Abstract
Background
The aim of this study was to determine if automated MRI Analysis Software (RAPID) can be used to identify stroke patients in whom reperfusion is associated with an increased chance of good outcome.
Methods
Baseline diffusion (DWI) and perfusion-weighted MRI scans (PWI) from DEFUSE (n=74) and EPITHET (n=100) were reprocessed with RAPID. Based on RAPID-generated DWI and PWI lesion volumes, patients were categorized according to three pre-specified MRI profiles that were hypothesized to predict benefit (Target Mismatch), harm (Malignant), and no effect (No Mismatch) from reperfusion. Favorable clinical response was defined as a NIHSS score of 0–1 or a ≥8 point improvement on the NIHSS score at day 90.
Results
In Target Mismatch patients reperfusion was strongly associated with a favorable clinical response (odds ratio 5.6; 95% CI 2.1–15.3) and attenuation of infarct growth (10±23 mL with reperfusion vs 40±44 mL without reperfusion p<0.001). In Malignant profile patients reperfusion was not associated with favorable clinical response (odds ratio 0.74; 95% CI 0.1–5.8) or attenuation of infarct growth (85±74mL with reperfusion vs 95±79 mL without reperfusion p=0.7). Reperfusion was also not associated with favorable clinical response (odds ratio 1.05; 95% CI 0.1–9.4) or attenuation of lesion growth (10±15 mL with reperfusion vs 17±30 mL without reperfusion p=0.9) in No Mismatch patients.
Conclusion
MRI profiles that are associated with a differential response to reperfusion can be identified with RAPID. This supports the use of automated image analysis software such as RAPID for patient selection in acute stroke trials.
Keywords: Stroke, tissue plasminogen activator, MRI
BACKGROUND
Treatment with intravenous tissue plasminogen activator (tPA), aimed at acutely restoring blood flow, reduces morbidity but its use is limited by the need to start treatment within 4.5 hours of stroke onset.1 It is, however, likely that the population of stroke patients who present beyond 4.5 hours is heterogeneous and includes some patients who would still benefit from reperfusion.2 Identification and effective treatment of the subgroup of patients who are likely to benefit would help reduce the burden of stroke. The combination of perfusion (PWI) and diffusion-weighted MRI (DWI) is one of the most promising techniques to select patients who are likely to benefit from reperfusion.3 The major drawback of this technique is that most currently available MRI processing algorithms require manual input and are therefore time-consuming and operator dependent. In order to overcome these barriers we designed RAPID, an operator-independent system for processing of PWI and DWI images. RAPID generates PWI and DWI maps, segments the PWI and DWI lesions, and calculates lesion volumes within 10 minutes of scan completion. These features make it an attractive tool for patient selection in acute stroke trials. The overall aim of this study was to compare RAPID, an automated processing system, to processing methods that have been used in prior stroke studies. We determined if it can be used to identify, based on PWI and DWI lesion data, patients for whom reperfusion is associated with an increased chance of good outcome. To achieve this goal imaging data from DEFUSE and EPITHET4, 5, two large prospective studies that have assessed the utility of MRI for patient selection, were reprocessed using RAPID. A pooled data-analysis was conducted on the reprocessed imaging data and the individual clinical data from both studies.
METHODS
Studies
Data from two studies, DEFUSE and EPITHET, were included in this pooled analysis.4, 5 Both studies were approved by local ethic committees and informed consent for participation was obtained from all patients in both studies. One patient who withdrew consent prior to treatment with study drug and one patient whose follow-up clinical outcomes (mRS and NIHSS scores) were not obtained are not included in this pooled dataset. DEFUSE was an open-label study of intravenous tPA administered in the 3–6 hour time-window and EPITHET was a randomized placebo-controlled study of intravenous tPA in the 3–6 hour time window. Supplemental table 1 (online only) documents the similarities and differences between the two studies.
Pooling methodology of imaging data
DWI and PWI data from DEFUSE and EPITHET were reprocessed in a standardized fashion using RAPID, an in-house developed automated image processing software package. Lesion volumes assessed using RAPID were compared with lesion volumes determined by the local DEFUSE and EPITHET investigators as part of their original analyses. Regression coefficients describing the relationship between RAPID and original DEFUSE volumes and between RAPID and original EPITHET volumes were calculated for DWI and PWI data separately.
DWI data
RAPID combines two independent methods to determine DWI lesion volumes: absolute and relative thresholding. The combination of these two methods was used to ensure that susceptibility pile-up artifacts, which appear hyperintense on b1000 images, were not falsely identified as acute stroke lesions. (See supplemental textbox 1 for a description of RAPID’s processing steps for DWI lesion segmentation.) Regions of interest (ROIs), generated automatically by RAPID, were reviewed by a single investigator (DdS), who manually excluded artifact from the ROI when indicated. DWI lesion volumes were calculated based on these corrected ROIs. This method differs from the method used in the original studies to assess DWI lesion volumes. In DEFUSE, DWI lesions were determined by a single reader using a semiautomated thresholding method in which voxels were included that exceeded the DWI signal intensity of a manually drawn control region in the contra-lateral hemisphere by more than three standard deviations. Artifacts were manually excluded from the lesion volume. In EPITHET the DWI lesion was calculated by averaging the results of two readers who independently drew manual outlines of the DWI lesion on the b1000 map.
PWI data
The original DEFUSE results were based on PWI maps generated by algorithms programmed in IDL and the original EPITHET results were based on PWI algorithms programmed in matlab. RAPID is programmed in C++. RAPID’s process of calculating PWI lesion volumes is described in supplemental textbox 2 and has recently been published in detail.6 The RAPID algorithm differs from the perfusion algorithms used in the original studies in three ways. First, RAPID has a built-in automated AIF selection tool, whereas the DEFUSE algorithm relied on a manually selected AIF and the EPITHET algorithm on a semi-automated AIF selection tool. Second, an AIF smoothing filter was used in the DEFUSE algorithm but not in the EPITHET and RAPID algorithms.7 Third, the EPITHET algorithm deconvolved the tissue signal using standard singular value decomposition (sSVD), whereas the DEFUSE and RAPID algorithms use circular deconvolution.6 ROIs corresponding to the perfusion lesion on Tmax maps, generated automatically by RAPID, were reviewed by a single investigator (DdS), who manually excluded artifact from the ROIs when indicated. PWI lesion volumes at four Tmax thresholds (>4sec, >6sec, >8sec, and >10sec) were derived from the corrected ROIs. PWITmax>6sec lesion volumes were used for determination of mismatch status.
Final infarct
Two investigators (SC and BC) independently reviewed all final infarct ROIs that were outlined by local investigators as part of the primary data analysis of the individual studies. Acute T2-weighted images were used to identify areas of pre-existing T2 hyperintensity. These areas were excluded from the final infarct. In cases where either of the secondary reviewers (SC and BC) felt that the ROI outlined by the local investigator was inaccurate, a final lesion determination was reached through an adjudication panel (SC, MM, ML).
MRA
For the pooled data analysis, two investigators (DdS and ML) jointly reviewed all MRA-ratings that were obtained by local investigators as part of the primary data analysis of the individual studies and condensed these primary data by consensus into the following two variables: 1) site of obstruction (ICA, MCA_M1, MCA_M2 or PCA) and 2) degree of obstruction (complete, partial, none).
Hemorrhagic Transformation/Parenchymal Hematoma
For the pooled analysis ECASS categories of hemorrhagic transformation/parenchymal hematoma were used.8 Presence of hemorrhage was adjudicated by a blinded committee in EPITHET and determined by a neuroradiologist in DEFUSE.
Definitions
MRI profiles
Each patient with a sufficient quality PWI and DWI at baseline was categorized into one of four mutually exclusive predefined MRI profiles: Malignant is defined as a DWI or PWITmax>8 lesion >100 mL; Small is defined as a DWI lesion <10 mL and a PWITmax>6 lesion <10 mL. Small lesion patients by definition do not qualify as mismatch which requires >10 mL difference between DWI and PWI lesion volumes; Target Mismatch includes all patients whose imaging does not meet criteria for the Malignant or Small profiles and who have a ratio of PWITmax>6 lesion volume / DWI lesion volume >1.2 and an absolute difference between PWITmax>6 lesion volume and DWI lesion volume >10 mL; and No Mismatch includes all patients who do not meet criteria for Target Mismatch, Malignant or Small lesion profile.
Reperfusion
Reperfusion definitions and assessments that were used in the original analyses of the individual studies have been adopted for the pooled analysis: for patients who were enrolled in DEFUSE a reduction in PWITmax>2 lesion volume of >30% and >10 mL between the baseline MRI and the 3–6 hour follow-up MRI qualifies as reperfusion; for EPITHET a reduction in PWITmax≥2 lesion volume of >90% and >10mL between the baseline MRI and the 5-day follow-up MRI qualifies as reperfusion. More stringent reperfusion criteria were used for EPITHET subjects because reperfusion was assessed at a later time-point (5 days) compared to DEFUSE (3–6 hours after tPA). Only patients with acceptable quality PWI data at baseline and at follow-up were included in reperfusion analyses. The effect of reperfusion was not assessed in patients with the “small lesion MR profile” because these patients, by definition, have a baseline PWI lesion volume that is too small (<10 mL) to qualify for reperfusion.
Recanalization is categorized as “none”, “partial” or “complete” for patients with evidence of a symptomatic vessel obstruction on their baseline MRA. Patients with any obstruction on baseline MRA and no improvement in MRA score on follow-up imaging are rated as “no racanalization”. Patients with complete obstruction on baseline MRA and partial obstruction on follow-up MRA are rated as “partial recanalization”. Patients with any obstruction on baseline MRA and no obstruction on follow-up MRA are rated as “complete recanalization”.
Favorable clinical response is defined as an improvement of 8 or more points on the NIHSS between baseline and 90 days or a score of 0 or 1 on the NIHSS at 90 days.
Good functional outcome is defined as a score of ≤2 points on the modified Rankin Scale at 90 days
Symptomatic ICH
The SITS-MOST sICH criteria were adapted for the pooled analysis.9
Lesion Growth is defined as the difference between the T2/FLAIR lesion volume on the final follow-up MRI (day 30 in DEFUSE; day 90 in EPITHET) and the DWI lesion volume on the baseline scan.
Statistical Analysis
Standard descriptive statistics and comparisons between groups were performed using a commercially available statistical software package (SPSS v17.0). Tests used included the chi-square and fisher exact tests for dichotomous variables and the Mann Whitney U and t tests for continuous data. Regression coefficients were also calculated using SPSS v17.0. Statistical tests were two-tailed and statistical significance was defined at α<0.05. Unadjusted pooled odds ratios to describe the relationships between reperfusion on various clinical outcomes were calculated using a fixed effect model in SPSS v17.0 and in Review Manager 5 (RevMan computer program, Version 5.0, Copenhagen: The Nordic Cochrane Centre). Adjusted odds ratios were calculated using a logistic regression model in SPSS v17.0. Criterion to enter variables in the model was set at α<0.1. A backward stepwise method was used to eliminate variables from the model.
RESULTS
Baseline characteristics of the DEFUSE and EPITHET study participants are listed in table 1. Compared to the DEFUSE patient cohort, patients enrolled in EPITHET were treated earlier, were more commonly hyperlipidemic, had larger DWI and PWI lesion volumes on their baseline MRI, and had larger mismatch volumes at their baseline evaluation.
Table 1.
| Baseline Characteristics | DEFUSE (n=74) | EPITHET (n=100) | p value |
|---|---|---|---|
| Female | 57% | 44% | 0.096 |
| Mean age ± SD | 71 ± 15 | 71 ± 13 | 0.779 |
| Hypertension | 45 (61%) | 71 (71%) | 0.159 |
| Diabetes mellitus | 20 (27%) | 22 (22%) | 0.444 |
| Hyperlipidemia | 18 (24%) | 40 (40%) | 0.030 |
| Current or past smoker | 31 (42%) | 37 (37%) | 0.513 |
| Median NIHSS (IQR) | 12 (8, 16) | 13 (8, 18) | 0.419 |
| Mean time to treatment in min ± SD | 324 ± 36 | 296 ± 46 | < 0.001 |
| Median baseline original DWI volume mL (IQR) | 10 (3, 27) | 20 (8, 45) | 0.001 |
| Median baseline RAPID DWI volume mL (IQR) | 6 (1, 22) | 12 (5, 33)a | 0.031 |
| Median baseline original PWI volume mL (IQR) | 49 (6, 97)b | 164 (87, 255)c | < 0.001 |
| Median baseline RAPID PWITmax>6 volume (IQR) | 34 (5, 86)b | 65 (23, 132)d | 0.003 |
| Median baseline original mismatch volume mL (IQR) | 26 (0, 69)b | 126 (60, 214)c | < 0.001 |
| Median baseline RAPID mismatch volume mL (IQR) | 16 (0, 68)b | 37 (12, 81)e | 0.003 |
|
| |||
|
Clinical outcomes at day 90
| |||
| Median NIHSS (IQR) | 4 (1, 12) | 5 (2, 16)c | 0.260 |
| mRS 0–2 | 37 (50%) | 42 (42%)c | 0.322 |
| mRS 0–1 | 25 (34%) | 30 (30%)c | 0.627 |
Data for 98 patients;
data for 67 patients;
data for 99 patients;
data for 95 patients;
data for 94
DWI data
RAPID was unable to process two cases. In both instances failure resulted from incorrect b-value settings of the diffusion images. Lesion volumes obtained with RAPID correlated highly with DWI lesion volumes obtained for the original DEFUSE (R2=0.87) and EPITHET (R2=0.84) analyses but were consistently smaller than original DWI lesion volumes. (supplemental figure A, online only). There was a greater difference between original and RAPID-derived DWI lesion volumes in the EPITHET dataset compared to the DEFUSE dataset (39% mean difference in lesion volume in EPITHET vs 22% mean difference in DEFUSE; p<0.01).
PWI data
RAPID was unable to generate an accurate baseline PWI lesion volume in 14 cases. Reasons for failure were severe head motion (n=5), no detectable gadolinium bolus (n=8) and scan not done (n=1). Five cases (one DEFUSE case and four EPITHET cases) whose baseline PWI data were considered technically adequate to determine a lesion volume in the original analyses were deemed of insufficient quality for data analysis using RAPID. These data were excluded from the pooled analysis. Two DEFUSE cases whose PWI data were deemed insufficient for the original analysis were successfully processed using RAPID and were included in the pooled analysis. The correlation between original and RAPID-derived PWI lesion volumes was higher for DEFUSE cases than for EPITHET (R2 is 0.84 for DEFUSE vs 0.56 for EPITHET; p<0.001) (supplemental figure B, online only). RAPID-derived PWI lesion volumes were smaller than the original PWI lesion volumes. The difference between original and RAPID-derived lesion volumes was greater for EPITHET cases (mean difference 55%) than for DEFUSE cases (mean difference 26%) (p<0.01).
MRA and Recanalization
Baseline MRA data are summarized in table 2. There was a trend towards more patients with MCA lesions in DEFUSE and more patients with ICA lesions or no MRA lesion in EPITHET (Chi-square=5.2; p=0.08). Complete recanalization as assessed on the early follow-up scan (obtained at 3–6 hours after treatment in DEFUSE and 3–5 days after treatment in EPITHET) occurred in 16 of 47 (34%) DEFUSE cases and 15 of 48 (31%) EPITHET cases (p=0.8). (Table 2)
Table 2.
MRA lesions at baseline and recanalization status at follow-up
| DEFUSE | EPITHET | |||||||
|---|---|---|---|---|---|---|---|---|
| MRA Lesion | Baseline N (%) | Follow-Up Recanalization Status | Baseline N (%) | Follow-Up Recanalization Status | ||||
| None | Partial | Complete | None | Partial | Complete | |||
| ICA complete occlusion | 6 (8%) | 17% | 83% | 0% | 15 (15%) | 60% | 40% | 0% |
| ICA partial occlusion | 6 (8%) | 83% | - | 17% | 5 (5%) | 100% | - | 0% |
| MCA M1 complete occlusion | 16 (22%) | 50% | 25% | 25% | 18 (18%) | 11% | 56% | 33% |
| MCA M1 partial occlusion | 12 (16%) | 58% | - | 42% | 7 (7%) | 14% | - | 86% |
| MCA M2 complete occlusion | 2 (3%) | 0% | 0% | 100% | 2 (2%) | 0% | 0% | 100% |
| MCA M2 partial occlusion | 2 (3%) | 0% | - | 100% | 1 (1%) | 0% | - | 100% |
| PCA occlusion | 3 (4%) | 33% | 0% | 67% | 0 (0%) | 0% | 0% | 0% |
| No lesion | 16 (22%) | 33 (33%) | ||||||
| Inadequate study | 11 (15%) | 19 (19%) | ||||||
Follow-up MRAs were obtained at 3–6 hours after treatment in DEFUSE and at 3–5 days after treatment in EPITHET. ICA indicates internal carotid artery; MCA M1 middle cerebral artery, first branch; MCA M2 middle cerebral artery, second branch; PCA posterior cerebral artery.
MRI mismatch profiles
Prior to standardization there was no difference in the percentage of Target Mismatch patients between EPITHET and DEFUSE (54% vs 49%, p=0.6), but the EPITHET study had more patients with the Malignant profile than DEFUSE (35% vs 9%, p<0.01) and fewer patients that had No Mismatch (6% vs 15%, p=0.05). These differences in baseline MRI profiles between EPITHET and DEFUSE remained largely unchanged following standardized processing of PWI and DWI images with RAPID software: The Target Mismatch profile was present in 53% of the EPITHET study population and 51% of the DEFUSE population (p=0.8); The Malignant profile was present in 25% and 9% (p=0.01); and No Mismatch in 10% and 15% (p=0.3) respectively. The inter-rater agreement in assessment of MR profile between original study criteria and RAPID was in the fair to good range (kappa 0.61) (Supplemental Table 2). Re-classification occurred in 40 cases. It was generally caused by the reduction in PWI lesion volume between the original assessment and the RAPID assessment. This led to a switch from Malignant profile to Target Mismatch in 12 cases, and from Target Mismatch to No Mismatch in 11 cases.
There were differences in baseline characteristics between the MRI profiles. Compared to patients with Target Mismatch, patients with the Malignant profile had their baseline MRI earlier (233±61 vs 263±46 min, p=0.03) and had larger DWI (60±44 vs 15±16 ccm, p<0.001) and PWI (182±50 vs 67±35 min, p<0.001) lesion volumes. Patients with the No Mismatch profile had smaller PWI lesions (20±12) than Target Mismatch patients (67±35, p<0.001).
Reperfusion
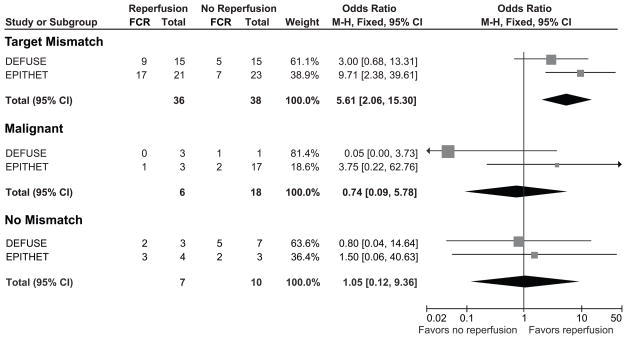
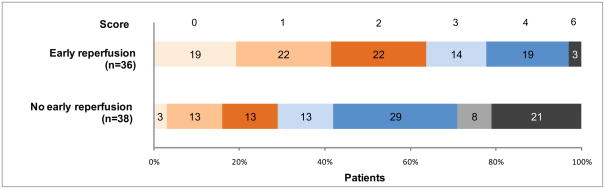
Reperfusion was strongly associated with a favorable clinical response in patients identified as having a Target Mismatch. The pooled odds ratio for favorable clinical response associated with reperfusion in Target Mismatch patients was 5.6 (95% CI 2.1–15.3; p=0.001) when considering all patients and 5.6 (95% CI 1.6–19.4; p=0.006) when considering only tPA treated patients (figure 1). Using a multivariate logistic regression model to adjust for an imbalance in baseline NIHSSS, the odds ratio for favorable outcome with reperfusion increased to 6.3 (95% CI 2.2–18.0; p=0.001). Reperfusion was also strongly associated with achieving good functional outcome (defined as a mRS 0–2) at 90 days in Target Mismatch patients. Sixty-four percent (23/36) of Target Mismatch patients with reperfusion achieved a good functional outcome compared to 29% (11/38) of Target Mismatch patients without reperfusion (p=0.003) (figure 2 and table 3). Percentages of poor functional outcome (mRS 5–6) were lower in Target Mismatch patients with reperfusion (3%) compared to Target Mismatch patients without reperfusion (29%; p=0.002).
Figure 1. Relationships between reperfusion and favorable clinical response in patients with the Target Mismatch, the Malignant and the No Mismatch profiles.
FCR indicates favorable clinical response, defined as a National Institutes of Health Stroke Scale score of 0–1 at day 90 or an improvement of ≥8 points on the NIHSS score between baseline and day 90. The forest plots demonstrate that reperfusion is associated with favorable clinical response in Target Mismatch patients (top), but not in patients with the Malignant (middle) and No Mismatch (bottom) profiles.
Figure 2. Modified Rankin Scale at 90 days in Target Mismatch patients.
The distribution of scores on the Modified Rankin Scale assessed at 90 days is shown for patients with a Target Mismatch. The statistical comparison of the outcomes was performed with the Mann-Whitney U test using all seven categories of the modified Rankin Scale (mRS). The outcomes of Target Mismatch patients with early reperfusion were more favorable compared to Target Mismatch patients without early reperfusion (p<0.001).
Table 3.
Clinical Outcomes Based on Magnetic Resonance Imaging Profiles and Reperfusion Status
| MRI profile | n | Me an Age (Yr) | Median Baseline NIHSS | Favorable Clinical Response (95% CI) | mRS of 0–2 at 90 days (95% CI) | SICH (95% CI) | mRS of 5–6 at 90 days or SICH (95% CI) |
|---|---|---|---|---|---|---|---|
| Target Mismatch with reperfusion | 36 | 75.2 | 15 | 72%(55–85)# | 64% (46–79)# | 0%(0–12) | 3% (0–16)* |
| Target Mismatch without reperfusion | 38 | 68.5 | 13 | 32%(18–49) | 29%(16–46) | 3%(0–15) | 29%(16–46) |
| Malignant with reperfusion | 6 | 81.3 | 19 | 17%(1–67) | 0%(0–48) | 50% (14–86)* | 83%(36–99) |
| Malignant without reperfusion | 18 | 67.3 | 17 | 17%(4–42) | 17%(4–42) | 0%(0–22) | 44%(22–67) |
| No Mismatch with reperfusion | 7 | 77.3 | 10 | 71%(30–95) | 57%(20–88) | 0%(0–44) | 14%(1–58) |
| No Mismatch without reperfusion | 10 | 66.8 | 8 | 70%(35–92) | 80%(44–96) | 10%(1–46) | 20%(4–56) |
| Small lesion profile | 26 | 71.1 | 8 | 65%(44–82) | 69%(48–85) | 0%(0–16) | 4%(0–22) |
| Unsuccessful baseline PWI | 14 | 73.4 | 12 | 36%(14–64) | 57%(30–81) | 0%(0–27) | 21%(6–51) |
| Unsuccessful follow-up PWI | 18 | 69.0 | 16 | 28%(11–54) | 22%(7–48) | 17%(4–42) | 56%(31–78) |
| All patients | 173 | 71.3 | 12 | 47%(39–55) | 46%(38–53) | 5%(2–9) | 24%(18–31) |
Favorable Clinical Response was defined as an improvement of 8 points or more in the National Institutes of Health Stroke Scale (NIHSS) score between baseline and 90 days or a score of 0–1 at 90 days. mRS indicates modified Rankin Scale; SICH, symptomatic intracerebral hemorrhage defined according to SITS-MOST criteria. Reperfusion was defined as a reduction in PWI lesion volume between baseline and early follow-up of >30% and >10 mL for DEFUSE subjects and >90% and >10 mL for EPITHET subjects. Mismatch profile was defined as a ratio between baseline PWITmax>6sec lesion and DWI lesion >1.2 and an absolute difference between baseline PWITmax>6sec lesion and DWI lesion > 10 mL.. Malignant profile was defined as a baseline DWI lesion >100 mL or a PWITmax>8sec lesion >100 mL. Target Mismatch was defined as Mismatch patients who did not have the Malignant profile. Small lesion profile was defined as a baseline DWI lesion <10 mL and a baseline PWITmax>6sec lesion < 10 mL.
p≤0.01 compared to patients with the same MR profile but without reperfusion
p≤0.001 compared to patients with the same MR profile but without reperfusion
In contrast, there was no association between reperfusion and favorable clinical response in patients with the Malignant or No Mismatch patterns. The pooled odds ratio for favorable clinical response following reperfusion was 0.74 (95% CI 0.1–5.8; p=0.8) for malignant patients and 1.05 (95% CI 0.1–9.4; p=1.0) for patients with No Mismatch when considering all patients (figure 1). These results remained largely unchanged when limiting the analysis to the subgroup of patients who were treated with tPA. The odds ratio for favorable clinical response following reperfusion was 0.5 (95% CI 0.1–4.5; p=0.5) for malignant patients and 1.4 (95% CI 0.1–12.6; p=0.8) for patients with No Mismatch. Percentages of poor functional outcome were similar in No Mismatch patients with and without reperfusion but trended higher in Malignant patients with reperfusion (83%) compared to Malignant patients without reperfusion (44%, p=0.2) (table 3).
Symptomatic intracerebral hemorrhage developed only in patients treated with tPA. sICH was not associated with reperfusion in Target Mismatch and No Mismatch patients but occurred more frequently in Malignant patients with reperfusion (50%) compared to Malignant patients without reperfusion (0%, p=0.01).
Lesion growth, defined as the difference between final infarct volume and the baseline RAPID-derived DWI volume, was attenuated by reperfusion in Target Mismatch patients, but not in Malignant and No Mismatch patients (table 4). These results were not altered by restricting the analysis to patients who were treated with tPA.
Table 4.
Lesion Growth according to MRI profile and reperfusion status
| MRI pattern | Lesion Growth with Reperfusion | Lesion Growth Without Reperfusion | p value* | ||||
|---|---|---|---|---|---|---|---|
| n | Mean(SD) | Median(IQR) | n | Mean(SD) | Median(IQR) | ||
| Target Mismatch | 31 | 10.4 (23.3) | 1.3 (−3.0, 16.7) | 27 | 40.1(43.9) | 26.8(5.8, 56.4) | <0.001 |
| Malignant | 2 | 84.6(74.4) | 84.6(32.0, ) | 12 | 95.3(79.2) | 81.7(40.3, 160.7) | 0.8 |
| No Mismatch | 7 | 10.1(14.7) | 6.0(−2.8, 21.1) | 7 | 16.7(30.5) | 11.5(−4.3, 21.1) | 0.9 |
by Mann-Whitney U test
DISCUSSION
This study has two important implications. First, the results indicate that assessment of DWI and PWI lesion volumes is highly dependent on the way the MRI images are processed and analyzed. This is illustrated by the fact that the methods used to determine DWI and PWI lesion volumes in the original DEFUSE study, the original EPITHET study, and this pooled analysis each yield different results. Thus, the use of DWI and PWI lesion criteria for patient selection in multi-center trials and, eventually, possibly also in clinical practice will require standardization of image processing methods across centers. Second, it demonstrates, in a large pooled cohort, that an automated MRI image analysis software suite (RAPID) can be used to identify patients for whom reperfusion is associated with an increased chance of good outcome. Consequently, RAPID processing of a baseline MRI study has the potential to determine if a patient is a good candidate to receive treatment aimed at restoring perfusion to the ischemic brain.
A standardized approach for DWI and PWI lesion volume assessment was applied. This was implemented by reprocessing and re-measuring the DWI and PWI lesion volumes using RAPID, an automated MRI analysis software suite. Previous studies that have pooled data from multiple sites have generally relied on volumetric assessments obtained locally.10 Given the many different steps involved in lesion volume measurement, this approach almost inevitably leads to wide heterogeneity in terms of lesion volume assessment. Our data illustrate this. For the DEFUSE data, the original DWI volumes were approximately 20% larger than corresponding RAPID DWI volumes, whereas the original EPITHET DWI volumes were almost 40% larger than RAPID volumes. Differences between DEFUSE and EPITHET in DWI lesion assessment are likely the result of differences in the methods used to identify infarct core on DWI/ADC maps and in the selection of regions of interest when manually outlining lesions.
A similar difference was seen for PWI assessments. The original DEFUSE PWI volumes were about 25% larger than corresponding RAPID PWI volumes, whereas the original EPITHET volumes were about 55% larger than the RAPID volumes. These differences are partially explained by the different Tmax thresholds used to identify tissue at risk in the three processing pipelines used to generate PWI maps. The Tmax threshold critically influences the volume of the PWITmax lesion; lower Tmax thresholds result in larger PWI lesions. Consequently, the shorter Tmax threshold used in EPITHET (Tmax≥2) compared to DEFUSE (Tmax>2) in part explains why the original EPITHET PWI lesions were larger than original DEFUSE PWI lesions.
Difference in the way the AIF was chosen (manual vs automatic) and the type of AIF filters used in the various PWI processing algorithms likely introduced additional variability.
As expected, the PWI volumes obtained by RAPID in this pooled analysis are smaller than original lesion volumes because RAPID uses a PWI-Tmax threshold of >6sec. This relatively stringent threshold was chosen for three reasons. First, due to differences in the processing algorithm, RAPID yields somewhat larger volumes than the software programs used in DEFUSE and EPITHET for any given Tmax threshold. Second, the use of shorter Tmax thresholds in RAPID resulted in the inclusion of artifacts within the PWI lesion, which precludes the ability of RAPID to automatically segment PWI lesions. Third, the choice of a longer and thus more stringent Tmax threshold reduces the inclusion of benign oligemia in the PWI lesion, which results in better correlations between the baseline PWI lesion volume and the final infarct volume in patients who do not reperfuse.11
This study demonstrates the feasibility of determining MRI profiles with automated image analysis software. RAPID’s success rate for determining MRI profiles was 92%. The 8% failure rate was related to poor quality of the original PWI data, either from severe patient motion (n=5) during image acquisition or because gadolinium contrast was not administered correctly (n=8). A newer beta-version of RAPID with improved motion correction capability is currently being tested and may further improve the success rate for determining MRI profiles in the acute stroke setting.
The results of RAPID-based patient selection in terms of patient outcome are encouraging and consistent with data from prior studies. The Target Mismatch was present in fifty-two percent of the patients in the pooled dataset. This percentage is comparable to data from a previous study that reported a Target Mismatch profile in 58–68% of patients eligible for recanalization therapy.12 The subgroup of patients with a Target Mismatch according to RAPID had a 5.6-fold increase in the odds of favorable outcome with reperfusion. Two other distinct MRI profiles, The Malignant profile and the No Mismatch profile, were present in 17% and 11% of patients, respectively. Consistent with prior data, patients with a Malignant MRI profile according to RAPID may be harmed by reperfusion.4 In this subgroup reperfusion was associated with an increased chance of sICH without an increase in the chance of favorable clinical outcome. Similarly, rates of favorable outcome did not differ depending on reperfusion status in patients with the No Mismatch profile. However, regardless of reperfusion, patients with the No Mismatch profile had a high chance (approximately 70%) of favorable clinical response, whereas patients with the Malignant profile had a very low chance of favorable clinical response (10–20%).
Given the consistency with prior studies in terms of patient selection and outcome, it should be noted that RAPID-based patient selection does not identify patients who are better candidates for reperfusion therapy than conventional MRI processing methods. Instead, the main advantage of RAPID lies in its automated processing which makes patient selection easier, faster and more consistent.
The main limitation of this study is the inability to standardize the assessment of reperfusion between the EPITHET and DEFUSE cohorts. In EPITHET reperfusion was assessed at 5 days based on >90% reduction in PWI volume, whereas in DEFUSE, reperfusion status was assessed 3–6 hours after administration of tPA based on >30% reduction in PWI volume. The percentage of reperfusion was similar in tPA-treated patient in EPITHET (56%) and DEFUSE (53%). Our estimates of the effect of reperfusion are probably conservative, because late assessment of reperfusion in EPITHET has likely diluted the effect of reperfusion in the pooled dataset.
In summary, significant heterogeneity can be introduced by the use of differing image processing and lesion segmentation methods. Such heterogeneity can be eliminated by the use of RAPID, an automated MR image processing suite that provides an objective assessment of a patient’s PWI and DWI lesion volumes. RAPID-generated quantitative DWI and PWI maps can be used to select patients in whom reperfusion is associated with an increased chance of good outcome. This makes automated image analysis software such as RAPID a potentially useful tool for patient selection in acute stroke trials.
Supplementary Material
Acknowledgments
Funding
This study was supported by the National Institutes of Health (NINDS, K23 NS051372). The DEFUSE study was supported by the National Institutes of Health (NINDS, RO1 NS39325; K24 NS044848). The EPITHET study was supported by National Health and Medical Research Council, Australia; National Stroke Foundation, Australia; Heart Foundation of Australia.
Footnotes
Conflicts of Interest
Steven Davis has served on advisory boards for Lundbeck, Boehringer Ingelheim, and Talls for which he has received compensation, and he has received honoraria for speaking at educational meetings and compensation for related travel expenses. Gregory Albers has served on advisory boards for Genentech and Lundbeck. Maarten Lansberg, Geoffrey Donnan, Bruce Campbell, Patricia Desmond, Deidre De Silva, Jun Lee, Jean Marc Olivot, Michael Mlynash, Roland Bammer, Soren Christensen, and Matus Straka declare that they have no conflicts of interest.
Contributor Information
Maarten Lansberg, Stanford University.
Jun Lee, Stanford University.
Soren Christensen, Royal Melbourne Hospital and University of Melbourne, Melbourne, Australia.
Matus Straka, Stanford University.
Deidre A De Silva, National Neuroscience Institute, Singapore General Hospital Campus, Singapore.
Michael Mlynash, Stanford University.
Bruce C Campbell, University of Melbourne, Melbourne, Australia.
Roland Bammer, Stanford University.
Jean-Marc Olivot, Stanford University.
Patricia Desmond, National Stroke Research Institute, Austin Health.
Stephen M Davis, Royal Melbourne Hospital and University of Melbourne, Melbourne, Australia.
Geoffrey A Donnan, Florey Neuroscience Institutes, Austin Health, University of Melbourne, Melbourne, Australia.
Gregory W Albers, Stanford University.
References
- 1.Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick J, Brott T, Frankel M, Grotta J, Haley EC, Kwiatkowski T, Levine SR, Lewandowski C, Lu M, Lyden P, Marler JR, Patel S, Tilley BC, Albers GW, Bluhmki E, Wilhelm M, Hamilton S ATLANTIS Trials Investigators, ECASS Trials Investigators, NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: Pooled analysis of atlantis, ecass, and ninds rt-pa stroke trials. The Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 2.Lansberg MG, Schrooten M, Bluhmki E, Thijs VN, Saver JL. Treatment time-specific number needed to treat estimates for tissue plasminogen activator therapy in acute stroke based on shifts over the entire range of the modified rankin scale. Stroke. 2009;40:2079–2084. doi: 10.1161/STROKEAHA.108.540708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qureshi AI, Kirmani JF, Sayed MA, Safdar A, Ahmed S, Ferguson R, Hershey LA, Qazi KJ Area ftBM, Erie County Stroke Study Group. Time to hospital arrival, use of thrombolytics, and in-hospital outcomes in ischemic stroke. Neurology. 2005;64:2115–2120. doi: 10.1212/01.WNL.0000165951.03373.25. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP for the DEFUSE Investigators. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Annals of Neurology. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 5.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, Chalk JB, Fink JN, Kimber TE, Schultz D, Hand PJ, Frayne J, Hankey G, Muir K, Gerraty R, Tress BM, Desmond PM. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. Lancet Neurology. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 6.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part i: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36:715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 8.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Second european-australasian acute stroke study investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study (sits-most): An observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 10.Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, Rovira A, Liebeskind DS, Gass A, Rosso C, Derex L, Kim JS, Neumann-Haefelin T. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Annals of neurology. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 11.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, Bammer R, Marks MP, Albers GW. Optimal tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang OY, Saver JL, Lee KH, Kim GM, Chung CS, Kim SJ, Ovbiagele B, Alger JR, Liebeskind DS. Characteristics of patients with target magnetic resonance mismatch profile: Data from two geographically and racially distinct populations. Cerebrovasc Dis. 2010;29:87–94. doi: 10.1159/000256653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.