Abstract
Introduction
Persistent STAT3 activation contributes to lung carcinogenesis. Survivin, one of STAT3-regulated genes, is antiapoptotic and confers cancer radioresistance.
Methods
We tested whether TG101209, a small-molecule inhibitor of JAK2 (a STAT3-activating tyrosine kinase), affected survivin expression and sensitized lung cancer to radiation. We investigated whether inhibition of JAK2 signaling with TG101209 can be used to reduce survivin expression and enhance radiosensitivity of lung cancer cells in vitro and tumor growth delay in vivo. JAK2 downstream signaling, including PI3-K/Akt and Ras/MAPK/ERK pathways, was also explored.
Results
TG101209 inhibited STAT3 activation and survivin expression, and sensitized HCC2429 (DER=1.34,p=0.002) and H460 (DER=1.09,p=0.006) cells to radiation in clonogenic assays. Radiation promoted phospho-Akt and phospho-ERK in H460 cells, while their levels were unchanged in HCC2429. After treatment with TG101209, phospho-ERK protein levels were reduced in both HCC2429 and H460 cells. HCC2429 cells transfected with K-Ras-12V mutant were more resistant to radiation- and TG101209-induced apoptosis than wild-type control cells. In vivo, addition of TG101209 to radiation in lung xenografts produced a significant tumor growth delay (>10 days) compared to radiation alone and was well tolerated. Immunohistochemistry staining of tumor sections showed that TG101209 increased apoptosis and decreased cell proliferation and vascular density, suggesting that TG101209 also has antiangiogenic effects.
Conclusions
TG101209 enhanced the effects of radiation in lung cancer in vitro and in vivo. This study suggests the potential utility of selecting lung cancer patients according to K-Ras mutation status for future clinical trials testing combination of TG101209 and radiotherapy.
Keywords: Survivin, JAK2, non-small cell lung carcinoma, radiation
Introduction
Lung cancer is the leading cause of cancer mortality in 2009 [1], despite advances in therapy. Radiotherapy plays an important role in lung cancer treatment, but the effectiveness of this modality is often limited by defects in cell death pathways. Research of novel sensitizing agents has focused on cancer-specific targets to enhance radiation therapy by making cancer cells more susceptible to death after radiation-induced damage. One target that was explored is survivin, the smallest member of the inhibitor of apoptosis protein (IAP) family, mainly because survivin is differentially expressed in tumors compared to normal tissues [2]. Indeed, it is overexpressed in most cancers, including lung, and is therefore deemed an attractive anticancer target either through inactivating the survivin protein or by stopping the production of survivin through inhibition of survivin gene expression. Accordingly, we and others have found that survivin prevents apoptosis and mediates resistance to radiation [3-6], and has some prognostic value for cancer patients, notably as an independent biomarker for disease recurrence and overall survival in patients with resected stage I/II non-small cell lung cancer (NSCLC) [7, 8].
Recently, studies have shown that one possible mechanism for the aberrant expression of survivin in cancer cells may result from activation of transcription factors to maintain survivin production [9]. Accordingly, survivin has been identified as a direct target of the Signal Transduction and Activator of Transcription-3 (STAT3), a member of a family of latent cytosolic transcription factors, in primary effusion lymphoma [9] and breast cancer [10]. Activation of STAT3 supports cell survival in association with survivin expression in gastric [11], breast and prostate cancer cells [12]. A strong association between STAT3 activation and survivin expression was found in cancer tissues [13, 14]. We confirmed this relationship in our previous study where we found that the use of a dominant-negative mutant of STAT3 reduced survivin transcription and subsequently sensitized breast cancer cells to radiation [15]. STAT3 is activated by phosphorylation of a conserved tyrosine residue (Tyr705) by kinases such as Janus kinase 2 (JAK2) in response to cytokine stimulation [16], subsequently promoting the dimerization of STAT3 monomers to render them in a transcriptionally-active conformation. STAT3 then translocates to the nucleus and binds to the promoter region of target genes and activates their transcription [17, 18]. While STAT3 activation is temporary in normal conditions, STAT3 is constitutively activated in many cancers and is commonly associated with a worse prognosis [19, 20]. Persistent activation of the JAK2/STAT3 signaling pathway contributes to carcinogenesis by promoting cell proliferation, metastasis, angiogenesis, and resistance to apoptosis. In this study, we examined whether TG101209, a new small-molecule inhibitor of JAK2, affected survivin expression in lung cancer, and its potential as a novel radiosensitizing agent for lung cancer.
Materials and Methods
Cell culture and chemicals
H460 (America Type Culture Collection, Rockville, MD) and HCC2429 (kindly provided by Dr. Tao Dang [21]) lung cancer cells were cultured in RPMI 1640 containing 10% fetal bovine serum and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C in a humidified cell culture chamber with 5% CO2. Since the American Type Culture Collection performs DNA profiling of cell lines and since all cells were cultured for less than 6 months before being reconstituted from frozen stocks, no additional authentication was done. Cells were maintained as monolayer cultures at 37°C in a humidified atmosphere of 5% CO2. TG101209 was obtained from TargeGen (Cambridge, MA).
Immunobloting
Cells were washed twice with ice-cold PBS and lysed in M-Per mammalian lysis buffer (Thermo Scientific, Rockford, IL, USA). The protein concentration of lysates was determined with the Bradford reagent (Bio-Rad, Hercules, CA, USA), and equal amounts of protein were subjected to SDS-PAGE of 10% or 15% gel. Separated proteins were transferred to a nitrocellulose membrane, which was exposed to 5% nonfat dried milk in 0.1% TBST for 1 h at room temperature before incubation overnight at 4°C with rabbit polyclonal antibodies to human survivin (1:2000 dilution; R&D Systems Minneapolis, MN, USA), to human STAT3 and phospho-STAT3 (1:1,000 dilution; Cell Signaling, Danvers, MA, USA), Caspase 3 (1:1,000 dilution; Cell Signaling), ERK and phospho-ERK (1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), Akt and phospho-Akt (1:1000 dilution; Cell signaling), HA (1:2,000 dilution; Roche Applied Science, Indianapolis, IN, USA) and actin (1:8000 dilution; Sigma, St Louis, MO, USA). The membrane was washed with TBS containing 0.1% Tween-20 before incubation for 1 hour at room temperature with horseradish peroxidase-conjugated goat antibodies to rabbit (Santa Cruz Biotechnology). Immune complexes were detected with chemiluminescence reagents (Perkin-Elmer Life Science).
Plasmids and transfections
pCGN and pCGN-HA-Kras-G12v were kindly provided by Dr. William Pao. HCC2429 cells were transfected 24 hours after being seeded in 6-well plates. Plasmids (1.5μg) in 100 μL of serum-free, antibiotic-free, opt-MEM (Invitrogen) were mixed with 5 μL Lipotectamine 2000 transfection reagent (Invitrogen), dissolved in 100 μL of the same medium and allowed to stand at room temperature for 20 min. The resulting 200 μL transfection solutions were added to each well containing 2 mL medium. Six hours later, the cultures were replaced with 2 mL fresh medium supplemented with 10% FBS and antibiotics. For western blot, cells were collected after an additional 48 hours. Drug or radiation treatment was performed 24 hours after tranfection and cells were collected at indicated time.
In vitro clonogenic assays
Exponentially growing cells were harvested by exposure to trypsin and plated in triplicate in 60 mm dishes containing 5 mL of complete medium in the presence of 1μM TG101209 or vehicle (final DMSO concentration of 0.1%; we confirmed that this DMSO concentration did not affect the proliferation of NSCLC cell lines). After incubation for 2 hours, cells were irradiated using a 137Cs irradiator (J.L. Shepherd and Associates). The dose rate was 1.8 Gy/min and dose range was 0 to 6 Gy. Fourty-eight hours after irradiation, cells were washed with PBS, cultured in drug-free medium for 7 to 8 days, fixed with 70% ethanol, and stained with 0.5 % crystal violet (Sigma, St. Louis, MO). Colonies containing >50 cells were counted. The dose enhancement factor (DER) was calculated as the dose (Gy) of radiation that yielded a surviving fraction of 0.2 for vehicle-treated cells divided by that for TG101209-treated cells (after correction for drug toxicity).
Tumor volume assessment
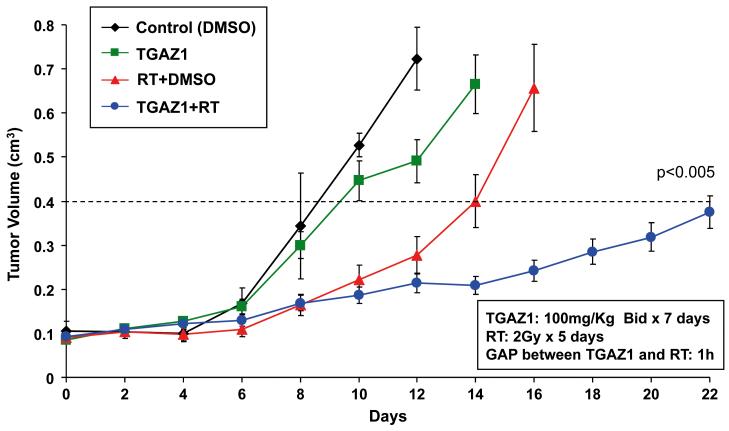
HCC2429 lung cancer cells (1×106 cells in 100 μL DMEM) were injected subcutaneously into the right flank of 5 to 6 week old female athymic nude (nu/nu) mice (Harlan Sprague Dawley Inc., Indianapolis, IN) using a 1-cc syringe with 27½-gauge needle. Tumors were grown for 6 to 8 days until average tumor volume reached 0.1 cm3. Treatment groups consisted of vehicle control, TG101209, RT, and combined TG101209 with radiation. Each treatment group contained 5 mice. TG101209 was administered orally at doses of 100 mg/kg BID for 7 consecutive days. Mice in radiation groups were irradiated 1 hour after TG101209 treatment with 2 Gy daily over 5 consecutive days. Tumors were irradiated using an X-ray irradiator (Therapax, Agfa NDT, Inc., Lewis Town, PA) while non-tumor parts of mice were shielded by lead blocks. Tumors were measured 2 or 3 times weekly in 3 perpendicular dimensions using a Vernier caliper. Tumor volumes were calculated using the modified ellipse volume formula (Volume = Height × Width × Depth/2). Tumor growth delay was calculated as the number of days required to reach a tumor volume of 0.4 cm3 for each treatment group relative to control.
Histological sections, vWF, Ki-67 and active caspase-3
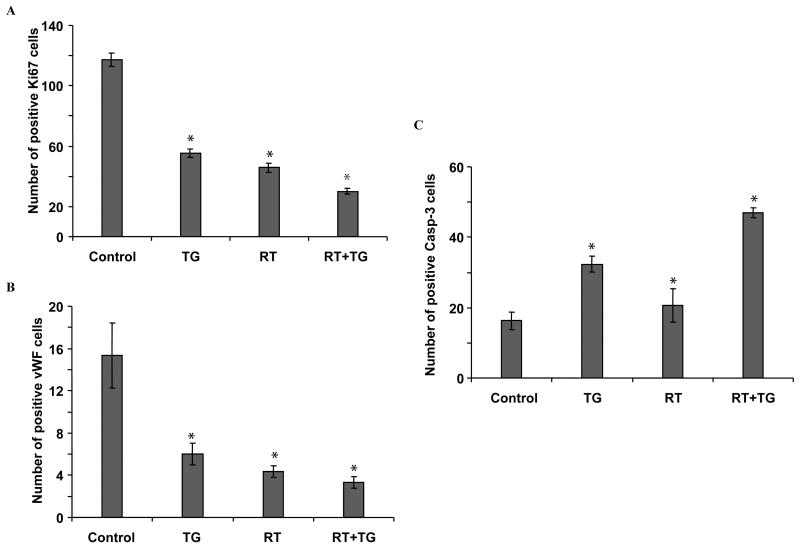
Mice were implanted with HCC2429 lung cancer cells as described above in the tumor volume studies. After 6-8 days, mice in the drug treatment group received an oral dose of 100 mg/kg TG101209 BID for 7 days, with or without radiation as described above in the tumor volume studies. After 7 days of treatment, tumors from each mouse were resected and fixed in paraffin. Slides from each treatment group were stained for Von Willebrand factor (vWF) using anti-vWF polyclonal antibody (Chemicon). Blood vessels were quantified by randomly selecting 400X fields and counting the number of blood vessels per field. This was done in triplicate and the average of the three counts was calculated. Staining for Ki67 (tumor proliferation) and active caspase-3 (apoptosis) were performed in the Vanderbilt University Pathology Core laboratory using standard protocols. The number of positive cells per 400X field were scored (by averaging multiple fields) and graphed by averaging three repeated experiments.
Statistical analysis
Standard error for all measured biological parameters is displayed in the appropriate figures. Student’s t-test was utilized to determine the significance between groups, using a statistical package. Significance was defined at the level of p < 0.05.
Results
TG101209 inhibits survivin and reduces phosphorylation of STAT3 in HCC2429 and H460 lung cancer cells
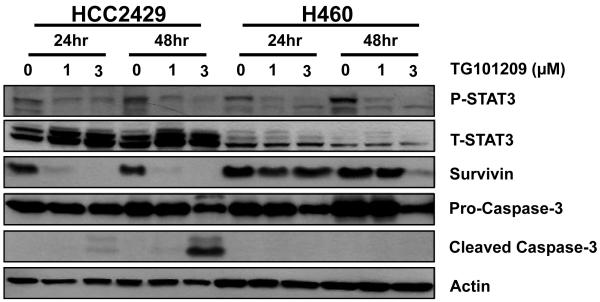
HCC2429 and H460 lung cancer cells were treated with varying concentrations (0-3 μM) of the JAK2 inhibitor TG101209 for varying lengths of time (0-48 h) to determine the optimal treatment conditions that would affect expression levels of total STAT3, phospho-STAT3 and survivin using western blot analysis. Because the goal of the study was to compare the response of the two cell lines to TG101209, the treatment dose was calibrated based on the more sensitive cell line, HCC2429. Therefore, 1 μM was chosen as the treatment dose as the HCC2429 cell line could not tolerate higher doses. Levels of apoptosis were assessed by caspase-3 cleavage. As shown in Fig. 1, 1 μM and 3 μM TG101209 reduced phospho-STAT3 in both cell lines while total STAT3 remained constant throughout with only a minor reduction after treatment with 3 μM TG101209 for 48 h. We also demonstrate that 1 μM of TG101209 is an adequate dose to inhibit pSTAT3, as there was no increased inhibition with 3 μM of TG101209 at the 24 h timepoint. Using similar treatment conditions, 1 μM TG101209 was able to inhibit survivin expression at both 24 h and 48 h in HCC2429 cells. In contrast, H460 cells required 3 μM TG101209 for 48 h to significantly reduce survivin. Finally, caspase-3 cleavage was observed in HCC2429 cells treated with 3 μM TG101209, particularly at 48 h, but was not detected in H460 cells using similar conditions.
Figure 1. TG101209 inhibits survivin and reduces phosphorylation of STAT3 in HCC2429 and H460 lung cancer cells.
HCC2429 and H460 cells were treated with 1 or 3 μM TG101209 for 24 or 48 h. Shown are results of western blots using labeled antibodies to phospho- and total STAT3, survivin, pro-caspase-3, and cleaved caspase-3. Actin was probed to demonstrate equal loading.
TG101209 results in radiosensitization of HCC2429 and H460 lung cancer cells in vitro
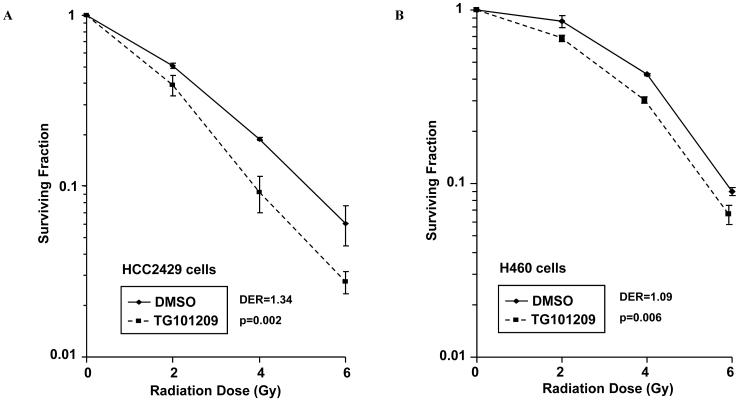
To investigate whether TG101209 radiosensitizes HCC2429 and H460 lung cancer cells, clonogenic assays were performed using 1 μM TG101209 with varying doses of radiation (0-6 Gy). Surviving colonies were quantified 8 days after irradiation to generate survival curves for both cell lines (Fig. 2). HCC2429 cells (Fig. 2A) receiving TG101209 had increased sensitivity to the lethal effect of radiation, with a drug enhancement ratio (DER) of 1.34 (p=0.002). Although statistically significant, H460 cells (Fig. 2B) only demonstrated a slight increase in radiosensitivity, as indicated by a DER of 1.09 (p=0.006). These data suggest that lower doses of radiation are required to achieve an equivalent anti-tumor effect when TG101209 is combined with radiation compared to radiation alone in vitro, particularly in HCC2429 lung cancer cells.
Figure 2. Radiosensitization of HCC2429 and H460 lung cancer cells by TG101209.
HCC2429 (A) or H460 (B) cells were treated with 1 μM TG101209 or DMSO for 2 h followed by irradiation with the indicated doses. Fourty-eight hour after radiation, drug-containing media was replaced with fresh media. After 8 days, surviving colonies were stained and scored. Shown are survival curves containing the mean ± the standard deviation of three separate, repeated experiments.
Effects of radiation and TG101209 on PI3K/Akt and Ras/MAPK/ERK pathways
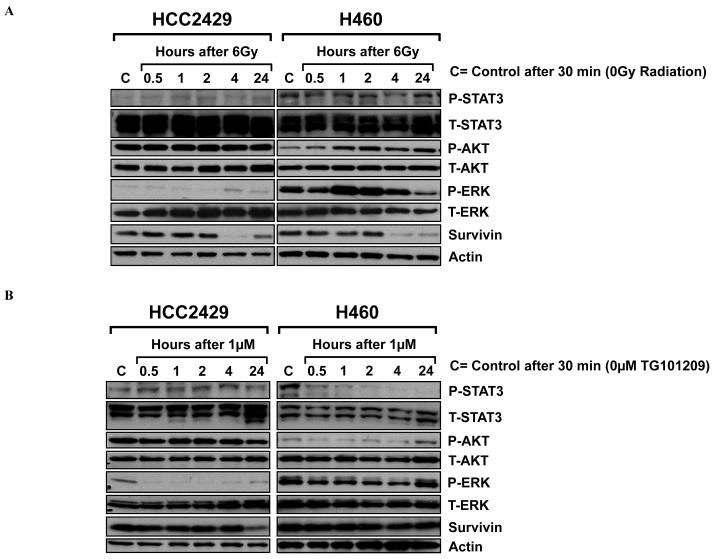
Our finding that TG101209 has greater effects in HCC2429 cells compared to H460 cells led us to investigate downstream proteins that can be affected by JAK2 including the phospho-inositide 3-kinase (PI3K)/Akt and the Ras/mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) pathways. As shown in Fig. 3A, radiation alone (6 Gy) promoted phospho-Akt and phospho-ERK expression in H460 cells. However, phospho-Akt levels were unchanged and phospho-ERK was only minimally detected in irradiated HCC2429 cells. After treatment with TG101209 (Fig. 3B), phospho-ERK and phospho-Akt protein levels were reduced by 30 min up to 4 h in H460 cells. This inhibition was not observed anymore at 24 h. In HCC2429 cells, phospho-ERK protein levels were reduced by 30 min after treatment with TG101209 while phospho-Akt was not affected. When HCC2429 and H460 cells were treated with a combination of radiation treatment and TG101209, there was a synergistic inhibition of phospho-ERK in treated H460 cells, whereas there was no significant difference between HCC2429 cells that were treated with combined treatment vs. a single agent (data not shown).
Figure 3. Effects of radiation and TG101209 on PI3K/Akt and Ras/MAPK/ERK pathways.
HCC2429 and H460 cells were treated with 6 Gy radiation (A) or 1 μM TG101209 (B) for 30 min, 1, 2, 4, and 24 h, or DMSO control. Shown are results of western blots using labeled antibodies to phospho- and total STAT3, phospho- and total Akt, phospho- and total ERK, and survivin. Actin was probed to demonstrate equal loading.
Differential effect of K-Ras mutation status on radiation- and TG101209-induced apoptosis in lung cancer cells
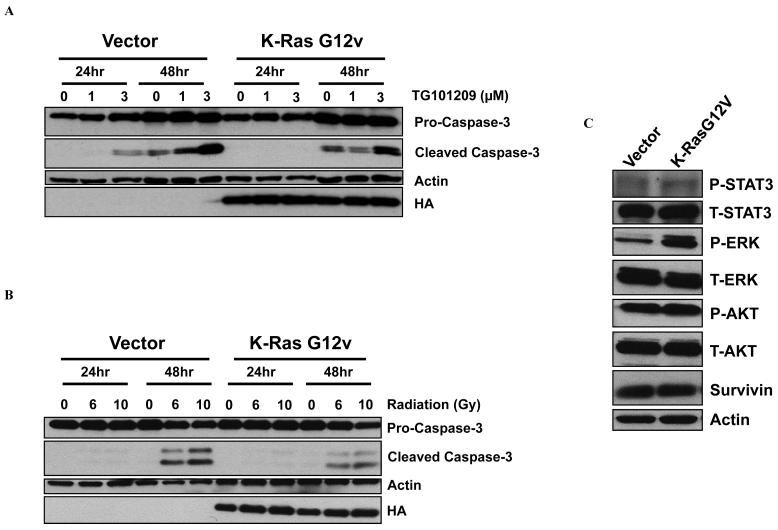
To further investigate the differential resistance of H460 cells to radiation-induced apoptosis compared to HCC2429 cells, we investigated whether the presence of a K-Ras mutation in H460 cells (and not in HCC2429) could be one potential factor that limits the induction of caspase cleavage. We transfected HCC2429 cells that are wild type K-Ras with a plasmid containing the K-Ras G12V mutation and tested caspase-3 cleavage by Western immunoblotting. As shown in Fig. 4A, HCC2429 cells transfected with K-Ras G12V mutation exhibited less apoptosis than HCC2429 control cells, as evidenced by decreased levels of cleaved caspase-3 after 1 or 3 μM TG101209. Similarly, 6 or 10 Gy radiation induced less caspase-3 cleavage in HCC2429 cells with K-Ras mutant as compared to HCC2429 vector control cells (Fig. 4B). This suggests diminished TG101209- and radiation-induced apoptosis in the presence of mutated K-Ras. In addition, a comparison of the protein profile between the K-Ras HCC2429 mutant cells and wild type HCC2429 cells revealed that phospho-ERK levels are greater in K-Ras mutant HCC2429 cells than in wild type controls (Fig. 4C).
Figure 4. Radiation- and TG101209-induced apoptosis is modulated by K-Ras mutation status in lung cancer cells.
HCC2429 K-Ras wild type cells were transfected with a plasmid containing the K-Ras G12V mutation and then were treated with either DMSO control, 1 or 3 μM TG101209 (A) or 0, 6 and 10 Gy radiation (B) for 24 and 48 h. Shown are results of Western blots using labeled antibodies to pro-caspase-3, cleaved caspase-3, and human influenza hemagglutinin (HA). Fourty-eight h after transfection, untreated wild type and K-Ras mutated HCC2429 cells were collected for Western blot and probed with antibodies to phospho- and total STAT3, phospho- and total ERK, phospho- and total Akt, and survivin. Actin was probed to demonstrate equal loading.
Combination of TG101209 and radiation extends tumor growth delay and is well-tolerated in vivo
To test whether the radiosensitizing effects of TG101209 on lung cancer cells in vitro could be translated in vivo, tumor growth was assessed in a mouse xenograft model in which HCC2429 lung cancer cells were injected into mouse hind limbs and treated as discussed in the material and methods section. Tumor growth delay was calculated as the number of days required to reach a tumor volume of 0.4 cm3 for treatment groups relative to control tumors. As shown in Fig. 5, tumor growth delay was significantly extended for mice receiving combination therapy of TG101209 and radiation compared to mice receiving radiation alone (~16 days vs. ~10 days, p=0.04). Notably, TG101209 treatment without radiation also significantly prolonged tumor growth, compared to no treatment (~3 days delay, p=0.04).
Figure 5. Combination of TG101209 and radiation prolongs tumor growth delay in lung cancer xenograft model.
HCC2429 lung cancer cells (1×106 cells in 100 μL DMEM) were injected subcutaneously into athymic nude mice. After 6 to 8 d, mice were treated with vehicle control, TG101209 (100 mg/kg BID for 7 consecutive days), radiotherapy, or combined TG101209 and radiotherapy (mice were irradiated 1 h after TG101209 treatment with 2 Gy daily for 5 consecutive days). Tumor growth delay as defined by the number of days required to reach a tumor volume of 0.4 cm3 was measured. Tumors were measured regularly and growth delay is calculated for treatment groups relative to control.
The body weights of all mice were monitored to assess the tolerability of systemic TG101209 therapy. As shown in Supplemental Fig. 1, body weight changes over the course of the experiment were minimal in all treatment groups, suggesting that TG101209 is well tolerated.
TG101209 reduces tumor proliferation and vascular density, and increases apoptosis in irradiated lung cancer mouse xenografts
To determine the mechanism that contributes to tumor growth delay extension following combination treatment in vivo, Ki67 proliferation index was examined using fixed tumor sections to quantify cellular proliferation. As shown in Fig. 6A, combined TG101209 and radiation treatment resulted in a ~4-fold reduction of proliferating cells compared to control (30 cells vs. 117 cells, p<0.001) and a 50% reduction compared to radiation alone (30 cells vs. 46 cells, p<0.001). Since tumor vasculature is an important target for cancer therapy, we assessed vessel density in tumor sections by staining for vWF. As is shown in Fig. 6B, the average number of vessels per microscopic field was lower for tumors treated with combination TG101209 and radiation than in tumors receiving radiation alone or control (3 vessels vs. 5 vessels vs. 15 vessels; p<0.001 for both comparisons). Finally, apoptosis was assessed using active caspase-3 staining (Fig. 6C). Because lymphocytes expressing caspase-3 can invade tumor tissue, we cannot exclude a sub-population of caspase-3-expressing lymphocytes, though we believe the number of cells would be small to negligible. Combined TG101209 and radiation treatment resulted in 47 positive apoptotic cells, compared to 20.7 in radiation alone (p<0.002) and 16.3 in control tumors (p<0.001). Representative photographs for fixed tumor sections stained for cellular proliferation (Ki67), vasculature (vWF), and apoptosis (active caspase-3) are presented in Supplemental Fig. 2.
Figure 6. TG101209 with radiation reduces Ki67 proliferative marker and blood vessel density, and increases apoptosis in lung tumor xenograft model.
Histological sections were obtained from the tumors of the mice in each treatment group from the tumor volume study. (A) Staining for Ki67 was performed to assess cell proliferation and the number of positive cells was scored and graphed by averaging three repeated experiments. (B) Slides were stained for Von Willebrand factor (vWF) using anti-vWF antibody to determine vessel density and the number of blood vessels per random 400x fields was scored and graphed by averaging three repeated experiments. (C) Staining for active caspase-3 was performed to measure apoptosis and the number of positive cells was scored and graphed by averaging three repeated experiments, *; p<0.05.
Discussion
In this report, we demonstrate the radiosensitizing effects of TG101209, a novel small molecule inhibitor of JAK2, in lung cancer models. We found that TG101209 was able to inhibit survivin, reduce phosphorylation of STAT3, and sensitize lung cancer cells to radiation in vitro. In lung cancer xenografts, the combination of TG101209 and radiation extended tumor growth delay and was well-tolerated. Analyses of tumor sections from in vivo experiments showed that TG101209 increased apoptosis while reducing tumor proliferation and vascular density.Survivin expression is notably absent in most terminally differentiated normal tissues, in contrast to its high expression in a variety of cancers [2]. Consistent with this observation, we detected high levels of survivin in both HCC2429 and H460 lung cancer cells in the absence of treatment (Fig. 1). Of particular interest, levels of survivin in lung cancer cells are among the highest found in human tumors [22], and its expression was found to be an independent biomarker for recurrence and survival in patients with NSCLC [8]. In addition, there is a growing body of evidence suggesting that persistent expression of survivin has an important role in radioresistance of malignant cells, likely through decreased apoptosis, which has been found in several tumor types, including colon [23], breast [24], and pancreas [25]. These characteristics make survivin an attractive target for cancer therapy and for radiosensitization. We previously found that overexpression of survivin leads to radiation resistance by inhibiting apoptosis and promoting cell survival, and survivin inhibition using antisense oligonucleotide enhanced the cytotoxic effects of radiation in H460 lung cancer cells [6].
In this study, we explored an alternative strategy to target survivin in lung cancer, based on previous findings that STAT3 regulates survivin transcription [10, 30] This likely mediates radiation resistance in breast cancer cells, as targeting phospho-STAT3 using a dominant-negative mutant subsequently sensitizes breast cancer cells to radiation [15]. In addition to its major role in hematologic malignancies, STAT3 is also activated in 55% of NSCLC tumors [31], and in most NSCLC cell lines in vitro [32], which was consistent with our results(Fig. 1 and 3). Because adenoviral delivery of dominant negative proteins may have only limited applications for cancer therapy [32], we targeted STAT3-mediated transcription of survivin using a small molecule JAK2 inhibitor, TG101209. Previous studies with TG101209 have focused on myeloproliferative disorders, mainly because of the primary role of JAK2 mutations such as V167F in those diseases [33-36].
In this study, we found that expression of survivin was inhibited at 24 and 48 h by 1 or 3 μM TG101209 and was positively associated with reduced level of phospho-STAT3 in HCC2429 cells (Fig. 1). In contrast to HCC2429 cells, we found the level of survivin to be only slightly reduced at 24 h in H460 cells and more greatly inhibited at 48 h using 3 μM TG101209 relative to control. This reduction in survivin paralleled the decreased phospho-STAT3 for both concentrations and time points. Similarly, there was a correlation between promotion of caspase-3 cleavage and reduction of survivin levels at 24 h in HCC2429 cells as opposed to H460 cells, which displayed higher survivin levels at both concentrations. Although a dose of 3 μM resulted in a significant decrease in survivin expression at 48 h, there was no detectable cleaved-caspase-3 in H460 cells, suggesting a more resistant apoptotic phenotype compared to HCC2429 cells. These data mirrored the clonogenic assay results (Fig. 2) where HCC2429 cells were more dramatically sensitized to radiation compared to H460 cells. These findings are consistent with other studies that show a correlation between the rate of apoptosis and intrinsic radiosensitivities of lung carcinoma cells [38].
One potential factor that might contribute to the enhanced resistance to apoptosis in H460 compared to HCC2929 cells is the presence of a K-Ras mutation found in H460 but not in HCC2429 cells [39]. To elucidate the role of this K-Ras mutation, we performed a K-Ras 12V mutant transfection in HCC2429 cells and analyzed TG101209- and radiation-induced caspase-3 cleavage. Interestingly, we found that radiation-induced apoptosis was considerably lower in K-Ras-transfected HCC2429 cells versus wild type controls, at both 24 and 48 h (Fig. 4B). Similarly, after TG101209 treatment (Fig. 4A), we were not able to detect cleaved caspase-3 at 24 h in K-Ras mutant cells compared to controls, and apoptosis was also reduced at 48 h. Taken together, these observations suggest that K-Ras mutation status is one potential factor associated with increased resistance to either radiation- or TG101209-induced apoptosis in lung cancer cells.
Another possible contributor to the increased radiation resistance in H460 cells may be the Ras/MAPK/ERK pathways, which may regulate STAT3 phosporylation through cross-talk [40]. As shown in Fig. 3A, phospho-ERK levels were higher in H460 cells than in HCC2429 cells, which might have contributed to increased resistance to radiation since activation of the ERK signaling pathway tends to be antiapoptotic [41]. Although radiation induces ERK activation in cells without K-Ras mutations [42], we found that phospho-ERK levels were significantly promoted in K-Ras mutant HCC2429 cells compared to vector control (Fig. 4C), which might induce inherent resistance to apoptosis and subsequent resistance to treatment. Indeed, about 30% of lung carcinomas contain activating K-Ras mutations, which are associated with resistance to various agents including EGFR tyrosine kinase inhibitors [41].
In this in vivo study (Fig. 6C), caspase-3 immunostaining was performed in tumor tissues. Following radiation treatment, there was a modest increase in active caspase-3 compared to control, confirming the relative resistance of lung tumors to radiation-induced apoptosis. With the combination of TG101209 and radiation, apoptosis was further increased compared to either radiation alone or drug alone, in an additive manner. Moreover, TG101209, similarly to radiation alone, was able to significantly reduce tumor cell proliferation compared to the control, while combination of both did not result in an additive effect (Fig. 6A). However, the addition of TG101209 to radiation synergistically extended tumor growth delay compared to radiation alone (Fig. 5), suggesting the potential of TG101209 as a radiation therapy enhancer in lung cancer.
In addition to its inhibitory role on JAK2, TG101209 has also been reported to inhibit JAK3, FLT3, and RET [36]. Therefore, we cannot conclude with complete certainty that JAK2 is wholly responsible for the radiosensitizing effect of TG101209. Although a role for FLT3 in survivin expression has not been described in lung cancer, one study has shown that FLT3-mediated signaling regulates survivin expression in acute myeloid leukemia [43]. Vargiolu et al. have shown that overexpression of RET inhibits a pro-apoptotic aryl hydrocarbon receptor-interacting protein (AIP)-survivin complex [44]. Thus, we assume inhibition of RET would induce an anti-apoptotic state, not a pro-apoptotic state as we have observed. Therefore, it is likely that any inhibition of RET by TG101209 was insufficient to disrupt association of the AIP-survivin complex. We are unaware of any publications indicating a role for JAK3 in the regulation of survivin expression. Taken together, our results indicate a strong role for abrogation of JAK2 in the radiosensitization of NSCLC with TG101209, though we cannot exclude down-regulation of other anti-apoptotic pathways such as PI3K or Ras/MEK.
This preclinical study supports the therapeutic potential of TG101209, a selective small molecule JAK2 inhibitor as a radiosensitizer for lung cancer. These findings also suggest that the effects of TG101209 involve the reduction of survivin expression levels and induction of apoptosis. Beneficial effects were confirmed in vivo where TG101209 plus radiation caused prolonged tumor growth delay compared to either radiation or drug alone. Further studies are needed to determine the clinical therapeutic potential of TG101209 in patients with NSCLC in combination with radiotherapy, and the value of K-Ras mutation status as a predictive factor for response to therapy and selection criteria.
Supplementary Material
Acknowledgments
This work was supported in part by National Cancer Institute grant 1R01 CA125842-01A1 and National Cancer Institute Lung Specialized Program of Research Excellence grant P50 CA90949.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 3.Chakravarti A, Zhai GG, Zhang M, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene. 2004;23:7494–7506. doi: 10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 4.Kami K, Doi R, Koizumi M, et al. Downregulation of survivin by siRNA diminishes radioresistance of pancreatic cancer cells. Surgery. 2005;138:299–305. doi: 10.1016/j.surg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Kappler M, Bache M, Bartel F, et al. Knockdown of survivin expression by small interfering RNA reduces the clonogenic survival of human sarcoma cell lines independently of p53. Cancer Gene Ther. 2004;11:186–193. doi: 10.1038/sj.cgt.7700677. [DOI] [PubMed] [Google Scholar]
- 6.Lu B, Mu Y, Cao C, et al. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res. 2004;64:2840–2845. doi: 10.1158/0008-5472.can-03-3547. [DOI] [PubMed] [Google Scholar]
- 7.Lu B, Gonzalez A, Massion PP, et al. Nuclear survivin as a biomarker for non-small-cell lung cancer. Br J Cancer. 2004;91:537–540. doi: 10.1038/sj.bjc.6602027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinohara ET, Gonzalez A, Massion PP, et al. Nuclear survivin predicts recurrence and poor survival in patients with resected nonsmall cell lung carcinoma. Cancer. 2005;103:1685–1692. doi: 10.1002/cncr.20951. [DOI] [PubMed] [Google Scholar]
- 9.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 10.Gritsko T, Williams A, Turkson J, et al. Persistent Activation of Stat3 Signaling Induces Survivin Gene Expression and Confers Resistance to Apoptosis in Human Breast Cancer Cells. Clinical Cancer Research. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 11.Kanda N, Seno H, Konda Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 12.Nam S, Buettner R, Turkson J, et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz N, Minton S, Cox C, et al. Activation of Stat3 in Primary Tumors from High-Risk Breast Cancer Patients Is Associated with Elevated Levels of Activated Src and Survivin Expression. Clinical Cancer Research. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 14.Schlette EJ, Medeiros LJ, Goy A, et al. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin Oncol. 2004;22:1682–1688. doi: 10.1200/JCO.2004.10.172. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Mutter RW, Cao C, et al. Inhibition of signal transducer and activator of transcription 3 activity results in down-regulation of Survivin following irradiation. Mol Cancer Ther. 2006;5:2659–2665. doi: 10.1158/1535-7163.MCT-06-0261. [DOI] [PubMed] [Google Scholar]
- 16.Zhong Z, Wen Z, Darnell J., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 17.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 18.Sasse J, Hemmann U, Schwartz C, et al. Mutational analysis of acute-phase response factor/Stat3 activation and dimerization. Mol Cell Biol. 1997;17:4677–4686. doi: 10.1128/mcb.17.8.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 20.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 21.Dang TP, Gazdar AF, Virmani AK, et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst. 2000;92:1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 22.Tamm I, Wang Y, Sausville E, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 23.Rodel C, Haas J, Groth A, et al. Spontaneous and radiation-induced apoptosis in colorectal carcinoma cells with different intrinsic radiosensitivities: survivin as a radioresistance factor. Int J Radiat Oncol Biol Phys. 2003;55:1341–1347. doi: 10.1016/s0360-3016(02)04618-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Iwamoto S, Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 25.Satoh K, Kaneko K, Hirota M, et al. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–278. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Asanuma K, Kobayashi D, Furuya D, et al. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1057–1062. doi: 10.1111/j.1349-7006.2002.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodel F, Hoffmann J, Distel L, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005;65:4881–4887. doi: 10.1158/0008-5472.CAN-04-3028. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara ET, Hallahan DE, Lu B. The Use of Antisense Oligonucleotides in Evaluating Survivin as a Therapeutic Target for Radiation Sensitization in Lung Cancer. Biol Proced Online. 2004;6:250–256. doi: 10.1251/bpo95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwasa T, Okamoto I, Suzuki M, et al. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–6504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 30.Haura EB, Zheng Z, Song L, et al. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–8294. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 31.Song L, Turkson J, Karras JG, et al. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 32.Mesri M, Wall NR, Li J, et al. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Fiskus W, Chong DG, et al. Cotreatment with panobinostat and JAK2 inhibitor TG101209 attenuates JAK2V617F levels and signaling and exerts synergistic cytotoxic effects against human myeloproliferative neoplastic cells. Blood. 2009;114:5024–5033. doi: 10.1182/blood-2009-05-222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma AC, Fan A, Ward AC, et al. A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp Hematol. 2009;37:1379–1386. e1374. doi: 10.1016/j.exphem.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Libani IV, Guy EC, Melchiori L, et al. Decreased differentiation of erythroid cells exacerbates ineffective erythropoiesis in beta-thalassemia. Blood. 2008;112:875–885. doi: 10.1182/blood-2007-12-126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pardanani A, Hood J, Lasho T, et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007;21:1658–1668. doi: 10.1038/sj.leu.2404750. [DOI] [PubMed] [Google Scholar]
- 37.Lee JW, Kim YG, Soung YH, et al. The JAK2 V617F mutation in de novo acute myelogenous leukemias. Oncogene. 2006;25:1434–1436. doi: 10.1038/sj.onc.1209163. [DOI] [PubMed] [Google Scholar]
- 38.Sirzen F, Zhivotovsky B, Nilsson A, et al. Spontaneous and radiation-induced apoptosis in lung carcinoma cells with different intrinsic radiosensitivities. Anticancer Res. 1998;18:695–699. [PubMed] [Google Scholar]
- 39.Sos ML, Fischer S, Ullrich R, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci U S A. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: Difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience. 2006;142:411–423. doi: 10.1016/j.neuroscience.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 41.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazlovitskaya EM, Linkous AG, Thotala DK, et al. Cytosolic phospholipase A2 regulates viability of irradiated vascular endothelium. Cell Death Differ. 2008;15:1641–1653. doi: 10.1038/cdd.2008.93. [DOI] [PubMed] [Google Scholar]
- 43.Fukuda S, Singh P, Moh A, et al. Survivin mediates aberrant hematopoietic progenitor cell proliferation and acute leukemia in mice induced by internal tandem duplication of Flt3. Blood. 2009;114:394–403. doi: 10.1182/blood-2008-11-188714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargiolu M, Fusco D, Kurelac I, et al. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J Clin Endocrinol Metab. 2009;94:2571–2578. doi: 10.1210/jc.2008-1980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.