Abstract
Vertebrate lens development depends on a complex network of signaling molecules to coordinate cell proliferation, migration and differentiation. In this study, we have studied the role of heparan sulfate in lens specific signaling by generating a conditional ablation of heparan sulfate modification genes, Ndst1 and Ndst2. In this mutant, N-sulfation of heparan sulfate was disrupted after the lens induction stage, resulting in reduced lens cell proliferation, increased cell death and defective lens fiber differentiation in later lens development. The loss of Ndst function also prevented the assembly of Fgf/Fgfr complexes on the lens cell surface and disrupted ERK signaling within the lens. We further demonstrated that Ndst mutation completely inhibited the FGF1 and Fgf3 overexpression phenotypes, but Kras reactivation was sufficient to reverse the Ndst deficient lens differentiation defect. The epistatic relationship between Ndst and FGF-Ras signaling demonstrates that FGF signaling is the predominant signaling pathway controlled by Ndst in lens development.
Keywords: Ndst, lens, heparan sulfate, FGF, Ras
INTRODUCTION
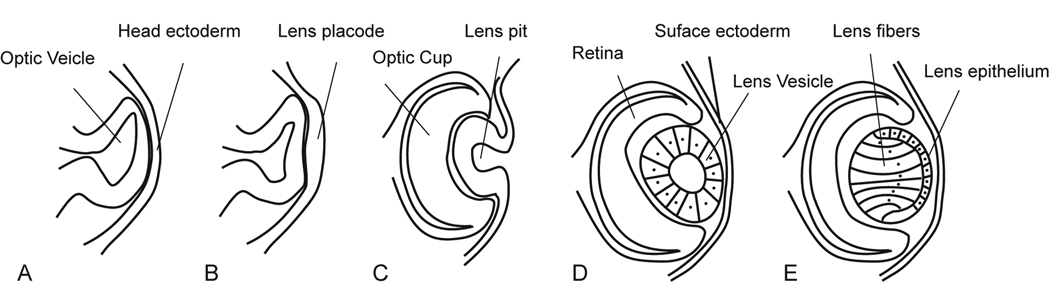
Vertebrate lens development requires a binary interaction between the optic vesicle and its overlying ectoderm (Swindell et al., 2008). The first noticeable morphologic event is the appearance of the optic vesicle, a neural ectodermal evagination of the diencephalon. The expanding optic vesicle comes in contact with the overlying head ectoderm, inducing the ectoderm to thicken into a cuboidal layer of cells known as the lens placode (Fig. 1A and B). Tissue specification continues as the optic vesicle invaginates to form a double-layered optic cup, while the lens placode invaginates to become the lens vesicle (Fig. 1C and D). Although all the cells in the early lens vesicle are capable of proliferation, soon after the vesicle forms, the cells in the posterior hemisphere of the vesicle withdraw from the cell cycle and elongate to form the primary lens fiber cells. Subsequent growth of the lens occurs by proliferation of the epithelial cells lining the anterior lens hemisphere, which continuously migrate toward the equator where they differentiate and elongate into secondary lens fiber cells (Fig. 1E).
Figure 1. Diagram of vertebrate lens development.
See text for details (adapted from Faber et al., 2002).
The proliferation and differentiation of the anterior lens epithelial cells into mature lens fibers is controlled by a complex array of signaling pathways. In explant culture experiments, Fgf1 and Fgf2 promoted lens epithelial cell proliferation and fiber cell differentiation in a dose dependent manner (McAvoy and Chamberlain, 1989). Consistent with this, transgenic mice overexpressing Fgfs in the lens consistently exhibited premature differentiation of the anterior lens epithelial cells and abnormal lens growth (Lovicu and Overbeek, 1998; Robinson et al., 1998; Robinson et al., 1995). Conversely, transgenic mice expressing a truncated form of Fgfr1 or a secreted form of Fgfr3 as a soluble receptor antagonist, exhibited lens fiber cell differentiation defects, while genetic ablation of Fgfr1–3 blocked lens fiber cell development. (Chow et al., 1995; Govindarajan and Overbeek, 2001; Robinson et al., 1995a; Stolen and Griep, 2000; Zhao et al., 2008). Studies have also demonstrated that BMP signaling is essential for cell differentiation during lens development. The BMP signaling antagonist, noggin, interfered with fiber cell elongation in explant culture, and this inhibition could be reversed by exogenous BMPs (Belecky-Adams et al., 2002). In transgenic mice, a dominant-negative BMP receptor Alk6 (Bmpr1b) inhibited lens fiber cell differentiation, while deletion of type I BMP receptors, Bmpr1a and Acvr1, abolished lens development (Faber et al., 2002; Rajagopal et al., 2009). Finally, both in vivo and in vitro studies have also implicated Wnt and Notch signaling in lens morphogenesis (Cain et al., 2008; Lyu and Joo, 2004; Ogino et al., 2008; Rowan et al., 2008; Smith et al., 2005; Stump et al., 2003).
We have studied lens development by focusing on heparan sulfate proteoglycans, which are known to play important roles in many of the signaling pathways described above. Heparan sulfate proteoglycans are glycoproteins with covalently linked sugar polymers, in which the glycosaminoglycan polysaccharide side chains are heavily modified by deacetylation, sulfation and epimerization (Esko and Selleck, 2002; Nybakken and Perrimon, 2002). These modifications allow heparan sulfate to interact with numerous signaling molecules, regulating their retention, transport and interactions with cell surface receptors. We have previously shown that a systemic knockout of Ndst1, an N-deacetylation/N-sulfation gene for heparan sulfate, disrupted both lens induction and lacrimal gland development (Pan et al., 2008; Pan et al., 2006). During early lens development, we showed that ERK signaling was down regulated in the Ndst1 mutants, but BMP and Wnt signaling appeared unaffected.
In this study, we examine the role of heparan sulfate in lens fiber development by disrupting Ndst-mediated heparan sulfate modification after lens induction. This leads to reduced lens epithelial proliferation, increased cell apoptosis and delayed primary lens fiber elongation. Consistent with the role of heparan sulfate in FGF signaling, the assembly of FGF/FGFR complexes on the lens cell surface is disrupted in Ndst null mutants. In a series of genetic epistasis experiments, we show that Ndst deletion completely prevents FGF-induced lens cell proliferation and differentiation, whereas constitutively activated Ras-ERK signaling restores Ndst–deficient lens fiber elongation. Therefore, Ndst deficiency predominantly disrupts FGF-Ras signaling during lens differentiation.
MATERIALS AND METHODS
Mice
To generate the lens-specific Ndst mutants, we crossed the previously described Ndst1flox mice and the Ndst2KO mice (kindly provided by Dr. Lena Kjellén, University of Uppsala, Uppsala, Sweden) with the Le-Cre mice (kindly provided by Dr. Ruth Ashery-Padan, Tel Aviv University, Tel Aviv, Israel and Dr. Richard Lang, Children's Hospital Research Foundation, Cincinnati, OH) (Ashery-Padan et al., 2000; Forsberg et al., 1999; Grobe et al., 2005). For genetic epistasis experiments, we used previously described transgenic FGF1 (OVE 371) and Fgf3 (OVE 391) mice, and obtained LSL-KrasG12D mice from the Mouse Models of Human Cancers consortium (MMHCC) Repository at National Cancer Institute (Robinson et al., 1998; Robinson et al., 1995b; Tuveson et al., 2004). The animals were maintained in mixed genetic background. All experiments were performed in accordance with institutional guidelines.
Histology and Immunohistochemistry
We performed routine hematoxylin and eosin histology according to a standard protocol. After digital imaging, the maximum area of the lens for each sample was measured using the ImageJ program (National Institute of Health, Bethesda, MD) and analyzed by the one-way ANOVA test as previously described (Pan et al., 2010).
For immunoflourescent staining, we used a standard antigen retrieval protocol (Pan et al., 2008; Pan et al., 2006), except that a Tyramide Signal Amplification kit (TSA™ Plus System, PerkinElmer, Waltham, MA) was used to amplify the phospho-ERK staining signal (Cai et al., 2010). The following antibodies were used: anti-E-Cadherin (U3254, BD Biosciences, San Jose, CA), anti-phospho-ERK1/2 (#4370, Cell Signaling Technology, Beverly, MA), anti-GFP (a gift from Dr. Pamela Silver, Harvard Medical School, Boston, MA), anti-Pax6 (PRB-278P, Covance, Berkeley, CA), anti-Prox1 (both from Covance, Berkeley, CA) and anti-heparan sulfate (10E4, Seikagaku, Tokyo, Japan). Anti-phospho-Smad1 (PS1) antibody was kindly provided by Peter ten Dijke (Leiden University Medical Center, Leiden, The Netherlands) and Carl-Henrik Heldin (Ludwig Institute for Cancer Research, Uppsala, Sweden). Anti-α, β and γ crystallins are kindly provided by Dr. Sam Zigler (National Eye Institute, Bethesda, DC).
Brdu analysis and TUNEL assays were also performed as previously described (Pan et al., 2008; Pan et al., 2006). Cell proliferation and apoptosis rates were calculated as BrdU- or TUNEL-positive cells versus DAPI-positive cells and analyzed by the Student’s t test.
Ligand and Carbohydrate Engagement (LACE) assay
The LACE assay was used to probe the in situ binding affinity of Fgf-Fgfr complexes to heparan sulfate on lens sections as previously described (Allen and Rapraeger, 2003; Pan et al., 2008; Pan et al., 2006). Recombinant Fgf1, Fgf3, Fgfr1b-Fc, Fgfr2b-Fc and Fgfr3b-Fc were obtained from R&D Systems, Minneapolis, MN.
RESULTS
Generation of lens-specific Ndst mutants
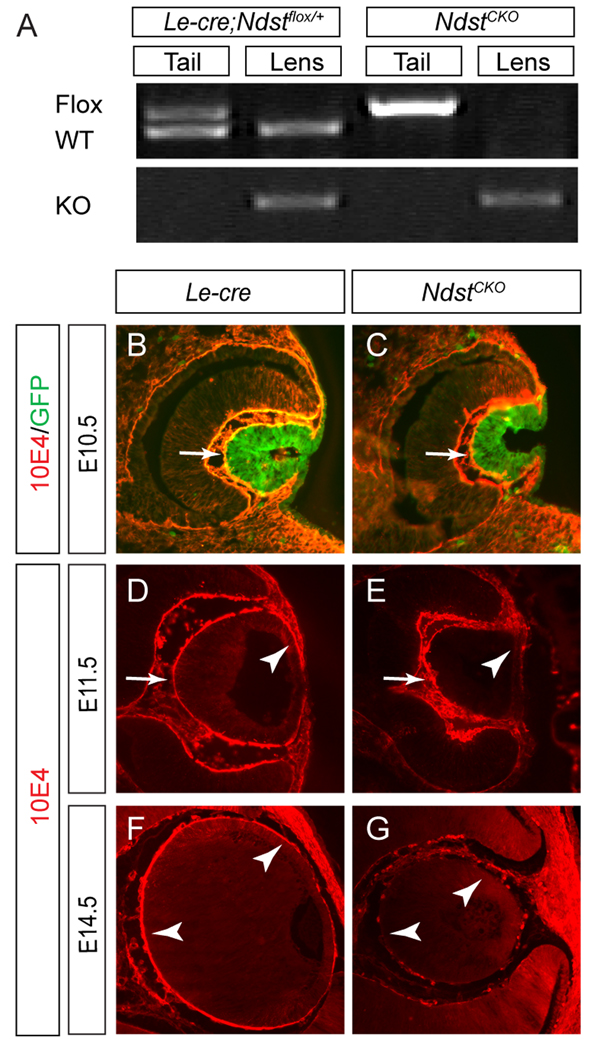
We have previously shown that both Ndst1 and, to a lesser extent Ndst2, are expressed in the E12.5 mouse lens (Pan et al., 2006). We thus crossed a floxed allele of Ndst1 and a knockout allele of Ndst2 with the Le-Cre transgene to ablate both genes in the lens. In the newborn Le-Cre;Ndst1flox/WT;Ndst2KO/KO (designated as Le-Cre;Ndstflox/+) pups, Ndst1flox and Ndst1WT alleles were detected by genotyping PCR in the tail DNA, whereas only the Ndst1KO and Ndst1WT alleles were found in the lens DNA (Fig. 2A). In the homozygous Le-Cre;Ndst1flox/flox;Ndst2KO/KO (designated as NdstCKO) pups, the Ndst1flox and Ndst1KO alleles were present exclusively in tail or lens DNA, respectively (Fig. 2A). These results demonstrated the lens-specific excision of the Ndst1flox alleles by the Le-Cre transgene in the NdstCKO mutants.
Figure 2. Disruption of heparan sulfate in NdstCKO mutants.
(A) Tail and lens DNA were collected from the new born Le-Cre;Ndst1flox/+;Ndst2KO/KO (designated as Le-Cre;Ndstflox/+) and the homozygous Le-Cre;Ndst1flox/flox;Ndst2KO/KO (designated as NdstCKO) pups. Genotyping PCR using primers to specifically detect the Ndst1flox allele (top band, upper panel), the Ndst1WT allele (lower band, upper panel) or the NdstKO allele (lower panel) confirmed that the complete conversion of the Ndst1flox allele to the NdstKO allele in the lens. (B–G) Although the Le-Cre transgene expression was detected in the E10.5 lens vesicle as shown by the GFP reporter (green fluorescence), heparan sulfate 10E4 staining (red fluorescence) appeared normal until E11.5 when staining was reduced specifically in the anterior NdstCKO lens cell basement membranes (arrowhead in E) and was not depleted in the entire NdstCKO lens until E14.5 (G). At least ten embryos of each genotype were tested for 10E4 staining.
We next examined the timing 2of heparan sulfate deficiency in the NdstCKO lens. At E10.5, Le-Cre was already active in the invaginating lens placode as shown by its bicistronic expression of GFP on the same transgene (Fig. 2B and C). Although we have previously shown that the antibody 10E4 recognizes Ndst-dependent sulfation of heparan sulfates (Pan et al., 2006), in both the control and the NdstCKO mutants, 10E4 staining in the basement membranes of the lenses were unchanged (Fig. 2B and C, arrows), suggesting that the heparan sulfate modification was unaffected at this stage. Only by E11.5 did the loss of 10E4 staining become visible at the anterior rim of the lens vesicle, but the posterior lens cells still retained heparan sulfate staining (Fig. 2D and E, arrows and arrowheads). At E14.5, the heparan sulfate 10E4 staining was now completely lost in all NdstCKO mutant lens cells (Fig. 2F and G, arrows). Thus, the apparent delay in heparan sulfate deficiency after Cre expression bypassed the lens induction defect previously observed in the systemic Ndst1 mutants (Pan et al., 2006), allowing us to examine the role of the Ndst genes in later lens development.
Ndst ablation resulted in severe lens defects
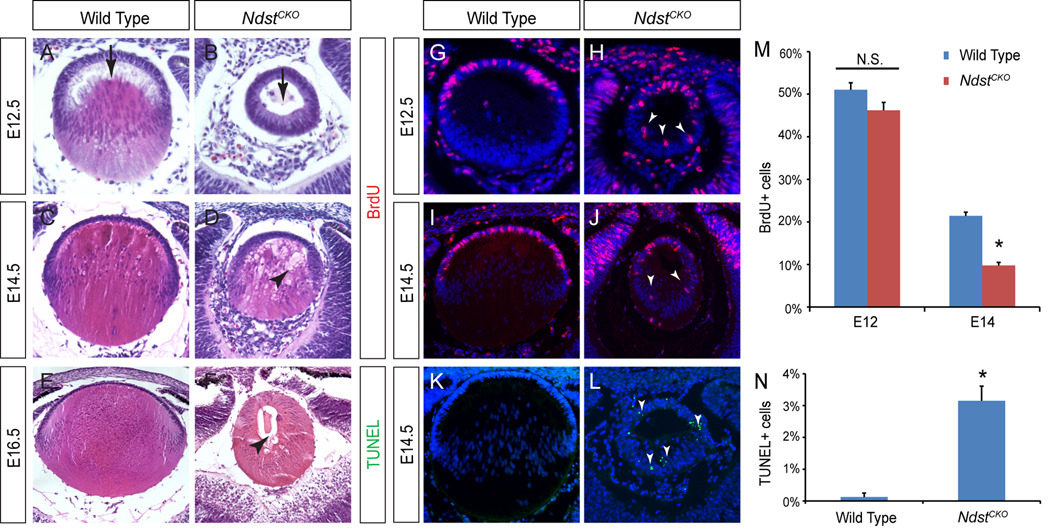
The NdstCKO lens phenotype first appeared after the closure of the lens vesicle. At E12.5, when the wild type primary lens fiber cells have elongated from the posterior lens vesicle toward the anterior rim, the NdstCKO lens remained a hollow vesicle (Fig. 3A and B, arrows). Although the elongated lens fiber cells were eventually found in E14.5 NdstCKO mutants, the fibers appeared disorganized with numerous vacuoles (Fig. 3C and D, arrowhead). At E16.5, the vacuoles were still present in the NdstCKO mutant lenses, which were also considerably smaller than the wild type controls (Fig. 3E and F, arrowhead). To further explore the lens size defects, we next measured lens cell proliferation by BrdU incorporation and apoptosis by TUNEL staining. At E12.5, the NdstCKO mutant had ectopic BrdU positive cells in the posterior lens vesicle, but in the anterior half of the lens, its BrdU index was statistically indistinguishable from that of the wild type (Fig. 3G, H and M, arrowheads). At E14.5, however, the percentage of BrdU positive cells at the anterior rim of the NdstCKO mutant lens was significantly reduced as compared to the wild type lens epithelial cells (Fig. 3I, J and M). In contrast, the percentage of TUNEL positive cells in the whole mutant lens were greatly increased (Fig. 3K, L and N, arrowheads). Therefore, both cell proliferation defects and apoptosis contributed to the NdstCKO lens phenotype.
Figure 3. NdstCKO mutant lens phenotype.
(A–B) At E12.5, primary lens fiber cell elongation was delayed in NdstCKO mutant lenses (arrows). (C–D) E14.5 NdstCKO mutant lens fibers were disorganized amid numerous vacuoles (arrowhead). (E–F) Significant reduction in lens size and persistent lens vacuoles (arrowhead) were observed in the E16.5 NdstCKO mutant. At least ten embryos of each genotype were used for histology analysis. (G–N) Cell proliferation as shown by BrdU incorporation was significantly reduced in the NdstCKO mutants at E14.5 but not at E12.5, while cell death as shown by TUNEL staining was strongly elevated (Students t-test: N.S., not statistically significant; *P < 0.01; n=3).
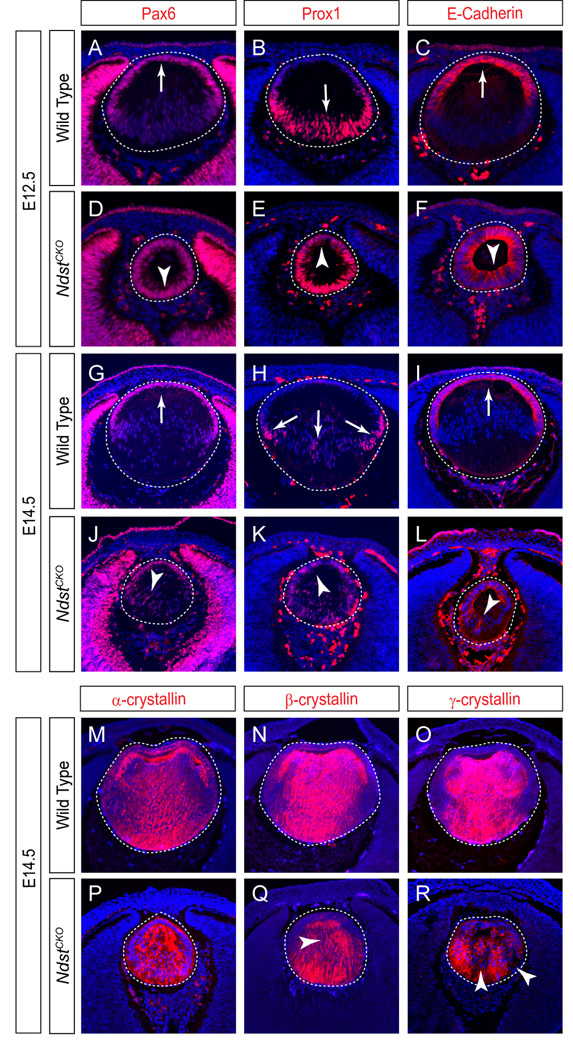
To further investigate the Ndst lens fiber cell elongation defects, we next examined molecular markers of lens development. Pax6, a critical transcription factor for lens determination and differentiation, was normally expressed throughout the nascent lens vesicle at E10.5, but became restricted to the anterior lens epithelium at E12.5 (Fig. 4A, arrow). In contrast, Pax6 expression persisted in the posterior lens vesicle of the E12.5 NdstCKO embryos (Fig. 4D, arrowhead). Prox1, another transcription factor uniformly expressed in early lens vesicle, was normally up regulated in the posterior of the E12.5 lens vesicle, where Prox1 promotes lens fiber cell differentiation (Fig. 4B, arrow). In the E12.5 NdstCKO mutant, Prox1 was again found in both the anterior and posterior of the lens vesicle (Fig. 4E, arrowhead). Finally, the whole NdstCKO mutant lens vesicle also maintained the expression of the epithelial marker, E-Cadherin, suggesting that the loss of Ndst function prevented the timely differentiation of the lens epithelium into lens fibers at this stage (Fig. 4C and F, arrow and arrowhead). The ectopic Pax6, Prox1 and E-Cadherin expression persisted at least until E14.5 (Fig. 4G–L, arrows and arrowheads), when lens fibers eventually arose in the NdstCKO mutant. Consistent with these molecular abnormalities, the intensities of β- and γ-crystallins also appeared to be reduced in the E14.5 NdstCKO lens (Fig. 4M–R, arrowheads). Notably, similar abnormal cell proliferation and apoptosis coupled with aberrant lens gene expression patterns (Pax6, Prox1, E-Cadherin and crystallins) have all been previously observed in the Fgfr1–3 deletion mutants (Zhao et al., 2008), suggesting that Ndst may be required for FGF signaling during lens differentiation.
Figure 4. Defective lens cell differentiation in NdstCKO mutants.
(A–F) In the E12.5 NdstCKO mutant, transcription factors Pax6 and Prox1 were misexpressed in the posterior and anterior lens vesicle, respectively. Consistent with this, the whole lens vesicle retains the lens epithelial marker, E-Cadherin. (G–L) Ectopic Pax6, Prox1 and E–Cadherin expression was reduced but still detectable at E14.5 (arrowheads). (M–R) α-, β-, and γ-Crystallins were present in the E14.5 NdstCKO mutant lens, but their levels appeared reduced compared to wild type controls (arrowheads). Three embryos of each genotype were tested for each staining.
Ndst mutation disrupted FGF signaling
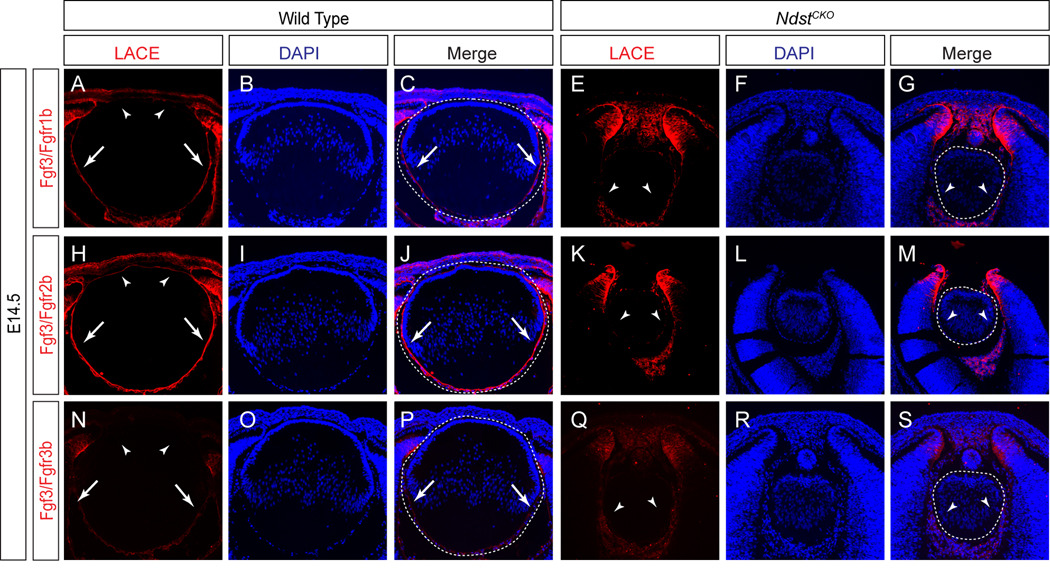
The strong phenotypic resemblance between Ndst and Fgfr mutants led us to further explore FGF signaling in NdstCKO lens. We thus performed the Ligand and Carbohydrate Engagement (LACE) assay by applying recombinant Fgf directly to lens tissue sections, together with the extracellular domains of Fgfrs conjugated to an Fc domain. Previous studies have demonstrated that endogenous heparan sulfate present on these sections can stabilize the in situ assembly of Fgf/Fgfr complexes, which are detectable by antibodies against the Fc domain (Pan et al., 2008; Pan et al., 2006). Fgf3 is known be expressed during eye development and transgenic expression of Fgf3 has been shown to promote lens fiber cell differentiation (Robinson et al., 1998; Wilkinson et al., 1989). Using the LACE assay, we observed strong binding of Fgf3/Fgfr2b complexes, weak binding of Fgf3/Fgfr1b complexes and almost no binding of Fgf3/Fgfr3b complexes on the basement membrane of wild type lens cell surfaces (Fig. 5A–C, H–J and N–P, arrows), which is consistent with the known binding pattern of Fgf3 with these specific Fgfr isoforms in cell culture studies (Ornitz et al., 1996). Interestingly, the intensities of the LACE signals in the lens appeared to follow an anterior-to-poster gradient, with the anterior lens epithelium exhibiting the weakest Fgf/Fgfr complex binding (Fig. 5A, H and N, arrowheads). In the E14.5 NdstCKO mutants, however, Fgf3/Fgfr complex binding was still present in the adjacent retina, despite being lost in the lens (Fig. 5E–G, K–M and Q–S, arrowheads). These results confirmed that N-sulfation of heparan sulfate by Ndst is necessary for Fgf3 binding to its cognate Fgfr partners on the lens cell surface.
Figure 5. Loss of Fgf/Fgfr interactions on the NdstCKO lens cell surface.
(A–S) In the presence of wild type heparan sulfate, the LACE assay showed that Fgf3 bound with increasing affinity with Fgfr1b and Fgfr2b, but not Fgfr3b, on the lens cell surface (arrows). Binding of Fgf3 to Fgfr1b and Fgfr2b was lost in the NdstCKO mutant lenses (arrowheads). The lenses are outlined in dotted ovals. Three embryos of each genotype were tested for each Fgf/Fgfr pair.
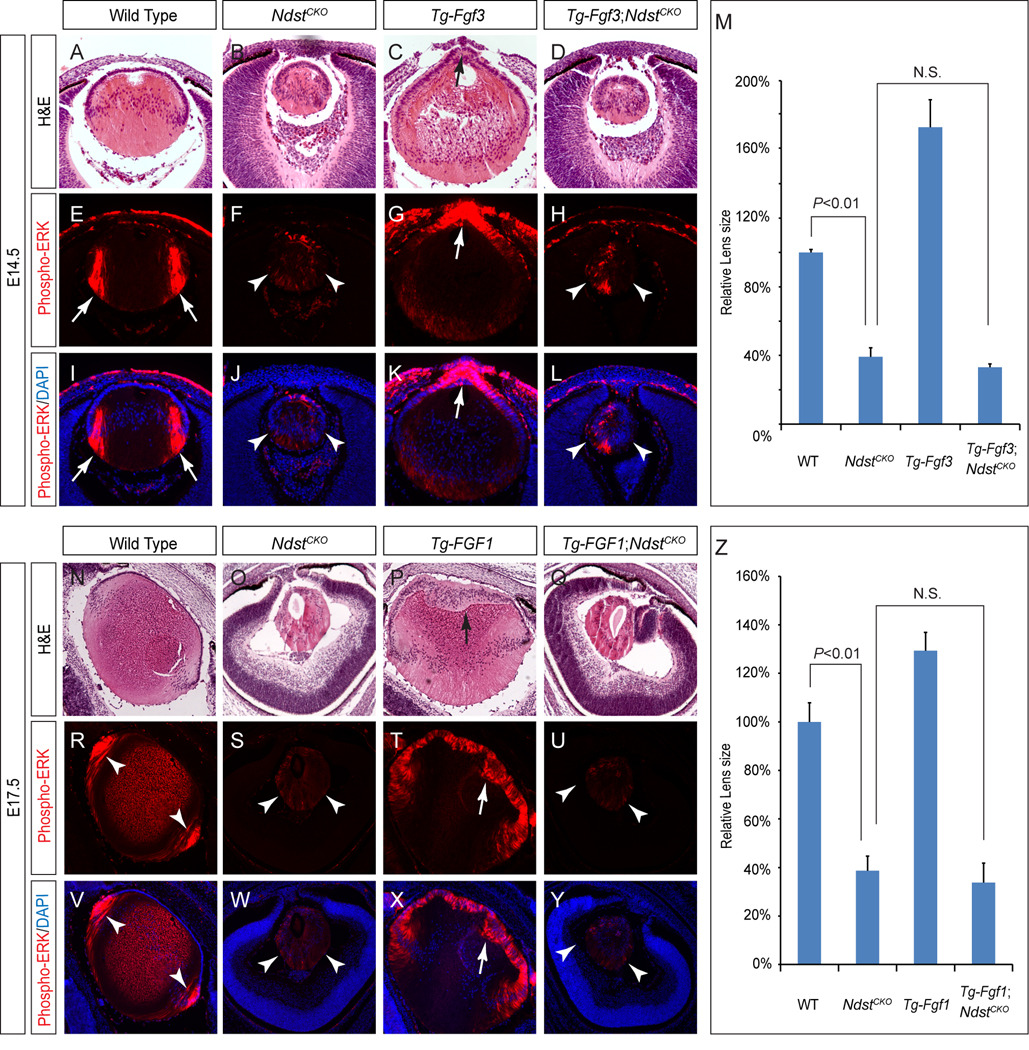
To investigate whether Ndst ablation disrupted FGF signaling during lens differentiation in vivo, we took advantage of a previously characterized transgenic line that overexpresses murine Fgf3 in the developing lens (henceforth denoted as Tg-Fgf3) (Robinson et al., 1998). In E14.5 wild type controls, endogenous FGF signaling is known to promote ERK phosphorylation in the bow region of the lens, where newly differentiated lens cells start to elongate into lens fibers (Fig. 6A, E and I, arrows). Such phospho-ERK signals were strongly diminished in the NdstCKO mutant lens, consistent with the idea that FGF signaling was down regulated (Fig. 6B, F and J, arrowheads). In contrast, Tg-Fgf3 embryos exhibited strongly elevated phospho-ERK levels at the anterior ridge of the lens, resulting in premature elongation of lens epithelial cells and protrusion of lens material into the anterior chamber of the eye (Fig. 6C, G and K, arrows). Remarkably, both the ERK hyper-phosphorylation and the premature lens differentiation were completely suppressed in the Tg-Fgf3; NdstCKO embryos, and the double mutant lenses remained much smaller than both the wild type and the Tg-Fgf3 lenses (Fig. 6D, H, L and M, arrowheads). Therefore, the Ndst mutation is epistatic to Fgf3 overexpression during lens development. To extend this finding to other FGF family members, we next crossed NdstCKO mutant mice with another transgenic line overexpressing a secreted form of human FGF1 (henceforth denoted as Tg-FGF1), since murine Fgf1 is also known to be present in the developing lens (Robinson et al., 1995b). At E17.5, while phospho-ERK activity was restricted in the wild type lens and greatly reduced in the NdstCKO mutant lens, ERK phosphorylation in the Tg-FGF1 lens was significantly elevated along the entire anterior rim, which expanded to acquire the morphology of the differentiated lens fibers (Fig. 6N–P, R–T and V–X, arrow and arrowheads). The Tg-FGF1; NdstCKO double mutants, however, still closely resembled the NdstCKO single mutant with respect to phospho-ERK staining and lens histology (Fig. Q, U, Y and Z, arrowheads). Taken together, these results demonstrated that both Fgf3 and FGF1 signaling require Ndst function in lens development.
Figure 6. Genetic epistasis between FGF signaling and Ndst.
(A–L)Transgenic overexpression of Fgf3 in the wild type lens led to premature lens cell differentiation (C, arrow) and increased ERK phosphorylation (G and K, arrows) at the anterior of the lens. However, both the small lens size and reduced phospho-ERK staining was not rescued in the Tg-Fgf3;NdstCKO embryos (D, H and L, arrowheads). (M) No statistical significance (N.S.) was observed for NdstCKO (n=4) and Tg-Fgf3;NdstCKO (n=8) by one way ANONVA test. (N–Y) Ndst mutation also completely blocked the lens anterior epithelial expansion (Q, arrow) and elevated ERK phosphorylation (U and Y, arrows) observed in the FGF1 transgenic overexpression lens (P, T and X, arrows). (R) The lens size of NdstCKO (n=6) mutants is greatly reduced compared to the wild type (n=4, P<0.01), but it was no different compared to that of Tg-FGF1;NdstCKO(n=4, N.S., no statistical significance) by one way ANONVA test.
Genetic epistasis between Ndst and Kras signaling
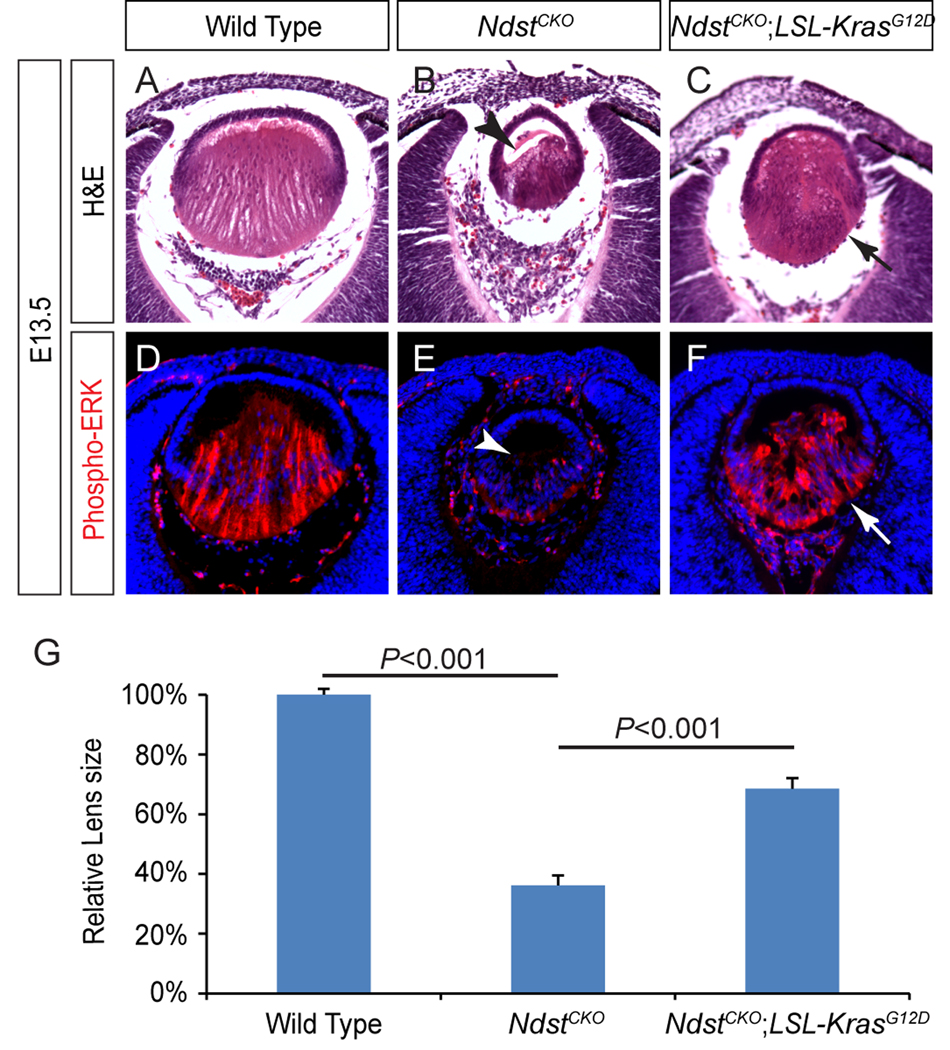
Having demonstrated that the Ndst mutation is epistatic to FGF signaling at the lens fiber cell differentiation stage, we next asked what is genetically downstream of Ndst. Considering that the Ras-MAPK pathway is one of the main effector pathways of FGF signaling, we chose to directly activate Ras signaling using an oncogenic allele of Kras (LSL-KrasG12D), which is normally silenced by a transcriptional stop cassette flanked by a pair of LoxP sites (Tuveson et al., 2004). When crossed with a Cre transgene, however, the stop cassette can be cleaved to allow tissue-specific expression of the constitutively active KrasG12D mutant. The advantage of this approach is that, as an allelic knock-in, KrasG12D is expected to be expressed at the normal physiological level, avoiding the confounding effects of Ras overexpression. Indeed, we did not observe any obvious lens phenotypes in the Le-Cre; LSL-KrasG12D mutants (Supplementary Figure 1). On the other hand, the E13.5 NdstCKO mutants exhibited deficient primary lens fiber elongation, leaving the anterior portion of the lens still empty (Fig. 7 B, arrowhead). As expected, ERK phosphorylation was also significantly reduced (Fig. 7E, arrowhead). In comparison, phospho-ERK staining was greatly elevated in the NdstCKO;LSL-KrasG12D lens. Importantly, lens fiber cells in the double mutant fully elongated to reach the anterior rim of the lens, resulting in a significant increase in the lens size (Fig. 7C, F and G). Therefore, Kras activation was sufficient to restore lens fiber elongation in the NdstCKO mutants.
Figure 7. Kras signaling reversed the Ndst mutant phenotype.
(A–F)The delay in lens fiber elongation in the E13.5 NdstCKO mutant (B, arrowhead) was reversed by activation of Kras signaling in the NdstCKO;LSL-KrasG12D lens (C, arrow). Consistent with this phenotypic rescue, phospho-ERK staining was also restored in the NdstCKO;LSL-KrasG12D lens (compare E and F, arrow and arrowhead). (G) Quantification of lens size. [One way ANOVA test: P<0.01 for the NdstCKO mutants (n=8) compared to either the wild type (n=10) or the NdstCKO;LSL-KrasG12D (n=10).]
DISCUSSION
Heparan sulfate proteoglycans have been implicated in numerous signaling pathways, but their functions in lens development are not well understood. We have previously shown that Ndst1-mediated heparan sulfate biosynthesis is required for FGF signaling during lens induction, but because of the early lens defects in the systemic Ndst1 knockout, the role of Ndst1 in later lens development had not been explored (Pan et al., 2006). Ablation of heparan sulfate 2-O sulfotransferase (Hs2st) has been reported to cause cataracts in mice (Bullock et al., 1998). Similarly, mutation of perlecan (HSPG2), a heparan sulfate core protein abundant in the lens capsule, is known to cause congenital cataracts in humans (Arikawa-Hirasawa et al., 2001). Rossi and colleagues removed the heparan sulfate attachment sites on murine perlecan, which resulted in lens capsule leakage and degeneration but not an embryonic developmental defect (Rossi et al., 2003). These results underlie the important function of heparan sulfate in maintaining the structural integrity of the lens. Nevertheless, heparan sulfate proteoglycans have been shown to closely associate with FGFs in the lens capsule (Lovicu and McAvoy, 1993; Schulz et al., 1997), suggesting these glycoproteins may be important for lens maturation.
In this study, we have generated a deletion of Ndst (Ndst1 and Ndst2) in the lens. Previous biochemical studies have demonstrated that the loss of Ndst prevents the N-sulfation of heparan sulfate and reduced its overall sulfation level (Holmborn et al., 2004). Consistent with this, we showed that the sulfation of heparan sulfate was disrupted in the Ndst conditional knockout mutants after the lens induction stage. This led to a delay in lens fiber cell differentiation and a significant reduction in the lens size. Importantly, we provided several lines of evidence to support that Ndst ablation primarily affected FGF signaling in lens development. First, we showed that both Fgfr/Fgfr interactions on the lens cell surface and ERK signaling within the lens cells were abrogated in Ndst mutants. Second, Ndst deletion completely suppressed the premature lens differentiation and abnormal lens growth induced by FGF transgenic overexpression. Third, we showed that reconstitution of Kras signaling could overcome Ndst deletion to restore lens fiber elongation. We should note that Ndst ablation did not affect lens placode development as recently reported in the Fgfr1/2 deletion mutant (Garcia et al., 2011). This is likely because of the timing of heparan sulfate proteoglycan depletion, as we have shown that heparan sulfate appeared to have an exceptionally slow turnover rate in the Ndst mutant lens. Finally, it has long been recognized that there exists an anterior-posterior gradient of FGF signaling across the developing lens (Lovicu and McAvoy, 2005), which is evident by the predominant ERK phosphorylation in the posterior but not the anterior lens (see Fig. 7D for example). Intriguingly, our LACE assay also revealed a similar pattern of heparan sulfate activities, by which Fgf/Fgfr interactions were stabilized strongly in the posterior lens, but only weakly in the anterior lens epithelium (See Fig. 6A, H and N). We would therefore like to propose that the differential specificities of heparan sulfate in the lens contributes to the anterior-posterior FGF signaling gradient, which in turn regulates the orderly proliferation, migration and differentiation of the anterior lens epithelial cells into posterior lens fiber cells.
The rescue of the Ndst lens defect by activated Kras further suggests that defective FGF-Ras signaling could account for most, if not all, of the Ndst mutant phenotype. This is surprising, considering that heparan sulfate is known to regulate multiple growth factor signals, including BMP and Wnt signaling, which are clearly important in lens differentiation. Nevertheless, our conclusion is supported by the phenotypic comparisons between Ndst and the previously reported FGF, BMP and Wnt signaling mutants at the lens fiber differentiation stage. For example, both the Ndst and the Fgfr1/2/3 mutants exhibited defective cell proliferation at E14.5 but not at E12.5, and their cell apoptosis rates were greatly elevated (Zhao et al., 2008). Furthermore, these two mutants maintained ectopic Pax6 and E-cadherin expressions during lens differentiation. This is in contrast to the disruption of the canonical Wnt signaling at this stage by deleting β-catenin, which resulted in the loss of Pax6 and E-cadherin expressions, but not TUNEL staining (Cain et al., 2008). Conditional knockout of a BMP receptor, Acvr1(Alk2), indeed caused abnormal cell death in the differentiating lens (Rajagopal et al., 2008). But unlike the Ndst mutant, the Acvr1 lens actually exhibited increased cell proliferation during lens maturation. Finally, we showed that the staining of phospho-Smad1, a downstream effector of BMP signaling, was unchanged in in the Ndst mutant lens (supplementary Figure 2), supporting that BMP signaling was unaffected. These results thus suggest, at least in lens development, the FGF pathway is the intercellular signaling pathway most sensitive to the loss of Ndst function.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Ruth Ashery-Padan, Carl-Henrik Heldin, Lena Kjellén, Richard Lang, Peter ten Dijke, Samuel Zigler for mice and reagents, and Jeffrey D. Esko for critical reading of the manuscript. The work was supported by the National Institute of Health (EY017061 to X.Z. and EY012995 to M.L.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen BL, Rapraeger AC. Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J Cell Biol. 2003;163:637–648. doi: 10.1083/jcb.200307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, Yamada Y. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 2001;27:431–434. doi: 10.1038/86941. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Feng GS, Zhang X. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci. 2010;30:4110–4119. doi: 10.1523/JNEUROSCI.4364-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain S, Martinez G, Kokkinos MI, Turner K, Richardson RJ, Abud HE, Huelsken J, Robinson ML, de Iongh RU. Differential requirement for beta-catenin in epithelial and fiber cells during lens development. Dev Biol. 2008.;321:420–433. doi: 10.1016/j.ydbio.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is required for development of primary lens fiber cells. Development. 2002;129:3727–3737. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- Garcia CM, Huang J, Madakashira BP, Liu Y, Rajagopal R, Dattilo L, Robinson ML, Beebe DC. The function of FGF signaling in the lens placode. Dev Biol. 2011;351:176–185. doi: 10.1016/j.ydbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–1627. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development. 2005;132:3777–3786. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmborn K, Ledin J, Smeds E, Eriksson I, Kusche-Gullberg M, Kjellen L. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J Biol Chem. 2004;279:42355–42358. doi: 10.1074/jbc.C400373200. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Localization of acidic fibroblast growth factor, basic fibroblast growth factor, and heparan sulphate proteoglycan in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol Vis Sci. 1993;34:3355–3365. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107:221–228. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- Nybakken K, Perrimon N. Heparan sulfate proteoglycan modulation of developmental signaling in Drosophila. Biochim Biophys Acta. 2002;1573:280–291. doi: 10.1016/s0304-4165(02)00395-1. [DOI] [PubMed] [Google Scholar]
- Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 2008;135:249–258. doi: 10.1242/dev.009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Feng GS, Zhang X. Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development. 2010;137:1085–1093. doi: 10.1242/dev.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Carbe C, Powers A, Zhang EE, Esko JD, Grobe K, Feng GS, Zhang X. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–310. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Dattilo LK, Kaartinen V, Deng CX, Umans L, Zwijsen A, Roberts AB, Bottinger EP, Beebe DC. Functions of the type 1 BMP receptor Acvr1 (Alk2) in lens development: cell proliferation, terminal differentiation, and survival. Invest Ophthalmol Vis Sci. 2008;49:4953–4960. doi: 10.1167/iovs.08-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal R, Huang J, Dattilo LK, Kaartinen V, Mishina Y, Deng CX, Umans L, Zwijsen A, Roberts AB, Beebe DC. The type I BMP receptors, Bmpr1a and Acvr1, activate multiple signaling pathways to regulate lens formation. Dev Biol. 2009;335:305–316. doi: 10.1016/j.ydbio.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995a;121:3959–3967. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Ohtaka-Maruyama C, Chan CC, Jamieson S, Dickson C, Overbeek PA, Chepelinsky AB. Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev Biol. 1998;198:13–31. doi: 10.1006/dbio.1998.8879. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995b;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan S, Conley KW, Le TT, Donner AL, Maas RL, Brown NL. Notch signaling regulates growth and differentiation in the mammalian lens. Dev Biol. 2008;321:111–122. doi: 10.1016/j.ydbio.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, McAvoy JW. Binding of FGF-1 and FGF-2 to heparan sulphate proteoglycans of the mammalian lens capsule. Growth Factors. 1997;14:1–13. doi: 10.3109/08977199709021506. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Griep AE. Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev Biol. 2000;217:205–220. doi: 10.1006/dbio.1999.9557. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Swindell EC, Liu C, Shah R, Smith AN, Lang RA, Jamrich M. Eye formation in the absence of retina. Dev Biol. 2008;322:56–64. doi: 10.1016/j.ydbio.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, McMahon AP. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989;105:131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, Madakashira BP, Thiels CA, Bechtle CA, Garcia CM, Zhang H, Yu K, Ornitz DM, Beebe DC, Robinson ML. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–288. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.