase. The average molecular weight of the polymer was determined by light scattering, UV, and RI, and calculated by ASTRA software (Mw = 9.6 × 103 g·mol−1, Mw/Mn=1.03).
In the second step, the terminal thiocarbonyl functional group of the copolymer was replaced with 2,4-dimethyl valeronitrile, as shown in Figure 2, using a procedure adapted after York et al. [37]. Briefly, 90 mg poly(HPMA)-TT (9.2 μmol) and 50 mg V-65 (20 times) were added to a 2 mL ampoule, which was then attached to the Schlenk line. After three vacuum-nitrogen cycles to remove the oxygen, 1 mL degassed methanol was added. The solution was purged with nitrogen for 20 min, and then the ampoule was sealed and kept stirring in oil-bath at 50 °C for 3 h. The copolymer was precipitated in cold anhydrous diethyl ether and purified by repeating twice the re-dissolving-precipitation cycle. The resulting powder with light-yellowish color was dried under vacuum. The content of TT in the copolymer was 7×10−4 mmol·mg−1, or 11.3 mol%, as determined by UV spectrophotometry in methanol (ε305=10860 L·mol−1·cm−1 [33]).
Figure 2.
Synthesis of β-sheet and RGD conjugated HPMA copolymers.
Finally, conjugation of the end-modified poly(HPMA)-TT (typically, 30 mg, 21 μmol) with the RGD and β-sheet peptides, Beta11A and Beta11B was accomplished using a ratio of TT : Beta11 / RGD = 1 : 1.1. The coupling took place in DMF, in 3 h, under gentle shaking at room temperature. After conjugation, the solvent was evaporated and the copolymer products were precipitated in cold diethyl ether, filtered, and lyophilized. Potentially unbound peptides were removed by dialysis against water for 24 h using a membrane with a molecular weight cut-off, MWCO, of 6000-8000 Da. The amount of peptide grafts in poly(HPMA)-g-Beta11,RGD copolymers was estimated by amino acid analysis (Table 1). For this analysis, the samples were hydrolyzed with 6 M HCl at 120 °C for 24 h in sealed ampoules. After drying, the hydrolysis products were derivatized with OPA/MPA and analyzed by analytical reverse phase (RP) HPLC equipped with fluorescence detection (exc. 229 nm, em. 450 nm), and a gradient elution with buffer A (0.005 M sodium acetate, pH 6.0) and buffer B (70% methanol in buffer A, pH 6.0).
Table 1.
Peptide content in complementary poly(HPMA)-g-Beta11,RGD copolymers
| Copolymer | GGRGDSP content | Beta11 grafts |
|---|---|---|
| poly(HPMA)-g-Beta11A,RGD | 13.7 wt.%, 3.7 grafts/macromolecule |
30.8 wt.%, 3.8 grafts/macromolecule |
| poly(HPMA)-g-Beta11B,RGD | 11.2 wt.%, 2.6 grafts/macromolecule |
24.5 wt.%, 2.6 grafts/macromolecule |
2.4. Circular dichroism spectroscopy
CD spectra were collected on an Aviv 410 CD spectrometer (Aviv Biomedical Inc., Lakewood, NJ). Samples of mixtures of β-sheet peptides Beta11A : Beta11B = 1 : 1, and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 (peptide grafts ratio) respectively, were prepared by dissolving a known mass of lyophilized products in DI water with pH adjusted to 7.0 with 1 N HCl or 1 N NaOH. Samples were incubated at room temperature for 2 h prior to CD measurements. Wavelength scans were recorded at 25 °C, from 250 to 200 nm, at 1 nm intervals and 3 s averaging time, using a 0.1 cm path length quartz cuvette. Spectra were averaged from three consecutive scans. Ellipticity was reported as mean residue ellipticity ([θ], deg·cm2·dmol−1), calculated as previously described [38].
2.5. Congo Red binding studies
Spectrophotometric analysis of CR binding to Beta11A : Beta11B = 1 : 1 and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 mixtures was performed on a Cary 400 Bio UV-vis spectrophotometer (Varian, Santa Clara, CA), using a 0.1 cm path length quartz cuvette. A 150 μM stock solution of CR in water/10% ethanol and solutions of peptides and copolymers in water (1 mg·mL−1) were used for sample preparation. Spectra were recorded between 700 and 390 nm, with a 1 nm sampling interval, on samples containing 4.7 μM CR that were incubated 2 h at room temperature before the spectral analysis.
2.6. Thioflavin T binding studies
ThT fluorescence measurements were performed on a LS-55 luminescence spectrometer (Perkin-Elmer, Waltham, MA) using a 1 cm path length quartz cuvette. A 100 μM stock solution of ThT in water was prepared and used together with solutions of peptide and copolymers in water (1 mg·mL−1) for sample preparation. The final ThT concentration in the samples was around 50 μM. Emission spectra were recorded from 460 to 540 nm, at an excitation wavelength of 440 nm, and 1 nm sampling interval. Spectra were averaged from 10 consecutive scans. ThT alone was used as the control.
2.7. Transmission electron microscopy
Complementary peptides and copolymer mixtures in water at pH 7 (0.1 mg·mL−1) incubated 24 h after preparation were analyzed for fibril formation on a Philips Tecnai transmission electron microscope at 100 kV accelerating voltage and magnifications ranging from 25000× to 360000×. The samples were absorbed for 20 s on carbon-coated 200-mesh copper grids, and then stained with uranyl acetate solution 4% (w/v) for another 20 s. The specimens were dried overnight before imaging.
2.8. Hydrogel preparation
Hydrogels were obtained by dissolving known amounts of lyophilized complementary peptides or copolymers in DI water with pH 7 adjusted with 1 N HCl or 1 N NaOH. For the preparation of the copolymer hydrogels, a ratio between complementary peptide grafts (Beta11A : Beta11B) of 1 : 1 was used. Typically, depending on the concentration, the gelation time varied from about 2 h for samples having a 3 wt.% concentration to about 24 h for samples at 1 wt.%.
2.9. Rheological characterization
Rheological studies of co-assembled complementary peptides or copolymer hydrogels were conducted on a TA-Instruments 550 Advanced Rheometer (New Castel, DE). A 20 mm diameter Peltier parallel plate and a 20 mm diameter/4 degrees cone-plate with a gap distance of 103 μm were used. Dynamic frequency and time sweep experiments were performed at 25 °C and 1% strain amplitude. Preformed hydrogel samples that were incubated for 24 h at room temperature before investigations were used for frequency sweep experiments at angular frequency of 0.1-10 rad·s−1, whereas gelation was monitored in time sweep experiments on freshly prepared samples. Time sweeps were 12 h long and were performed at a frequency of 6 rad·s−1, with data collected every 30 min. During measurements, evaporation was prevented by insulating the sides of the parallel plate with standard low-viscosity mineral oil. Concentrations ranging from 1 to 3 wt.% were employed. Evolution of storage modulus, G’ as well as loss modulus, G” with frequency or time for gels with different concentrations was obtained.
2.10. Fourier transform infrared spectroscopy
To induce the formation of apatite, hydrogel samples of complementary peptides or copolymers (3 wt.%) were immersed in SBF for up to 2 weeks, following a protocol previously reported by Kokubo et al. [32]. The ion concentration was maintained in the systems by changing the SBF every day. After 2 weeks, the samples were retrieved, washed thoroughly with DI water to remove loosely attached minerals and soluble ions, and lyophilized. The powder samples obtained were then analyzed by ATR-FTIR spectroscopy. Spectra were acquired from 4000 to 500 cm−1 and averaged from 16 scans.
2.11. Scanning electron microscopy
Hydrogel samples of Beta11A : Beta11B = 1 : 1 and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 (3 wt.%), before and after mineralization, prepared as described above, were shock-frozen in liquid nitrogen for 10 min and then lyophilized overnight. The samples were coated with gold under argon atmosphere for 30 s before imaging. Their morphology was examined with a Hitachi S-2460N SEM at an accelerating voltage of 25 kV and magnifications from 50× to 500×. An energy dispersive spectrometer (EDS) was used in conjunction with SEM for elemental analysis of the deposited mineral phase.
2.12. MC3T3-E1 cell culture
MC3T3-E1 Subclone 4 preosteoblast cells were purchased from ATCC (Manassas, VA) and cultured according to the manufacturer’s specifications. Cells were cultured with α-MEM supplemented with 10% fetal bovine serum (FBS), and incubated at 37 °C and 5% CO2. The media was changed every two days throughout the study. Subconfluent cells of passages 5 to 7 were detached using 0.25% trypsin EDTA (Gibco Invitrogen), collected, centrifuged to a pellet, re-suspended and counted. Cells were seeded at a density of 1·105 cells·mL−1 on 2-D hydrogel surfaces, which were presterilized by exposure to UV light for 24 h. For cell entrapment in hydrogels and creation of a 3-D structure, a suspension of preosteoblasts in α-MEM was mixed with a sterile solution of poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 in DI water at pH 7. A viscoelastic hydrogel of 3 wt.% concentration containing 1·106 cells·mL−1 formed at room temperature. Before investigation, seeded and embedded cells were cultured in α-MEM with 10% FBS and incubated at 37 °C and 5% CO2 for various periods of time.
2.13. Cell viability assay
At 24, 48, 72, 120, and 168 h, the 2-D and 3-D hydrogel-cell constructs were treated with the LIVE/DEAD assay (Molecular Probes – Invitrogen, Eugene, OR) according to the manufacturer’s instructions and analyzed for cell viability, distribution and attachment using fluorescence microscopy. This assay is based on the use of Calcein AM and ethidium homodimer-1 (EthD-1). Calcein AM can penetrate the membranes of living cells where it is hydrolyzed by active endogenous esterase into green fluorescent calcein, therefore it serves as a marker of viable cells. On the other hand, EthD-1 is excluded by living cells, but is taken up easily by cells with damaged membrane and produces a bright red fluorescence upon binding to nucleic acids. A stock solution containing 1 μM calcein AM and 2 μM EthD-1 in α-MEM was prepared and used for staining the cells in 2-D and 3-D constructs 30 min before investigation with confocal microscope. Micrographs of stained cells at magnifications ranging from 10× to 80× were obtained on an Olympus FV1000-XY inverted confocal microscope equipped with lasers for excitation at 488 and 546 nm and a desktop computer with Velocity software installed for reconstruction of 3-D images from acquired sectional Z stacks.
3. Results and discussion
3.1. Design of hybrid hydrogels based on complementary HPMA copolymers grafted with β-sheets and RGD
Previously we have shown that conjugation of a short β-sheet peptide (Beta11) as grafts on poly(HPMA) results in the formation of fibril nanostructures at acidic pH and, with increasing concentration, of hybrid hydrogels [36]. Encouraged by this result, we anticipated that mixing two HPMA copolymers containing complementary β-sheet peptides conjugated as grafts on different polymer chains would also form a gel, but under neutral pH conditions. The complementary peptide sequences, named Beta11A and Beta11B, were designed following Aggeli’s model of P11-3 and P11-4 [39], with replacement of glutamine (Gln) with threonine (Thr), and different distribution of charges, as shown in Table 2. Thr is a polar residue with high propensity for β-sheets and it was chosen to enhance the water solubility of the peptides. Phenylalanine (Phe) and tryptophan (Trp) were used because they provide the hydrophobic interactions needed for promoting the hierarchical self-assembly of the peptides. Oppositely charged amino acid residues, arginine (Arg) in Beta11A, and glutamic acid (Glu) in Beta11B, were expected to facilitate the self-assembly of the two complementary graft copolymers at neutral pH through electrostatic interactions. The extra negative charge (Glu) in Beta11B was used with the intention to stabilize the mixed system against flocculation and play an important role in biomineralization. In addition, an RGD peptide (NH2-Gly-Gly-Arg-Gly-Asp-Ser-Pro-amide) was conjugated to the polymer as grafts to promote preosteoblast cell adhesion and long-term survival. We selected the sequence Arg-Gly-Asp-Ser-Pro for its high receptor affinity and selectivity [40]. The Gly-Gly dipeptide spacer was intended to decrease the steric hindrance of the polymer backbone and increase the availability of the RGD peptide for binding to the cell integrin. We anticipated that the RGD peptide would not disturb the self-assembly of the β-sheet grafts and would not interfere with the template mineralization of the bone-like apatite.
Table 2.
Complementary peptide structures
| Peptide | Amino acid sequence 1 2 3 4 5 6 7 8 9 10 11 |
|---|---|
| P11-3 [39] | CH3CO-Gln-Gln-Arg-Phe-Gln-Trp-Gln-Phe-Gln-Gln-Gln-NH2 |
| Beta11A [36] | CH3CO-Thr-Thr-Arg-Phe-Thr-Trp-Thr-Phe-Thr-Thr-Thr-NH2 |
| P11-4 [39] | CH3CO-Gln-Gln-Arg-Phe-Glu-Trp-Glu-Phe-Glu-Gln-Gln-NH2 |
| Beta11B | CH3CO-Thr-Thr-Glu-Phe-Thr-Trp-Thr-Phe-Glu-Thr-Thr-NH2 |
The peptides were synthesized by solid-phase peptide synthesis and attached to different poly(HPMA) backbones via TT functional groups. A schematic diagram for the synthesis of β-sheet and RGD grafted poly(HPMA) is shown in Figure 2. Upon mixing, the self-assembly of the complementary graft copolymers into hydrogels was expected to occur at neutral pH through the specific association of the mutually attractive Beta11A and Beta11B peptide modules. The possibility of two oppositely charged grafts to find each other in the mixture was significantly enhanced by the flexibility of the poly(HPMA) backbone [41]. The co-assembly process described above was greatly influenced by factors such as density of the grafts, concentration and incubation time.
3.2. Characterization of fibers self-assembled from poly(HPMA)s grafted with complementary β-sheets and RGD
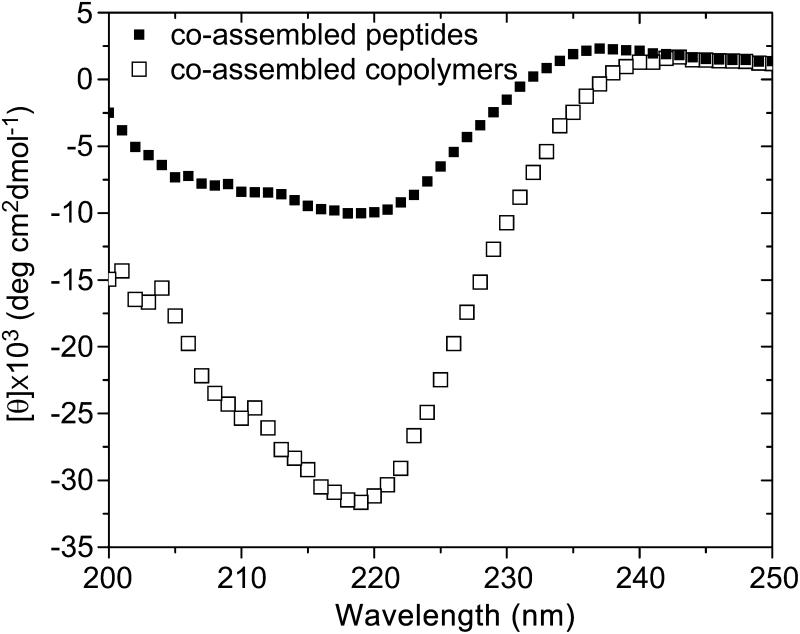
Mixing the aqueous dilute solutions of oppositely charged peptides and graft copolymers, respectively, at pH 7, induced the formation of fibrils with a hierarchic β-sheet structure. Increasing concentration induced the formation of viscoelastic materials. CD spectral studies performed on Beta11A : Beta11B = 1 : 1 and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 systems showed the negative minimum near 218 nm, characteristic to peptides adopting the β-sheet conformation (Figure 3). For similar peptide concentrations (1 mg·mL−1), the ellipticity of the complementary copolymers system was extensively enhanced comparing to the complementary peptides, presumably due to a stabilizing effect that the polymer backbone has on the self-assembly of the β-sheet grafts. In comparison, the β-sheet CD spectrum of peptide mixture was slightly altered, indicating that the peptides adopt alternative conformations.
Figure 3.
CD spectra of Beta11A : Beta11B = 1 : 1 and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD = 1 : 1 in water at pH 7 (1 mg·mL−1).
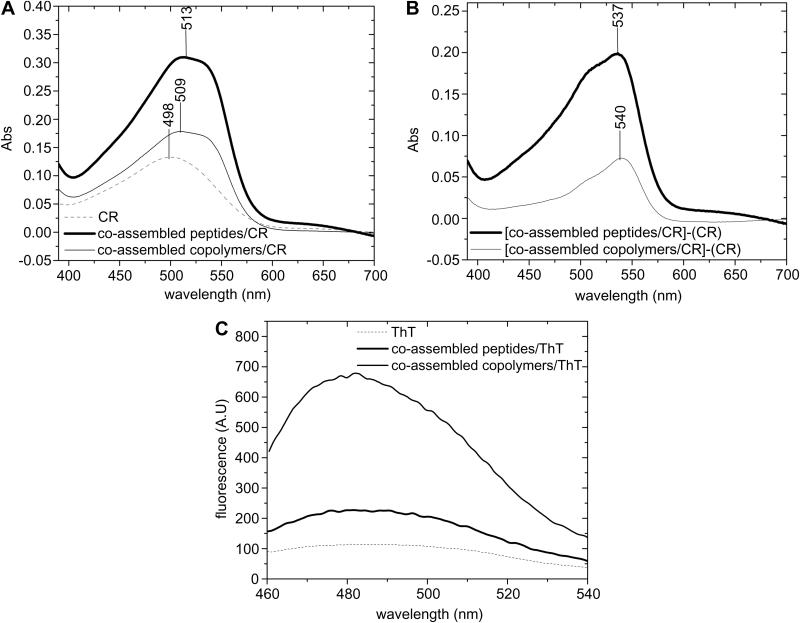
Formation of β-sheet fibrils was further tested for CR binding by spectroscopic band-shift assay. Experiments showed that dye binding was accompanied by a significant increase in absorption intensity, but also produced a hyperchromic red shift of the absorption maxima from 498 nm for CR alone to 513 nm for complementary peptides, and 509 nm for complementary copolymers (Figure 4 A). The points of maximal spectral difference (λmax) at 537 and 540 nm further indicated the presence of the fibrils in both systems (Figure 4 B), λmax at 520-540 nm being a characteristic feature of β-sheet fibrils [42]. It should be also noted that the large molecule of CR requires a high number of grafts self-assembled into β-sheets, hardly encountered in the copolymers system, which might explain the characteristic shift to lower wavelengths in this case.
Figure 4.
(A) CR binding assay; (B) Differential spectra of [(Beta11A:Beta11B)/CR]-CR and [(poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD)/CR]-CR showing the points of maximum absorption; (C) Fluorescence emission spectra (λexc=440 nm) of ThT binding assay.
On the other hand, the smaller ThT molecule is able to bind even four or five associated β-strands [43], therefore ThT binding assay was used to test formation of the β-sheet fibrils in the complementary copolymers system. A 6-fold increase in fluorescence intensity was observed when ThT bound to complementary copolymers fibrils (Figure 4, C). By comparison, binding of ThT to complementary peptide fibrils produced only 1.5-fold increase in fluorescence intensity. This result is in good agreement with previously published data on poly(HPMA)-g-β-sheet copolymers that showed similar behavior [36].
β-Sheet fibril formation, proved by CR and ThT binding assays, was confirmed by TEM imaging. Micrometer-long fibrils were detected in both, complementary peptide and complementary copolymer samples (Figure 5). Individual fibrils were a few tens of nm in width and μm in length, characteristic of amyloid morphology. Both co-assembled systems exhibited a crosslinked network structure, consistent with their ability to organize scaffolds from interconnecting nanofibers. The crosslinked network structure was found to be denser when increasing the sample concentration (Figure 5, C).
Figure 5.
TEM images of (A) Beta11A : Beta11B fibrils; and (B), (C) poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD fibrils.
3.3. Characterization of hydrogels self-assembled from complementary HPMA copolymers grafted with β-sheets and RGD
While the designed complementary peptides have relatively poor solubility in water, their conjugation as grafts on different poly(HPMA) chains improved solubility; this enabled the formation of hybrid hydrogels to be mediated by the co-assembly of the β-sheets. Complementary peptide and complementary copolymer solutions having a concentration of 3 wt.% gelled in minutes, whereas lower concentrations needed hours of incubation at room temperature. When agitated, the fluidity of the formed hydrogels increased, but upon cessation of the agitation, their viscosity restored on standing, in a time dependent manner.
A series of rheological measurements were conducted to evaluate the viscoelastic properties of the hydrogels and the kinetics of network formation. Frequency sweeps conducted at 1% strain amplitude showed that the storage modulus, G’ (a measure of the material elastic response to stress), is independent of frequency (ω) within the tested range, displaying plateau-like curves at 3 wt.% concentration, an indication that gels were formed in both, peptide and copolymer, co-assembled systems (Figure 6, A,B). Values of G’ were an order of magnitude greater than the loss modulus, G” (a measure of the material viscous response to stress). Compared to the Beta11A : Beta11B =1 : 1 hydrogels, co-assembled copolymer hydrogels of similar concentrations were stiffer; while the plateau value of the elastic modulus, G’, was around 400 Pa for copolymer gel at 3 wt.% concentration, for peptide gel at similar concentration it was only 100 Pa. Crossover points, or gel points (G’ = G”), where the properties change from a dominant viscous, liquid-like behavior to a dominant elastic, solid-like state [44], were identified in both systems at lower concentrations.
Figure 6.
Dynamic frequency sweeps showing the frequency dependence of G’ and G” from 0.1 to 10 rad·s−1 of (A) co-assembled peptides, and (B) co-assembled copolymers hydrogels at different concentrations. The arrows indicate the crossover points (gel points).
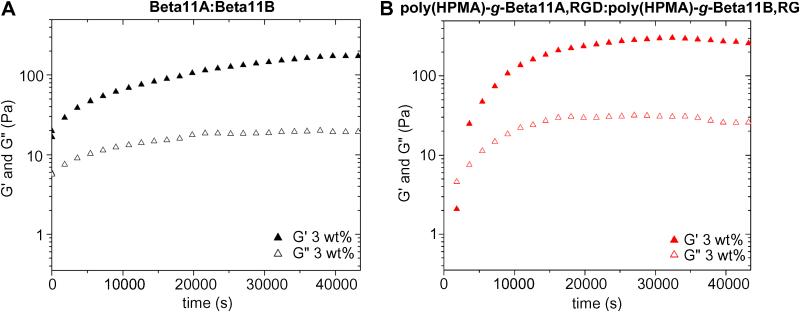
Change of G’ and G” as a function of time was also measured using time sweep experiments performed on systems with a concentration of 3 wt.%, at 6 rad·s−1 frequency and a strain of 1% amplitude. The experiments showed that G’ of the complementary peptide solution displayed a plateau-like dependency with respect to time, an indication that the gel formed almost instantaneously after mixing of the co-assembling peptides (Figure 7, A). On the contrary, G’ of the complementary copolymers solution rose to 20 Pa in a few min and reached a plateau value of around 300 Pa after 4 h (Figure 7, B). This behavior is consistent with a gelation mechanism characterized by an initial burst phase of elasticity caused by initial interaction of peptide grafts, followed by a slower growth phase during which reorganization of the β-sheet fibrils occurs [45]. Over time, the homogeneity of the system increased, and consequently the elasticity of the gel reached its plateau. It should be noted that the gels needed relatively long periods of time to recover from the strain applied during the time sweep experiments. Because shorter recovery periods prevented the optimal formation of the gels after being disrupted, 30 min was chosen as the interval for the collection of every data point. G’ of the gel was therefore allowed to reach its new plateau value, 75% of the value estimated from frequency sweep experiments.
Figure 7.
Time sweeps showing the evolution of G’ and G” as a function of time for (A) co-assembled peptide hydrogels, and (B) co-assembled copolymer hydrogels, at 3 wt.% concentration.
Although not strong enough in itself at 3 wt%, to be a bone grafting material, the hybrid hydrogels could be applied as bone regeneration material by combining it with osteoconductive inorganic HA and preosteoblast bone cells as described below. In fact, Elisseeff and others have demonstrated the suitability of hydrogels with similar mechanical properties in musculoskeletal tissue engineering [46].
3.4. Mineralization in hybrid hydrogels
Electron microscopy investigations on similar poly(HPMA)-g-β-sheet systems evidenced the layered packing of the copolymer fibrils and the lamellar morphology of the gels [36]. Based on the previous results, the main target of this work was to produce an anisotropic bone biomimetic composite material in which the nucleation, growth, and orientation of the inorganic component, HA crystals, are controlled by the β-sheet template. The morphology of the co-assembled peptide and co-assembled copolymer lyophilized hydrogel networks, before and after mineralization in SBF, was observed by SEM (Figure 8). Compared to the peptide-based hydrogel (Figure 8 A), the copolymer hydrogel (Figure 8 B) showed an anisotropic porosity with specific pore orientation which could be used to achieve HA crystals alignment or oriented cellular migration in 3-D (Figure 8 B). Because we obtained a rather isotropic organization of the pores in the absence of the polymer, we believe that the freeze-drying process was not responsible for creating this well-defined micro-architecture. However, one factor that might contribute to porous structure formation could be phase separation between the peptide and the poly(HPMA), resulting in continuous, oriented pores throughout the gel matrix. The SEM images (Figure 8 A,B) showed that the pores were in the range of 10-20 μm. According to previous reports, they should be at least the size of the cell in order for bone cells and nutrients to migrate into the scaffold [47]. Because the pores were larger than the preosteoblasts, the microscale features of these co-assembled copolymer hydrogels were expected to provide sufficient nutrient and osteoblast cellular infusion, while maintaining structural integrity.
Figure 8.
SEM micrographs of (A) Beta11A : Beta11B, and (B) poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD lyophilized hydrogels before mineralization; SEM images of HA crystals deposited on the surface of poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD (C), (D) and EDS spectra corresponding to minerals (E) inside and (F) on the edge of the pores.
Resulting from biomimetic apatite growth in the hydrogel, a composite, interpenetrating network of inorganic coating and polymeric matrix was generated by extensive treatment for 2 weeks in SBF. Precipitation of the apatite within the micropores of the hydrogels generally preserved the overall architecture of the scaffolds. With the understanding that the lyophilization treatment can influence the morphology, evaluation of the inorganic coating on the co-assembled peptides hydrogel indicated a poorly crystalline or amorphous calcium phosphate phase, as further confirmed by EDS and FTIR. In contrast, deposition from SBF in the co-assembled copolymers hydrogel resulted in the formation of characteristic crystalline apatite coatings composed of spherical aggregates, situated on the edge of the hydrogel pores (Figure 8 C,D). The highly ordered molecular template of co-assembled poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD presumably promoted the template-driven mineralization of HA-like crystals. The crystalline mineral phase was more likely to grow and aggregate on the surface of hybrid hydrogel scaffold, which had more anionic carboxylate and hydroxyl groups. EDS analysis carried out on the surface of the hybrid hydrogels mineralized in SBF showed that, indeed, the calcium (Ca)/phosphorus (P) ratio of the deposited minerals was 1.7, close to Ca/P=1.67 ratio found in HA or carbonated HA [19], whereas in the mineralized peptide hydrogels was around 2, corresponding to a tetracalcium phosphate mineral [48]. The other major elements of mineralized hybrid hydrogel scaffold consisted of carbon (C), oxygen (O) from the organic material, and sodium (Na), chlorine (Cl), and potassium (K) from the deposited mineral. Whereas HA crystals covered mainly the edges of pores in the hybrid hydrogel (Figure 8 D,E,F), halide (NaCl) crystals were predominant on the surface of the mineralized peptides hydrogels. Presumably, the calcium phosphates did not attach to the peptide gels as they did in the hybrid hydrogels and were therefore washed away when the scaffolds were rinsed with DI water before lyophilization. It is also possible that the chemistry of the hybrid matrix, with more anionic functional groups compared to the complementary peptides hydrogel, promoted a strong interaction with Ca2+ ions which initialized nucleation of calcium phosphate mineral phase, leading to templated mineralization. The flexibility of the HPMA polymer chain could contribute to an increased accessibility of the anionic groups at the surface and could allow the Ca2+ bound chains to attain a conformation favoring the formation of apatite crystals, as previously shown for templated mineralization of N-acryloyl amino acid hydrogels [49].
SEM showed that calcium phosphates formed on peptide hydrogel versus copolymer hydrogel were different. However, additional measurements by FTIR were necessary to confirm the nature of these crystals. For both samples, FTIR analysis showed intense absorption bands in the 1300-800 cm−1 region, where characteristic phosphate peaks appear (Figure 9). The peak at 1060 cm−1, identified in the mineralized peptides hydrogel spectrum, was associated with an amorphous calcium phosphate phase, whereas the peak at 1038 cm−1 was assigned to the vibration in the PO −34 group of HA [50]. It was speculated that the higher level of organization in co-assembled copolymer hydrogel over the peptide hydrogel, favored the crystallization of the HA-like mineral from the amorphous calcium phosphate precursors, hence the shift in the characteristic phosphate peak. This speculation is based on the observation that dicalcium phosphate dihydrate (brushite, DCPD, CaHPO4•2H2O) can act as precursor to the formation of apatite phase from acidic solutions in vitro [51].
Figure 9.
ATR-FTIR spectra of (A) SBF, (B) co-assembled peptide gel, (C) co-assembled copolymer gel and (D) co-assembled peptide and (E) co-assembled copolymer hydrogels mineralized in SBF.
3.5. Hybrid hydrogels as scaffolds for bone cells
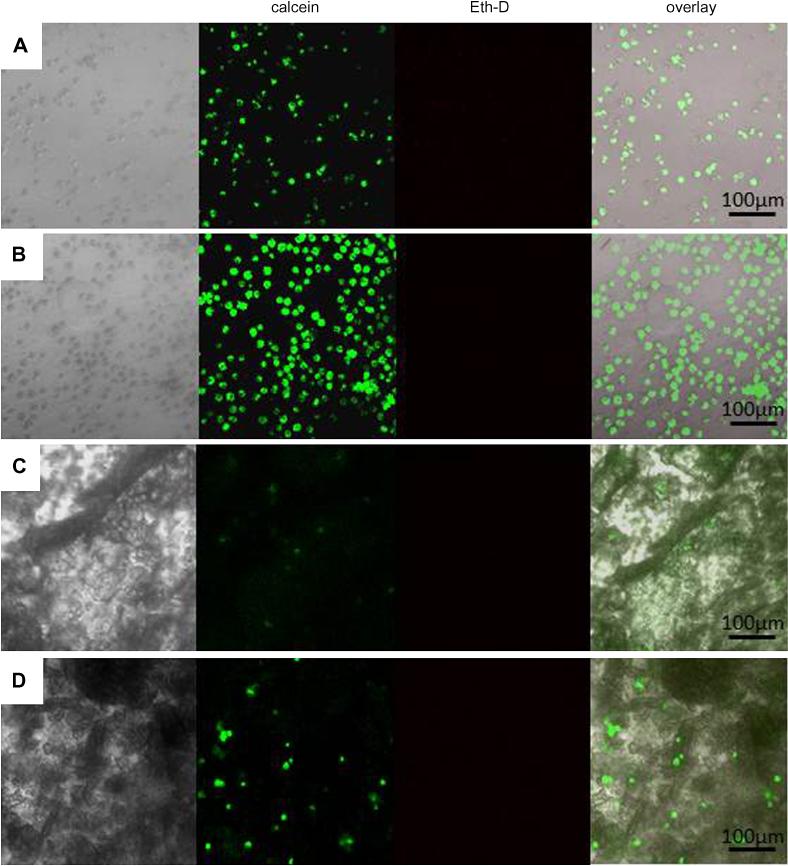
The potential of poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD hydrogel as bioactive 2-D and 3-D scaffolds for MC3T3-E1 preosteoblast cells was investigated. Co-assembled peptide hydrogel, Beta11A : Beta11B, was used as control. Cells were either seeded or encapsulated inside the hydrogels and their morphology and viability was checked at different time periods; viability was determined by Live/Dead assay using confocal microscopy. The 3-D cell-hydrogel constructs formed within minutes; this is favorable for the homogeneity of the cell distribution throughout the hydrogels. Even after one week of culture, most cells were viable in both 2-D and 3-D hybrid hydrogels, as shown by the intense green fluorescence, and only few were identified by the red color as being dead (Figure 10). Uniform spatial distribution of the cells in the scaffolds was observed not only in the 3-D constructs, but also in 2-D when cells were seeded on the surface of the gel, an indication of the cells migration through the pores that acted as guidance channels from the surface to inside.
Figure 10.
Confocal microscopy images of preosteoblasts seeded (A), (B) and embedded (C), (D) in Beta11A : Beta11B hydrogel (A), (C) and poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD hydrogel (B), (D) at 1 week-culture.
It is known that cells do not attach to highly hydrated synthetic hydrogels. Therefore, to encourage cell attachment, we modified our copolymer-based hydrogels by incorporation of a cell adhesion RGD peptide, as grafts attached to the HPMA copolymer backbone. Although cells survived in the co-assembled copolymer hydrogel, as well as on its surface, in general, they did not attach. The spindle-like or polyhedral characteristic shapes of attached MC3T3-E1 preosteoblasts were rarely identified, the majority of the cells maintaining a spherical or ellipsoidal shape. Cells in the copolymer hydrogel showed some interaction with the scaffold evidenced by formation of fine filopodia, but spreading was not induced, despite incorporation of RGD motif as a possible adhesion site. RGD concentration (minimum 2.5 mM required [52]), but also ligand molecular structure, density and spatial distribution may all influence cell adhesion [53]. Previously, it was shown that cell receptors could only access RGD peptides that are present 10 nm or more apart from each other in the gel [53]. However, due to the random nature of the coupling process, as well as the flexibility of the polymer chain, the precise location of the RGD ligands within the hydrogel matrix proposed in this study was difficult to predict. As a result, it was suggested that the steric hindrance of binding caused by closely distributed RGD grafts on the polymer backbone prevented attachment of most of the MC3T3-E1 cells to the hydrogel scaffold. Another possible factor that impeded the spreading of the cells may be the mechanically weak cell adhesion platform provided by the hydrated gel, since cell elongation needs a highly rigid surface which withstands the traction forces generated during the process [54]. A rough comparison was made between the numbers of MC3T3-E1 cells on co-assembled peptide hydrogels without RGD at various days of culture with MC3T3-E1 cells grown on hybrid hydrogels containing RGD. Both substrates displayed similar increase in cell number during first days, however, compared with the peptide hydrogels, RGD-conjugated hybrid hydrogels promoted faster cell proliferation after 5 days in culture, a clear indication of the RGD role in regulating the cell function.
Although optimization and further toxicity studies are needed, the cell viability, and proliferation migration responses suggest that the poly(HPMA)-g-Beta11A,RGD : poly(HPMA)-g-Beta11B,RGD hydrogel is not cytotoxic, thus it could potentially be useful in developing an engineered scaffold for bone regeneration.
4. Conclusions
Conjugation of complementary β-sheet peptides as grafts on different poly(HPMA) chains, followed by mixing of the copolymers, resulted in formation of a hybrid hydrogel. CD spectroscopy and CR and ThT binding studies showed that the material adopted a β-sheet structure given by the peptides grafts that served as units of assembly for fibrils formation. TEM investigation of the fibrous network indicated its ability to organize scaffolds from physically crosslinked, interconnecting nanofibers. Oscillatory rheology demonstrated that differences in concentration and incubation time, as well as the presence of external disturbing factors (strain), influenced the bulk mechanical properties of the self-assembled gels. Although not strong enough in itself to serve as a bone grafting material, the co-assembled hybrid hydrogel was characterized by anisotropic porosity, as shown by SEM, and therefore, it could be a useful tool to control mineralization of bone-like apatites and distribution of bone cells when applied as a scaffold for tissue engineering. Based on SEM, EDS and FTIR analyses, HA-like crystals were created onto anionic β-sheet templates by incubating the hybrid hydrogel in SBF for 2 weeks. Preliminary in vitro studies showed that long-term culture maintained cell viability and promoted cell proliferation, an indication that the hydrogel is not cytotoxic, and once optimized, could be used as a bone scaffold. The data acquired so far suggest that templated mineralization of hybrid poly(HPMA) hydrogels with β-sheet structure may be a promising approach for the construction of bone grafting materials in vitro. Further studies are required to examine whether the co-assembled hybrid hydrogel with β-sheet structure proposed herein plays a significant role in promoting osteoinduction in vivo.
Acknowledgements
This research was supported in part by NIH grants EB 005288 and GM69847. We thank Drs. Vladimir Hlady, Pavla Kopečková, Jihua Liu, and Kui Luo for valuable discussions, and Dr. Patrick Kiser for the use of rheometer and FTIR instruments.
References
- [1].Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- [2].Puppu D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–440. [Google Scholar]
- [3].Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- [4].Cushing MC, Anseth KS. Hydrogel cell cultures. Science. 2007;316(5828):1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- [5].Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Betz MW, Yeatts AB, Richbourg WJ, Caccamese JF, Coletti DP, Falco EE, et al. Macroporous hydrogels upregulate osteogenic signal expression and promote bone regeneration. Biomacromolecules. 2010;11(5):1160–1168. doi: 10.1021/bm100061z. [DOI] [PubMed] [Google Scholar]
- [7].Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jia X, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol Biosci. 2009;9(2):140–156. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim SS, Park MS, Jeon O, Choi CY, Kim BS. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(8):1399–1409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- [10].Turco G, Marsich E, Bellomo F, Semeraro S, Donati I, Brun F, et al. Alginate/hydroxyapatite biocomposite for bone ingrowth: A trabecular structure with high and isotropic connectivity. Biomacromolecules. 2009;10(6):1575–1583. doi: 10.1021/bm900154b. [DOI] [PubMed] [Google Scholar]
- [11].Kino R, Ikoma T, Yunoki S, Nagai N, Tanaka J, Asakura T, et al. Preparation and characterization of multilayered hydroxyapatite/silk fibroin film. J Biosci Bioeng. 2007;103(6):514–520. doi: 10.1263/jbb.103.514. [DOI] [PubMed] [Google Scholar]
- [12].Song J, Saiz E, Bertozzi CR. A new approach to mineralization of biocompatible hydrogel scaffolds: An efficient process toward 3-dimensional bonelike composites. J Am Chem Soc. 2003;125(5):1236–1243. doi: 10.1021/ja028559h. [DOI] [PubMed] [Google Scholar]
- [13].Song J, Malathong V, Bertozzi CR. Mineralization of synthetic polymer scaffolds: A bottom-up approach for the development of artificial bone. J Am Chem Soc. 2005;127(10):3366–3372. doi: 10.1021/ja043776z. [DOI] [PubMed] [Google Scholar]
- [14].Iwatsubo T, Sumaru K, Kanamori T, Shinbo T, Yamaguchi T. Construction of a new artificial biomineralization system. Biomacromolecules. 2006;7(1):95–100. doi: 10.1021/bm0504476. [DOI] [PubMed] [Google Scholar]
- [15].Watanabe J, Akashi M. Novel biomineralization for hydrogels: Electrophoresis approach accelerates hydroxyapatite formation in hydrogels. Biomacromolecules. 2006;7(11):3008–3011. doi: 10.1021/bm060488h. [DOI] [PubMed] [Google Scholar]
- [16].Lebourg M, Antón JS, Ribelles JL Gomez. Hybrid structure in PCL-Hap scaffold resulting from biomimetic apatite growth. J Mater Sci: Mater Med. 2010;21(1):33–44. doi: 10.1007/s10856-009-3838-6. [DOI] [PubMed] [Google Scholar]
- [17].Zhang R, Ma XP. Porous poly(L-lactic acid)/apatite composites created by biomimetic process. J Biomed Mater Res. 1999;45(4):285–293. doi: 10.1002/(sici)1097-4636(19990615)45:4<285::aid-jbm2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- [18].Bonzani IC, George JH, Stevens MM. Novel materials for bone and cartilage regeneration. Curr Opin Chem Biol. 2006;10(6):568–575. doi: 10.1016/j.cbpa.2006.09.009. [DOI] [PubMed] [Google Scholar]
- [19].Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108(11):4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhang W, Liao SS, Cui FZ. Hierarchical self-assembly of nano-fibrils in mineralized collagen. Chem Mater. 2003;15(16):3221–3226. [Google Scholar]
- [21].Jang JH, Castano O, Kim HW. Electrospun materials as potential platforms for bone tissue engineering. Adv Drug Deliv Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- [22].Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- [23].Galler KM, Cavender A, Yuwono V, Dong H, Shi S, Schmalz G, et al. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng: Part A. 2008;14(12):2051–2058. doi: 10.1089/ten.tea.2007.0413. [DOI] [PubMed] [Google Scholar]
- [24].Huang Z, Sargeant TD, Hulvat JF, Mata A, Bringas P, Jr, Koh CY, et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J Bone Miner Res. 2008;23(12):1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fan D, Lakshminarayanan R, Moradian-Oldak J. The 32 kDa enamelin undergoes conformational transitions upon calcium binding. J Struct Biol. 2008;163(1):109–115. doi: 10.1016/j.jsb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Takeuchi A, Ohtsuki C, Miyazaki T, Kamitakahara M, Ogata S, Yamazaki M, et al. Heterogeneous nucleation of hydroxyapatite on protein: structural effect of silk sericin. J R Soc Interface. 2005;2(4):373–378. doi: 10.1098/rsif.2005.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen CL, Rosi NL. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew Chem Int Ed. 2010;49(11):1924–1942. doi: 10.1002/anie.200903572. [DOI] [PubMed] [Google Scholar]
- [28].Semino CE. Self-assembling peptides: From bio-inspired materials to bone regeneration. J Dent Res. 2008;87(7):606–616. doi: 10.1177/154405910808700710. [DOI] [PubMed] [Google Scholar]
- [29].Kirkham J, Firth A, Vernals D, Boden N, Robinson C, Shore RC, et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res. 2007;86(5):426–430. doi: 10.1177/154405910708600507. [DOI] [PubMed] [Google Scholar]
- [30].Takeuchi A, Ohtsuki C, Kamitakahara M, Ogata S, Miyazaki T, Tanihara M. Biomimetic deposition of hydroxyapatite on a synthetic polypeptide with a β-sheet structure in a solution mimicking body fluid. J Mater Sci: Mater Med. 2008;19(1):387–393. doi: 10.1007/s10856-007-3179-2. [DOI] [PubMed] [Google Scholar]
- [31].Segman-Magidovich S, Grisaru H, Gitli T, Levi-Kalisman Y, Rapaport H. Matrices of acidic β-sheet peptides as templates for calcium phosphate mineralization. Adv Mater. 2008;20:2156–2161. [Google Scholar]
- [32].Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [33].Šubr V, Ulbrich K. Synthesis and properties of new N-(2 hydroxypropyl)methacrylamide copolymers containing thiazolidine-2-thione reactive groups. React Funct Polym. 2006;66:1525–1538. [Google Scholar]
- [34].Mitsukami Y, Donovan MS, Lowe AB, McCormick CL. Water-soluble polymers. 81. Direct synthesis of hydrophilic styrenic-based homopolymers and block copolymers in aqueous solution via RAFT. Macromolecules. 2001;34(7):2248–2256. [Google Scholar]
- [35].Kopeček J, Bažilová H. Poly[N-(2-hydroxypropyl)methacrylamide]. 1. Radical polymerization and copolymerization. Eur Polym J. 1973;9:7–14. [Google Scholar]
- [36].Radu-Wu LC, Yang J, Wu K, Kopeček J. Self-assembled hydrogels from poly[N-(2-hydroxypropyl)methacrylamide] grafted with β-sheet peptides. Biomacromolecules. 2009;10(8):2319–2327. doi: 10.1021/bm9005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].York AW, Huang F, McCormick CL. Rational design of targeted cancer therapeutics through the multiconjugation of folate and cleavable siRNA to RAFT-synthesized (HPMA-s-APMA) copolymers. Biomacromolecules. 2010;11(2):505–514. doi: 10.1021/bm901249n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yang J, Xu C, Kopečková P, Kopeček J. Hybrid hydrogels self-assembled from HPMA copolymers containing peptide grafts. Macromol Biosci. 2006;6:201–209. doi: 10.1002/mabi.200500208. [DOI] [PubMed] [Google Scholar]
- [39].Aggeli A, Bell M, Carrick LM, Fishwick CWG, Harding R, Mawer PJ, et al. pH as a trigger of peptide β-sheet self-assembly and reversible switching between nematic and isotropic phases. J Am Chem Soc. 2003;125(32):9619–9628. doi: 10.1021/ja021047i. [DOI] [PubMed] [Google Scholar]
- [40].Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [41].Kamei S, Kopeček J. Prolonged blood circulation in rats of nanospheres surface-modified with semitelechelic poly[N-(2-hydroxypropyl)methacrylamide] Pharmaceutical Res. 1995;12(5):663–668. doi: 10.1023/a:1016247206531. [DOI] [PubMed] [Google Scholar]
- [42].Shahi P, Sharma R, Sanger S, Kumar I, Jolly RS. Formation of amyloid fibrils via longitudinal growth of oligomers. Biochemistry. 2007;46(25):7365–7373. doi: 10.1021/bi7001136. [DOI] [PubMed] [Google Scholar]
- [43].Biancalana M, Makabe K, Koide A, Koide S. Molecular mechanism of thioflavin-T binding to the surface of β-rich peptide self-assemblies. J Mol Biol. 2009;385(4):1052–1063. doi: 10.1016/j.jmb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tung CYM, Dynes PJ. Relationship between viscoelastic properties and gelation in thermosetting systems. J Appl Polym Sci. 2003;27(2):569–574. [Google Scholar]
- [45].Ramachandran S, Tseng Y, Yu YB. Repeated rapid shear-responsiveness of peptide hydrogels with tunable shear modulus. Biomacromolecules. 2005;6(3):1316–1321. doi: 10.1021/bm049284w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Varghese S, Elisseeff JH. Hydrogels for musculoskeletal engineering. Adv Polym Sci. 2006;203:95–144. [Google Scholar]
- [47].Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factorsy. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- [48].Mathew M, Takagi S. Structures of biological minerals in dental research. J Res Natl Inst Stand Technol. 2001;106(6):1035–1044. doi: 10.6028/jres.106.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Phadke A, Zhang C, Hwang YS, Vecchio K, Varghese S. Templated mineralization of synthetic hydrogels for bone-like composite materials: Role of matrix hydrophobicity. Biomacromolecules. 2010;11(8):2060–2068. doi: 10.1021/bm100425p. [DOI] [PubMed] [Google Scholar]
- [50].Sauer GR, Wu LNY, Iijima M, Wuthier RE. The influence of trace elements on calcium phosphate by matrix vesicles. J Inorg Biochem. 1997;65(1):57–65. doi: 10.1016/s0162-0134(96)00080-3. [DOI] [PubMed] [Google Scholar]
- [51].Liu G, Zhao D, Tomsia AP, Minor AM, Song X, Saiz E. Three-dimensional biomimetic mineralization of dense hydrogel templates. J Am Chem Soc. 2009;131(29):9937–9939. doi: 10.1021/ja903817z. [DOI] [PubMed] [Google Scholar]
- [52].Yang F, Williams CG, Wang D, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005;26(30):5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [53].Brinkerhoff C, Linderman J. Integrin dimerization and ligand organization: key components in integrin clustering for cell adhesion. Tissue Eng. 2005;11(5-6):865–876. doi: 10.1089/ten.2005.11.865. [DOI] [PubMed] [Google Scholar]
- [54].Ito F, Usui K, Kawahara D, Suenaga A, Maki T, Kidoaki S, et al. Reversible hydrogel formation driven by protein-peptide-specific interaction and chondrocyte entrapment. Biomaterials. 2010;31(1):58–66. doi: 10.1016/j.biomaterials.2009.09.026. [DOI] [PubMed] [Google Scholar]