Abstract
Objective
Optimal care for most patients with advanced ovarian cancer generally includes both surgery and chemotherapy. Little is known about the proportion of women in the US who receive combination care or the sequence in which this care is delivered. This study evaluated patterns of care, frequency of completion of recommended therapy and factors associated with sequencing of therapy.
Methods
Using the Surveillance, Epidemiology and End-Results data we identified a cohort of 8211 women aged 65 and above with stage III/IV epithelial ovarian cancer diagnosed between 1995–2005. Receipt of chemotherapy or surgery was identified using Medicare claims. Logistic regression was used to evaluate factors associated with sequencing of treatment and the receipt of surgery.
Results
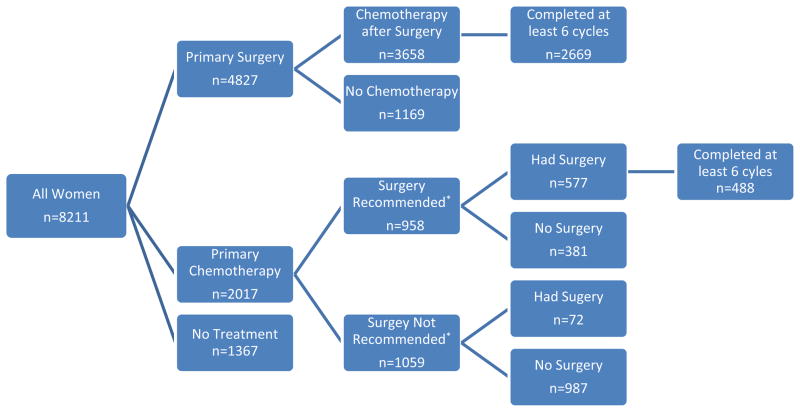
3241 (39.1%) had surgery and at least 6 cycles of chemotherapy in either order. Surgery was performed initially in 4827 (58.8%) women and 3658/4827 (75.8%) had subsequent chemotherapy. 2017 (24.6%) had primary chemotherapy and 649/2017 (32.2%) of these women had subsequent surgery. Advanced age, African American race, stage IV disease, non-married status and increasing medical comorbidity were all associated with the failure to receive both surgery and at least 6 cycles of chemotherapy (all p<0.01).
Conclusions
The majority of women with advanced ovarian cancer in the Medicare population do not receive both combination therapy with surgery and at least 6 cycles of chemotherapy. A large proportion of women are receiving chemotherapy as primary treatment for advanced ovarian cancer, and the majority of these patients do not have cancer-directed surgery.
Keywords: Ovarian Neoplasms, Aged, Guideline Adherence, Chemotherapy, Surgery
Introduction
Ovarian cancer is the most lethal gynecologic malignancy and the fourth leading cause of cancer death among women in the US. In 2010 an estimated 21,880 American women will be newly diagnosed with ovarian cancer and 13,850 women will die of the disease[1]. Survival in epithelial ovarian cancer is strongly related to stage of disease, and the majority of patients present with advanced stage (III/IV) disease at the time of diagnosis. Advances in the treatment of ovarian cancer in the past twenty years have been associated with an improvement in the likelihood of 5-year survival from 34.8% in 1975 to 45.6% from 1999–2006[2]. This increase is thought to be largely a result of advances in ovarian cancer-directed surgery and the use of platinum based chemotherapy[3].
Current guidelines from the National Comprehensive Cancer Network (NCCN, 2010) and earlier ones issued by the National Institutes of Health (NIH, 1994) recommend that primary treatment for most patients with advanced ovarian cancer should include primary debulking surgery (PDS) with a maximal cytoreductive effort and at least 6 cycles of systemic chemotherapy [4, 5]. Despite these recommendations, previous studies have suggested that many women with ovarian cancer may not receive recommended surgical procedures [6, 7].
Administering chemotherapy as a treatment for advanced ovarian cancer prior to planned surgery is referred to as neoadjuvant chemotherapy (NAC), and the practice is controversial and generally reserved for women who are poor surgical candidates [8, 9]. The administration of chemotherapy without the intent to proceed to surgery is considered palliative chemotherapy. The proportion of patients nationwide with advanced ovarian cancer primarily treated with palliative chemotherapy has not been well described as these patients are often excluded from studies.
The primary purpose of this study is to describe the receipt and sequencing of surgery and chemotherapy in the primary treatment of advanced ovarian cancer in the US Medicare population. This analysis provides an assessment of how recommended therapies are being utilized in the general community and how this has changed over time. The secondary aims are to identify factors associated with the receipt of chemotherapy as a primary treatment for ovarian cancer and to determine the factors associated with the receipt of both ovarian cancer-directed surgery and completion of 6 cycles of chemotherapy in this population.
Methods
Data Source
Internal Review Board approval was obtained from the Human Subjects Division of the University of Washington (IRB 37473). Data for this analysis came from a linkage between the Surveillance Epidemiology, End Results (SEER) database provided by the National Cancer Institute (NCI) and Medicare healthcare claims records provided by the Center for Medical Services (CMS)[10]. The SEER database is derived from the records of cancer registries that served approximately 14% of the US population in 1995 and 26% in 2005 and include an estimated 97% of incident cancer cases in these areas.[2] [11] Of persons over the age of 65 in the SEER database, 93% were identified in the Medicare enrollment file and their records were successfully matched to SEER records in the linkage process [10].
Cohort Selection
This study identified all women over the age of 65 in the SEER-Medicare database diagnosed with ovarian cancer from January 1, 1995 to December 31, 2005. Women were included if they had American Joint Cancer Committee (AJCC) stage III or IV ovarian cancer (n=13,998). Patients listed as AJCC unstaged in SEER but who were classified as advanced disease were also included and classified as stage distant, not otherwise specified (NOS). Women were excluded if they had a diagnosis based on autopsy or death certificate only (n=17), borderline or non-invasive pathology (n=58), disease that was not pathologically confirmed (n=874), non-epithelial malignancies (n=100), or a second primary malignancy diagnosed any time in the six months before or after the date of the ovarian cancer diagnosis (n=439). Women had to be continuously covered by Medicare parts A+B and not be enrolled in an HMO from the 12 months prior to diagnosis and at least 9 months following diagnosis (4264 excluded). HMO claims files are not available in this dataset thus necessitating the exclusion of these patients.
Patient Characteristics
Sociodemographic variables, including age, size of area of residence and race, were collected from SEER data. SEER registries were categorized according to geographic region. Median household income from zip code of residence was categorized into quartiles and used as a proxy for socioeconomic status and was derived from 2000 census data. Tumor stage, grade and histology were determined from SEER. Comorbidity score was determined using claims for the 12 months prior to ovarian cancer diagnosis to calculate the Deyo adaptation [12] of the Charleson comorbidity index, [13] which allows for the use of administrative claims data to determine a comorbidity score[14].
Treatment Identification
Medicare claims data were used to identify treatment because they are more specific than the SEER treatment variables. Because SEER reports only the month of diagnosis, all patients were assigned the 15th as the day of diagnosis. Medicare claims were available through December 31, 2007 and were searched from 60 days prior to the date of diagnosis up to 1 year after the date of diagnosis to identify treatment.
Receipt of chemotherapy was identified if either the inpatient record, outpatient file or physician claims indicated that chemotherapy was given (Supplement 1). A previous study demonstrated that when Medicare claims do not identify a specific chemotherapeutic agent, 97.1% of the patients with ovarian cancer actually received a platinum-based agent[15]. Thus, when a specific agent was not identified in this analysis, we assigned a platinum agent. Chemotherapy claims were grouped into “weeks of received therapy” according to the dates of the claims. The number of weeks with chemotherapy claims was used to approximate the number of cycles of chemotherapy. Surgical treatment for ovarian cancer was identified in the MEDPAR files using ICD-9 procedure codes and in the physician claims using CPT codes indicating surgical resection of the primary tumor (Supplement 2)[16]. A very limited number of patients (360/8211) had a treatment start date in the SEER files and no evidence of treatment as defined above in the Medicare files. These patients were classified as untreated in this analysis as treatment could not be verified and specific treatment modalities could not be identified.
For this analysis patients were classified as having received primary debulking surgery (PDS) or primary chemotherapy based on the first claim identified. When both surgery and chemotherapy were identified within the same claim as the patient’s first therapy, the patient was classified as having received PDS. The patients who received initial chemotherapy were further stratified by surgical intent at the time of diagnosis as reported in SEER (Figure 1). A variable is available within the SEER database that categorizes cancer directed surgery as performed, recommended but not performed and not recommended. It further breaks down some of these categories to indicate a reason such as not performed, patient refused or reason unknown. This variable was in use during the entire study period and has no missing data. We dichotomized this variable into surgery recommended (including performed and all recommended but not performed categories) and surgery not recommended and used this to help delineate surgical intent as previously described[7]. Women who had surgery recommended were labeled as having received neoadjuvant chemotherapy (NAC) and those not recommended surgery were labeled as having received palliative chemotherapy (PC).
Figure 1. Treatment sequencing among women for advanced ovarian cancer in Medicare claims.
*Surgery recommended as identified by SEER at the time of diagnosis
Provider Characteristics
Provider specialty was determined from both Medicare files and AMA files. Medicare claims data indicate a provider specialty code; however, this code in isolation has been demonstrated to identify less that 50% of gynecologic oncologists23. Physician unique provider identification numbers from Medicare files were linked with files obtained from the American Medical Association to increase the ability to identify gynecologic oncologists, a technique that is estimated to identify over 80% of gynecologic oncologists23. All medical records were searched from 2 months prior to the diagnosis date through one month and six months following diagnosis date. Patients were classified as having any contact with a gynecologic oncologist if any claim was present from a specialist classified as a gynecologic oncologist during this period.
Statistical Methods
The Chi squared test was used to compare the frequency distributions of categorical variables. Multivariate logistic regression was performed to model the factors that predicted treatment groups. Robust standard errors were used. All p values are 2 sided. Grade of tumor was unavailable for a large proportion of the patients and was not used in the model. The final multivariate model adjusted for all factors that were associated in the univariate analysis (age, race, marital status, median household income (reported as quartiles), geographic region, stage, histology, comorbidity) as well as diagnosis year and size of geographic area of residence. STATA SE version 11.0 (College Station, TX) was used for all calculations.
Results
Of 8211 women with advanced epithelial ovarian cancer, 4827 (58.8%) were treated with primary debulking surgery (PDS), 2017 (24.6%) were treated with primary chemotherapy, and 1367 (16.6%) had no evidence of either surgery or chemotherapy. Demographic, clinical and pathological characteristics of these groups are shown in Table 1. Women treated with PDS tended to be younger than those treated with primary chemotherapy and those who did not get any treatment. Untreated women were relatively more likely to be of non-white race, be unmarried and have a lower median household income. Women treated with primary chemotherapy were much more likely than those treated with primary surgery to have stage IV disease and to have a comorbidity score >0. Untreated women had a median survival of 1.38 months and only 15.3% lived for at least 6 months.
Table 1.
Demographic, Clinical and Pathologic Characteristics by Treatment Group*
| All Primary Surgery n (%) | All Primary Chemo n(%) | No Treatment n (%) | Primary Chemo with Surgery recommended n(%) | |
|---|---|---|---|---|
| No. of patients | 4,827 (58.79) | 2,017 (24.56) | 1,367 (16.65) | 958 (47.49 of PC) |
|
| ||||
| Age (years) | p<0.01¥ | p=0.07€ | ||
|
| ||||
| Mean (SD) | 75.5 (6.10) | 76.8 (6.20) | 81.48 (6.96) | 75.64 (5.91) |
| 65–69 | 1,066 (22.08) | 326 (16.16) | 91 (6.66) | 188 (19.62) |
| 70–74 | 1,383 (28.65) | 497 (24.64) | 173 (12.66) | 268 (27.97) |
| 75–79 | 1,282 (26.56) | 560 (27.76) | 274 (20.04) | 270 (28.18) |
| 80–84 | 731 (15.14) | 429 (21.27) | 372 (27.21) | 171 (17.85) |
| 85+ | 365 (7.56) | 205 (10.16) | 457 (33.43) | 61 (6.37) |
|
| ||||
| Race | p<0.01¥ | p=0.22€ | ||
|
| ||||
| White | 4,352 (90.16) | 1,777 (88.10) | 1,181 (86.39) | 843 (88.00) |
| Black | 230 (4.76) | 130 (6.45) | 123 (9.00) | 60 (6.26) |
| Other | 174 (3.60) | 77 (3.82) | 47 (3.44) | 39 (4.07) |
|
| ||||
| Median Household Income | p<0.01¥ | p<0.01€ | ||
|
| ||||
| First Quartile | 1,140 (23.62) | 437 (21.67) | 384 (28.09) | 176 (18.37) |
| Second Quartile | 1,158 (23.99) | 480 (23.80) | 326 (23.85) | 224 (23.38) |
| Third Quartile | 1,136 (23.53) | 492 (24.39) | 334 (24.43) | 260 (27.14) |
| Fourth Quartile | 1,194 (24.74) | 506 (25.09) | 261 (19.09) | 246 (25.68) |
|
| ||||
| Marital Status | p<0.01¥ | p<0.88€ | ||
|
| ||||
| Married | 2,190 (45.37) | 804 (39.86) | 329 (24.07) | 502 (52.40) |
| Not Married | 2,499 (51.77) | 1,164 (57.71) | 997 (72.93) | 431 (44.99) |
|
| ||||
| Region | p=0.01¥ | p=0.58€ | ||
|
| ||||
| Northeast | 933 (19.33) | 441 (21.68) | 263 (19.24) | 196 (20.46) |
| Midwest | 1,016 (21.05) | 354 (17.55) | 303 (22.17) | 193 (20.15) |
| South | 677 (14.03) | 294 (14.58) | 209 (15.29) | 121 (12.63) |
| West | 2,201 (45.60) | 928 (46.01) | 592 (43.31) | 448 (46.76) |
|
| ||||
| Area of Residence | p=0.18¥ | p<0.01€ | ||
|
| ||||
| Large Metropolitan | 2,762 (57.22) | 1,188 (58.90) | 770 (56.33) | 579 (60.44) |
| Metropolitan | 1,290 (26.72) | 552 (27.37) | 368 (26.92) | 256 (26.72) |
| Urban | 293 (6.07) | 119 (5.90) | 83 (6.07) | 67 (6.99) |
| Less Urban | 381 (7.89) | 127 (6.30) | 121 (8.85) | 13 (1.36) |
| Rural | 101 (2.09) | 31 (1.54) | 25 (1.83) | 13 (1.36) |
|
| ||||
| Stage | p<0.01¥ | p<0.01€ | ||
|
| ||||
| III | 3,401 (65.05) | 813 (40.31) | 435 (31.82) | 482 (50.31) |
| IV | 1,599 (33.13) | 1,149 (56.97) | 879 (64.30) | 454 (47.39) |
| “Distant” NOS | 88 (1.82) | 55 (2.73) | 53 (3.88) | 22 (2.30) |
|
| ||||
| Grade | p<0.01¥ | p<0.01€ | ||
|
| ||||
| I/II | 871 (18.04) | 149 (7.39) | 78 (5.71) | 98 (10.22) |
| III/IV | 2,983 (61.80) | 650 (32.23) | 281 (20.56) | 426 (44.47) |
| Unknown | 970 (20.10) | 1,218 (60.39) | 1,008 (73.74) | 434 (45.30) |
|
| ||||
| Histology | p<0.01¥ | p<0.01€ | ||
|
| ||||
| Serous/Adenocarcinoma | 3,674 (76.11) | 1,647 (81.66) | 1,035 (75.71) | 797 (83.19) |
| Mucinous | 197 (4.08) | 71 (3.52) | 62 (4.54) | 25 (2.61) |
| Endometroid | 304 (6.30) | 31 (1.54) | # | 21 (2.19) |
| Clear Cell | 78 (1.62) | 16 (0.79) | # | 13 (1.36) |
| Other Epithelial | 574 (11.89) | 252 (12.49) | 248 (18.14) | 102 (10.65) |
|
| ||||
| Comorbidity Score | p<0.01¥ | p<0.14€ | ||
|
| ||||
| 0 | 3,271 (67.76) | 1,197 (59.35) | 637 (46.60) | 615 (64.20) |
| 1 | 1,023 (21.19) | 488 (24.19) | 402 (29.41) | 217 (22.65) |
| 2 | 326 (6.75) | 184 (9.12) | 178 (13.02) | 76 (7.93) |
| 3+ | 207 (4.29) | 148 (7.34) | 150 (10.97) | 50 (5.22) |
Not all totals add up to 100% because of rounding and missing data.
p values are using Chi2 comparing differences among the 4 groups: all primary surgery, all primary chemo, treatment NOS and no treatment
p values compare the group all primary surgery to primary chemotherapy with surgery recommended
cell contents suppressed due to n<10 for confidentiality
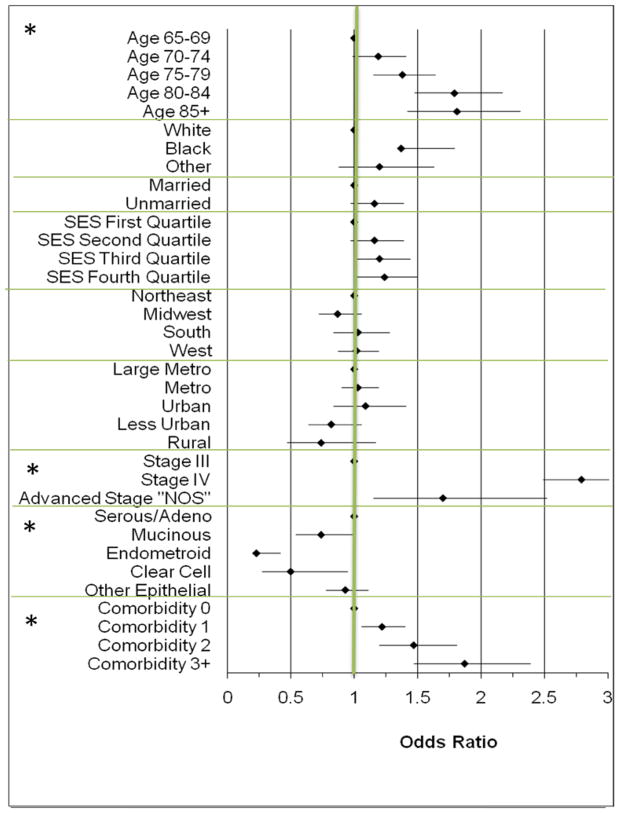
Factors associated with the primary use of chemotherapy compared to primary surgery in advanced ovarian cancer are shown in Figure 2. Older women were more likely to be primarily treated with chemotherapy, especially women in the 80–85 and 85+ age categories compared to women 65–70 (Odds Ratio [OR] 1.78; 95% confidence interval [CI] 1.47–2.15 and OR 1.78; 96%CI 1.39–2.25 respectively). African-American women were more likely than white women to be treated with chemotherapy first (OR 1.32; 95%CI 1.01–1.72). Women with stage IV disease were almost 3 times as likely to receive primary chemotherapy (OR 2.79; 95%CI 2.49–3.14) compared to women with stage III disease. Women with serous tumors were more likely to be treated with primary chemotherapy than women with mucinous, endometroid or clear cell tumors. Increasing comorbidity score was associated with the increasing utilization of primary chemotherapy.
Figure 2. Factors Associated with the Odds of Being Treated With Primary Chemotherapy (n=2017) Compared to Primary Surgery (n=4827) Among Women Receiving Treatment for Advanced Ovarian Cancer.
All Odds Ratios are multivariate and adjusted for age, race, marital status, median household income (reported as quartiles in above figure), geographic region and size, stage, histology, comorbidity and diagnosis year. Odds >1 indicate an increased likelihood of being treated with primary chemotherapy compared to primary surgery. Graph made using Microsoft Excel [29] * indicates p<0.01 for category
Only 3658 (75.8%) of the women in the PDS group had evidence of chemotherapy after surgery in the year following diagnosis. The median number of cycles was 8 (range 1–48) and 2669 (55.3%) received at least 6 cycles of postoperative chemotherapy. Among the women who received chemotherapy after PDS, 74.8% had at least one cycle including both platinum and a taxane. Among the 4222 women who lived at least 60 days from diagnosis, 3576 (84.7%) had evidence of chemotherapy after surgery, and 2668 (63.3%) had at least 6 cycles. Among the 2017 women treated with primary chemotherapy, 649 (32.2%) went on to surgery and the median time to surgery was 17 weeks. Women having chemotherapy followed by surgery had a median of 4 cycles of chemotherapy prior to surgery, and 89% of these women received additional chemotherapy postoperatively with a median of 6 cycles (range 0–48) following surgery. Among the 1759 women who lived at least 60 days following diagnosis, 643 (36.6%) had evidence of surgery following primary chemotherapy. Of the 8211 women in the cohort, 4307 (52.5%) had evidence of ovarian cancer directed surgery and at least 1 cycle of chemotherapy and 3184 (46.5%) had at least 3 cycles of chemotherapy. Only 3214 (39.1%) received ovarian cancer-directed surgery and 6 cycles of chemotherapy in either sequence in the year following diagnosis.
In the primary chemotherapy group 958 women had surgery recommended (Neoadjuvant Chemotherapy [NAC] group) and 1059 women did not (Palliative Chemotherapy [PC] group, Figure 1). In the NAC group, 577 (59.6%) of women went on to have surgery as compared with only 72 (6.8%) in the PC group. When women in the NAC group are compared to women in the PDS group (Table 1) previously observed differences in age, race, geographic region and comorbidity score were no longer present. Of the 897 women in the NAC group who lived at least 60 days, 572 (63.8%) went on to have ovarian-cancer directed surgery.
Of women treated with PDS, 54.5% had a gynecologic oncologist involved in their care within 1 month of diagnosis and 59.9% of the time by 6 months. Only 42.6% of women treated with NAC had care by a gynecologic oncologist at 1 month but by 6 months this proportion increased to 60.5%. Women receiving palliative chemotherapy were only seen by a gynecologic oncologist 25.6% and 32.5% of the time, by one and six months following diagnosis respectively.
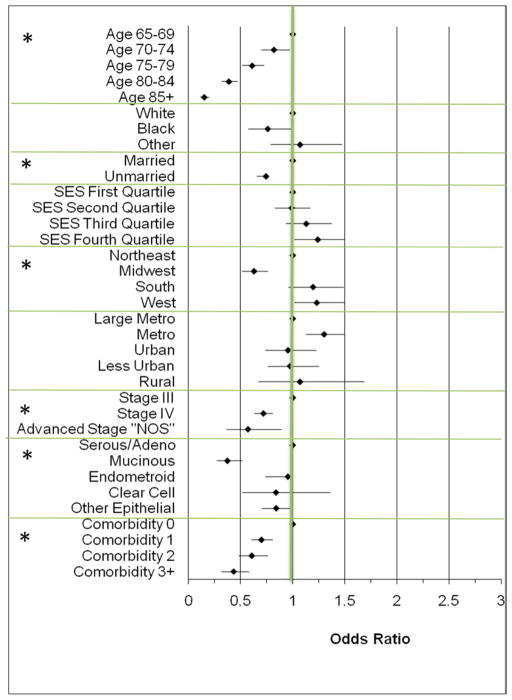
Multivariate analysis of factors associated with the receipt of both ovarian cancer-directed surgery and at least 6 cycles of chemotherapy (in either order) are shown in Figure 3. This analysis included all women who were treated with either PDS (n=4827) or NAC (n=958) of whom 3157 (54.57%) completed at least 6 cycles of chemotherapy and had surgery. Advancing age, not being married, African American race, stage IV disease and increasing comorbidity score were all associated with a decreased likelihood of completing therapy. There was not a large difference in the proportion of women completing therapy based on sequencing of therapy, with 2669 (55.2%) in the PDS group and 488 (50.95%) in the NAC group having surgery and at least 6 cycles of chemotherapy.
Figure 3. Factors Associated with the Odds of Receiving Both Surgery and 6 Cycles of Chemotherapy in any Sequence (n=3157) Among Women Receiving Treatment with Primary Surgery or Primary Chemotherapy with Surgery Recommended (n=5785) for Advanced Ovarian Cancer.
All Odds Ratios are multivariate and adjusted for age, race, marital status, median household income (reported as quartiles above), geographic region and size, stage, histology, comorbidity and diagnosis year. Odds <1 indicate a decreased likelihood having the combination of surgery and 6 cycles of chemotherapy in any order. * indicates p<0.01 for the category
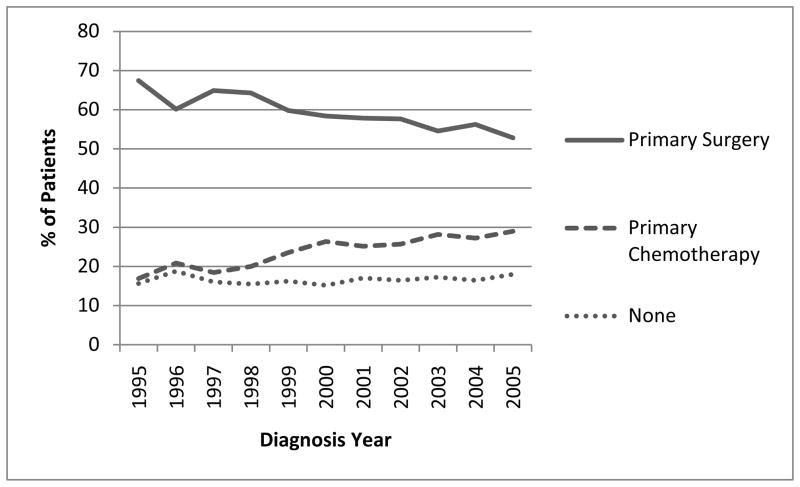
The use of primary debulking surgery decreased over time, from 67.5% for women diagnosed in 1995 to 52.8% in 2005 (Figure 4). There was a corresponding increase in the use of primary chemotherapy, with an increase in the odds of use of 6.7% per year from 1995 to 2005 (OR 1.067; 95%CI 1.046–1.088). Among women treated with either PDS or NAC, the odds of completing at least 6 cycles of chemotherapy and surgery increased during the study period by 8.6% per year (OR 1.086, 95%CI 1.065–1.108). Among women receiving chemotherapy, there was a large increase in the proportion receiving at least one cycle of both a platinum and taxane agent from to 32.35% in 1995 to 85.3% in 2005. Over 98.7 % of all patients receiving chemotherapy were treated with a platinum agent for at least one cycle during treatment.
Figure 4.
Time trends in Medicare claims in the treatment of advanced ovarian cancer
Discussion
Guidelines for primary treatment of advanced ovarian cancer from the National Comprehensive Cancer Network (NCCN) and National Institutes of Health (NIH) recommend primary debulking surgery (PDS) with maximal cytoreductive effort followed by at least 6 cycles of systemic platinum based chemotherapy, or alternatively neoadjuvant chemotherapy followed by interval cytoreductive surgery. Failure to receive surgery and platinum-based chemotherapy has been associated with a decrease in survival for women with advanced ovarian cancer [17, 18]. Our study of US women over 65 diagnosed from 1995–2005 found that only 39.1% had evidence of surgery and at least 6 cycles of chemotherapy in either sequence. About one-fourth of patients (24.6%) were treated primarily with chemotherapy, and only 32.2% of these patients went on to have surgery. This study is the first large US population-based report on the sequencing of surgery and chemotherapy in the treatment of women with advanced ovarian cancer and it demonstrates a concerning discrepancy between guideline-recommended care and treatment that is actually received.
A recent summary of European studies suggested a similarly low frequency of implementation of recommended care for women with advanced ovarian cancer[19]. The lack of adherence to guidelines is one of the major barriers to the widespread practice of evidence-based medicine [20]. The uptake of guidelines is inhibited by a number of factors, including lack of awareness by clinicians and by clinicians’ lack of agreement and acceptance of the existing guidelines[21]. The latter may be of particular importance in the treatment of advanced ovarian cancer. Results of a survey published in 2004 by Chen et al. of US gynecologic oncologists demonstrated a discordance between physician beliefs and recommendations in ovarian cancer management [22].
Despite recent publications emphasizing the importance of primary debulking surgery in advanced ovarian cancer [23, 24], we observed a striking decrease in the use of primary debulking surgery from 1995–2005. During this same period of time we observed an increase in the use of platinum and taxane agents in the treatment of advanced ovarian cancer. These trends suggest that while there is uptake in the community in the utilization of recommended chemotherapeutic agents over time, the same is not true of surgery for advanced ovarian cancer. This suggests that there is either an increasing lack of consensus as to the benefit of initial surgery or an increase in other barriers to the receipt of surgery. In this population there was little variation over time in the proportion of patients who refused recommended surgery, ranging from 0.5–2% throughout the study period.
We did not exclude women who died early on in the study period. This likely resulted in the inclusion of women who had such advanced disease and medical infirmity that treatment was not likely considered feasible or safe. This fact likely contributes to the finding that only 39.1% of women in this study received both a surgical operation and at least 6 cycles of chemotherapy. We were unable to directly document treatment discontinuation due to complications or disease progression. The associations we observed between increasing age, stage and comorbidity score and the inability to complete surgery and 6 cycles of chemotherapy support the hypothesis that many women may be unable to complete therapy due to disease progression or medical complications. Many of the women in our cohort had a number of characteristics that predict an inability to complete treatment and “guideline” therapy may be inappropriate in these circumstances. However, it seems unlikely that 60.9% of women were too ill or had such advanced disease that treatment was not indicated or could not be completed. When we limited the analysis to women who lived at least 60 days from diagnosis, we still observed that only 84.7% of women having primary surgery had any chemotherapy, and only 63.2% completed at least 6 cycles. Similarly among women having NAC who lived at least 60 days from diagnosis, only 63.8% had ovarian cancer directed surgery.
Apart from the 1.4% of the patients who refused surgery according to SEER, no other information is directly available to provide insight into what proportion of these patients simply refused therapy. Given the association we observed between marital status and the ability to complete recommended treatment it seems likely that social support and other personal circumstances are closely linked with a patients’ ability to complete treatment. Further investigation is needed to determine the reasons that so many women did not receive standard therapy, and to direct the efforts to improve the quality of care.
Previous publications have suggested an increasing tendency to administer primary chemotherapy in elderly patients[25]. We found a similar pattern in this study, with a strong correlation observed between increasing age and the use of primary chemotherapy. A previous study of women treated with NAC suggested that socioeconomic factors such as race and insurance status may have contributed to the use of NAC[26]. It seems less likely in this cohort that financial obstacles played a large role in the lack of surgery as may be observed in other settings, as patients were all covered by Medicare. Our data does not allow the determination of secondary insurance coverage to further explore this question. Previous studies have examined the role of geographic variation in the administration of surgery and chemotherapy in ovarian cancer[7]. We identified a similar geographic variation in the completion of therapy including surgery. This variation may be related to the decreased availability of gynecologic oncologists in certain regions of the country and this could represent an important barrier to the receipt of recommended surgery.
There are several limitations to this analysis. The use of claims data to identify treatment and comorbidity is likely to result in some under ascertainment of treatment actually received. However, previous studies have determined a high level of agreement between Medicare data billing data and chart review in the identification of either surgery or chemotherapy among cancer patients [15, 27]. Any treatment performed that was not billed to Medicare would not be detected in this database. In this dataset there were a small number of patients (<5%) that had an indication of surgery or other treatment in the SEER database that did not have evidence of this treatment in Medicare claims. This may represent patients that had treatment that was not billed through Medicare and thus would be misclassified as having received no treatment. The use of weeks of claims to estimate treatment cycles is likely to result in some inaccuracy given the broad spectrum of codes included in defining the receipt of chemotherapy. Some important clinical information was unavailable that may account for some of the observed variability, such as information regarding completeness of surgical debulking or performance status. Our study population is limited to women over the age of 65. However, from 2003–2007 the median age at diagnosis for ovarian cancer was 63 years and 45.7% of women were 65 years or older when diagnosed[2]. The accuracy of the “surgery recommended” variable in SEER has not been documented in the literature and no information about specialty or experience with ovarian cancer is available about the provider (s) making this determination. This variable has been used by other investigators [7, 28] to aid in the delineation of surgical intent.
In conclusion, the current study demonstrates that only 39.1% of women over 65 in the Medicare population from 1995–2005 received what is considered standard care for women with advanced ovarian cancer, surgery and at least 6 cycles of chemotherapy in either sequence. This observation suggests that there is an opportunity for improvement in the care of women with advanced ovarian cancer.
Supplementary Material
Acknowledgments
This work was supported by the Marsha Rivkin Center for Ovarian Cancer Research. Dr Thrall is the recipient of the Scientific Scholar Award from the Rivkin Center. This work is also supported by the National Cancer Institute (NCI) at the National Institutes of Health, Dr. Thrall is the recipient of an NCI-funded postdoctoral fellowship (T32-CA009515-26). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
This study used the linked Surveillance Epidemiology, and End Results (SEER)-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors would also like to acknowledge the members of the Surgical Outcomes Research Collaborative at the University of Washington for critical feedback and advice on methodology and project design.
Footnotes
Presented at the Forty Second Annual Meeting of the Society of Gynecologic Oncologists, Orlando, Florida, March 6–9, 2011.
Conflict of Interest Statement
The authors have no conflicts of interest to report.
References
- 1.Institute NC. Cancer Facts and Figures - 2010. In: Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2007. 2010. [Google Scholar]
- 2.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. Institute NC. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: 2010. [Google Scholar]
- 3.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988–2001. Gynecol Oncol. 2008;109:203–9. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.NCCN. 2010 Ovarian Fallopian Tube and Primary Peritoneal Carcinomas. 2010. Practice guidelines in Oncology v.2. [Google Scholar]
- 5.National Institutes of Health Consensus Development Conference Statement. Ovarian cancer: screening, treatment, and follow-up. Gynecol Oncol. 1994;55:S4–14. doi: 10.1006/gyno.1994.1333. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006;103:383–90. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Fairfield KM, Lucas FL, Earle CC, Small L, Trimble EL, Warren JL. Regional variation in cancer-directed surgery and mortality among women with epithelial ovarian cancer in the medicare population. Cancer. 2010 doi: 10.1002/cncr.25242. in press. [DOI] [PubMed] [Google Scholar]
- 8.van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J, Pecorelli S. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1995;332:629–34. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg LE, Rodriguez G, Hurteau JA. The role of neoadjuvant chemotherapy in treating advanced epithelial ovarian cancer. J Surg Oncol. 2010;101:334–43. doi: 10.1002/jso.21482. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Warren JL, Legler JM. Med Care. Vol. 40. 2002. Assessing comorbidity using claims data: an overview; pp. IV-26–35. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, Trimble EL, Bodurka DC, Bristow RE, Carney M, Warren JL. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 17.Chemotherapy in advanced ovarian cancer: an overview of randomised clinical trials. Advanced Ovarian Cancer Trialists Group. BMJ. 1991;303:884–93. doi: 10.1136/bmj.303.6807.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 19.Verleye L, Vergote I, van der Zee AG. Patterns of care in surgery for ovarian cancer in Europe. Eur J Surg Oncol. 2010 doi: 10.1016/j.ejso.2010.06.006. in press. [DOI] [PubMed] [Google Scholar]
- 20.Timmermans S, Mauck A. The promises and pitfalls of evidence-based medicine. Health Aff (Millwood) 2005;24:18–28. doi: 10.1377/hlthaff.24.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Learman LA, Weinberg V, Powell CB. Discordance between beliefs and recommendations of gynecologic oncologists in ovarian cancer management. Int J Gynecol Cancer. 2004;14:1055–62. doi: 10.1111/j.1048-891X.2004.14602.x. [DOI] [PubMed] [Google Scholar]
- 23.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, Guile MW, Bristow RE, Aghajanian C, Barakat RR. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, Podratz KC, Cliby WA. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstet Gynecol. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 25.Bruchim I, Altaras M, Fishman A. Age contrasts in clinical characteristics and pattern of care in patients with epithelial ovarian cancer. Gynecol Oncol. 2002;86:274–8. doi: 10.1006/gyno.2002.6759. [DOI] [PubMed] [Google Scholar]
- 26.Chase DM, Rincon A, Deane M, Tewari KS, Brewster WR. Socioeconomic factors may contribute to neoadjuvant chemotherapy use in metastatic epithelial ovarian carcinoma. Gynecol Oncol. 2009 doi: 10.1016/j.ygyno.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40:IV-43–8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 28.Farjah F, Wood DE, Yanez ND, 3rd, Vaughan TL, Symons RG, Krishnadasan B, Flum DR. Racial disparities among patients with lung cancer who were recommended operative therapy. Arch Surg. 2009;144:14–8. doi: 10.1001/archsurg.2008.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark ODB. [Web site last accessed 8/2001];Forest plots in excel software(Data sheet) 2001 www.evidencias.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.