Abstract
Purpose
To evaluate the effectiveness of mitigation of acute ionizing radiation damage by mitochondria-targeted small molecules.
Materials and Methods
We evaluated the nitroxide-linked alkene peptide isostere JP4-039, the nitric oxide synthase inhibitor-linked alkene peptide esostere MCF201-89, and the p53/mdm2/mdm4 inhibitor BEB55 in radiation mitigation by clonogenic survival curves with the murine hematopoietic progenitor cell line 32D cl 3, human bone marrow stromal (KM101) and pulmonary epithelial (IB3) cell line. The p53 dependent mechanism of action was tested with p53 +/+ and p53 −/− murine bone marrow stromal cell lines. C57BL/6 NHsd female mice were injected I.P. after 9.5 Gy total body irradiation (TBI) with JP4-039, MCF201-89, or BEB55 individually or in combination.
Results
Each drug, JP4-039, MCF201-89, or BEB55, individually or as a mixture of all 3 compounds, increased the survival of 32D cl 3 cells and IB3 cells significantly over control irradiated cells (p=0.0021, p=0.0011, p=0.0038, and p=0.0073, respectively), and (p=0.0193, p=0.0452, p=0.0017, and p=0.0019 respectively). KM101 cells were protected by individual drugs (p=0.0007, p=0.0235, p=0.0044, respectively). JP4-039 and MCF201-89 increased irradiation survival of both p53+/+ (p=0.0396 and p=0.0071, respectively) and p53−/− cells (p=0.0007 and p=0.0188 respectively), while BEB55 was ineffective with (p53−/−) cells. Drugs administered individually or as a mixtures of all 3 after TBI significantly increased mouse survival (p=0.0234, 0.0009, 0.0052 and 0.0167 respectively).
Conclusion
Mitochondrial targeting of small molecule radiation mitigators decreases irradiation-induced cell death in vitro and prolongs survival of lethally irradiated mice.
Keywords: radiation injury, mitigation, antioxidant, nitric oxide synthase inhibitor, p53 inhibitor, mitochondrial-targeting
INTRODUCTION
The development of effective radiation damage mitigators (delivered after radiation exposure, but before appearance of clinical syndromes of radiation toxicity) requires an understanding of both pathophysiology and subcellular specific targeting (1– 3). Ionizing irradiation induces nuclear DNA strand breaks and initiates a molecular damage response which includes mitochondrial transport and activation of both pro-apoptotic and anti-apoptotic signals, the time course of which allows the potential introduction of small molecule mitigators (4–8).
Superoxide and nitric oxide are both induced by ionizing radiation, forming the potent reactive oxygen species (ROS) and reactive nitrogen species such as peroxynitrite. Molecular effects of ROS in mitochondria include oxidation of cardiolipin, which triggers cytochrome c release and caspase activation, resulting in apoptosis (9). A mitochondrial targeted antioxidative transgene product Manganese Superoxide Dismutase (MnSOD), delivered by plasmid liposomes, is an effective ionizing irradiation damage protector, but requires hours for in vivo gene expression making it impractical as a mitigator (8, 10– 12).
As a first strategy for rapid radiation mitigation, we focused on the biochemistry of the peroxinitrite. We designed two potential therapeutic agents. The translocation of the nitroxide 4-hydroxy-2,2,6,6-tetramethyl piperidine-1-oxyl (TEMPOL) to the mitochondria increases cytoprotection from oxidative stress, since TEMPOL can act as an effective scavenger of electrons and SOD mimic as well as due to radical scavenging properties of its one electron radiation product hydroxylamine (13). Furthermore, by attaching a Gramicidin S (GS) derived peptide isostere sequence to 4-aminotempo (4-AT), i.e. generating the nitroxide JP4-039, we were able to enhance its radioprotection capacity in vitro (4, 14–16).
Since radiation induced peroxynitrite formation requires nitric oxide (17), we constructed a nitric oxide synthase (NOS) inhibitor targeted to the mitochondria by using the same peptide isostere linkage mechanism. The conjugate MCF201-89 is composed of a 2-amino-5,6-dihydro-6-metyl-4H-1,3-thiazine (AMT) NOS inhibitor (I), linked to the same hemigramicidin S derivative peptide isostere as JP4-039.
An alternative independent strategy for mitochondria-targeted radiation mitigation was tested using a small molecule inhibitor of p53 binding to mdm2 and mdm4 (18– 21), with the goal to enhance the bioavailability of p53 and enhance cellular repair processes (22, 23). We hypothesized that cell death would be mitigated by keeping p53 concentration high at the mitochondrial membrane in radiation damaged cells through disruption of the negative regulator complexes p53/mdm2 and p53/mdm4. Furthermore, we hypothesized that the p53/mdm2/mdm4 inhibitor (BEB55) might show additive or synergistic effects when combined with other radiation damage mitigators such as mitochondrial-targeted GS-nitroxides and/or GS-NOS inhibitors.
MATERIALS AND METHODS
Synthesis of JP4-039 (targeted nitroxide) and MCF201-89 (targeted NOS inhibitor)
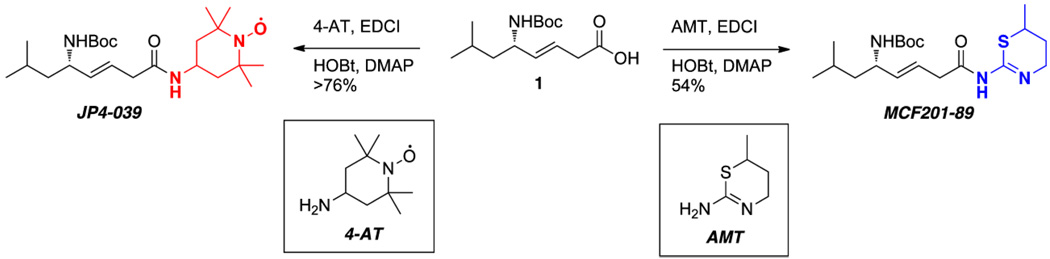
The synthesis of JP4-039 (a GS derived peptide isostere sequence linked to 4-AT) has been previously described (29). MCF201-89 was synthesized in using an analogous approach (Figure 1). In both cases, the N-Boc protected leucylglycine (E)-alkene peptide isostere 1 was coupled to 4-AT or AMT in the presence of carbodiimide (EDCI), hydroxybenzotriazole (HOBt), and dimethylaminopyridine (DMAP) to give JP4-039 or MCF201-89, respectively.
Figure 1. Targeted nitroxide JP4-039 (C23H42N3O4•, MW=424.60) and its nitroxide building block, 4-AT, compared to targeted NOS inhibitor MCF201-89 (C19H33N3S30, MW=383.55) and its 6-methyl-5,6-dihydro-4H1,3-thiazin-2-amine building block, AMT.
Both MCF201-89 and JP4-039 contain the identical GS derived alkene peptide isostere acyl component that facilitates translocation to the mitochondria.
Design and Synthesis of P53/MDM2/MDM4 inhibitors
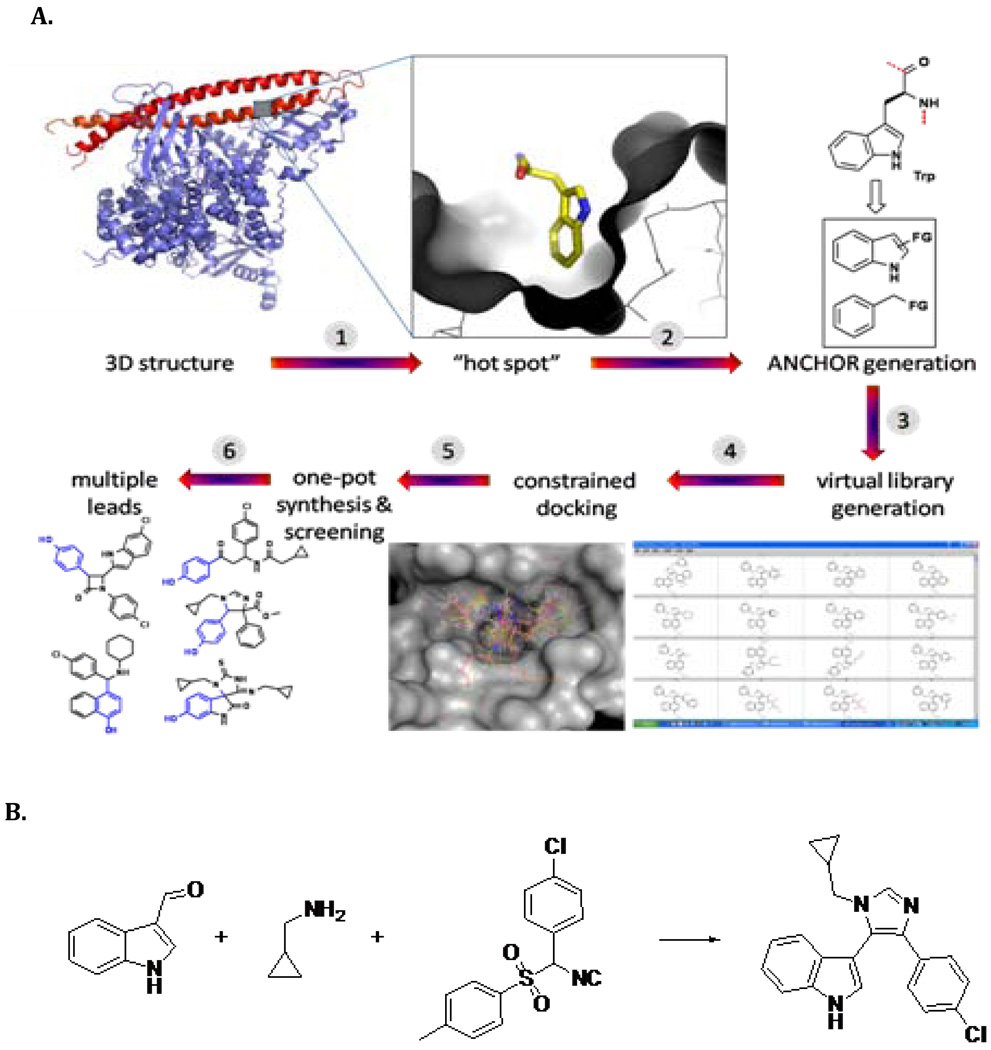
The imidazolo-indole derivative BEB55 was recently designed as the parent of a novel class of mdm2 and mdm4 binders, by using an advanced process for predicting and producing antagonists of cancer relevant protein-protein interactions (25, 26) (Figure 2). A virtual library was based on several MCR (multicomponent reaction chemistry) scaffolds. The library was then docked into the PPI interface allowing compounds “anchor” to be aligned. Inspection of the docking was used to choose compounds for synthesis and screening (27–29). The synthesis of BEB55 is shown in Figure 2. The binding constant of BEB55 to mdm2/4 was weak micromolar (∼60 uM) as determined by FP and NMR assays.
Figure 2. Design and synthesis of p53/mdm2/mdm4 inhibitors.
A) An amino acid “anchor” with specific interaction on the PPI interface of mdm2/mdm4 proteins. Next, virtual libraries of anchors were generated on several chemical-accessible MCR scaffolds. These compound libraries are then docked into the PPI interface whereby the compounds “anchor” align with the protein “anchor” as a starting pose. Manual inspection of the docking results or automatic scoring functions was then used to choose compounds for synthesis and screening. B) Synthesis of BEB55.
1H-Indole-3-carbaldehyde (940 mg, 6.48 mmol), cyclopropylmethanamine (460 mg, 6.48 mmol), 1-chloro-4-(isocyano(tosyl)methyl)benzene (2.0 g, 6.55 mmol) and piperazine (560 mg, 6.48 mmol) were added together in 10 mL of MeOH. The mixture was heated at reflux for 12 h. The solvent was then removed under reduced pressure and the residue was purified by chromatography on SiO2 (EtOAc/PE=x:y) to give colorless BEB55 (0.9 g, 44%).
In Vitro Studies
Clonogenic radiation survival curves using 32D cl 3 cells, a murine hematopoietic progenitor (8) and murine p53+/+ and p53−/− bone marrow stromal cell lines (30) have previously been described. Briefly, the 32D cl 3 interleukin-3 (IL-3) dependent murine hematopoietic progenitor cell line was derived from a long-term bone marrow culture of a C3H/HeJ mouse. Cells were passaged in 15% WEHI-3 cell conditioned medium (as a source of IL-3), 10% fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT), and McCoy’s supplemented medium according to published methods (8). The human bone marrow stromal cell line KM101 (16) and lB3 bronchial epithelial cell line (31) have previously been described. KM101 cells were passaged weekly in 24 cm3 Falcon plastic flasks in McCoy’s 5A modified medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% FBS (Hyclone Laboratories, Logan, UT). IB3 cells were passaged twice weekly in standard Dulbecco's modified Eagle's medium (DMEM) (Lonza, Allendale, NJ), supplemented with 10% FBS (Hyclone laboratories, Logan, UT), 1% L-glutamine (GIBCO BRL, Gaithersburg, MD) and 1% penicillin-streptomycin (GIBCO BRL, Gaithersburg, MD) on uncoated 75 cm3 tissue culture Falcon flasks in a 5% CO2 incubator at 37 °C for 48–72 hours to reach 80% confluency.
Cells from each cell line were suspended at 1 × 105 cells/mL and irradiated with 0 to 8 Gy. Preliminary in vitro studies showed a dose optimization of 10 µg/mL for each of the 3 drugs dissolved in 50% DMSO in PBS. JP4-039, MCF 201-89, or BEB55 were added to the 32D cl3 cells 10 min after irradiation. Irradiated IB3, KM101 and p53 +/+ or −/− cells were treated with drugs as in the 32D cl 3 cells experiments (8), then cells were plated in quadruplet and incubated in a high-humidity incubator at 37°C with 95% air/5% CO2 for 7 days, at which time the cells were stained using crystal violet and colonies of greater than 50 cells were counted. Each experiment was carried out 3 separate times on three separate days. Data were analyzed using linear quadratic and single-hit, multi-target models (32). The dose reduction factor (DRF) for each of the 3 drugs was calculated as the ratio of the dose giving 50% cell survival in the treated group divided by the dose at 50% survival in the control cell group.
Mice and In Vivo Studies
Adult female C57BL/6 NHsd mice (20 to 22 g, Harlan Sprague Dawley, Chicago, IL) were housed according to IACUC protocols at the Hillman Cancer Center (University of Pittsburgh Cancer Institute). All protocols were approved by the IACUC of the University of Pittsburgh. Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh. C57BL/6NHsd mice (n=15 per group) were irradiated with 9.5 Gy TBI to achieve the (LD 50/30) dose using a Gamma beta irradiation dose rate (74 cGy/min)and received an intraperitoneal injection from 10 min to 24 hours later (depending on the experiment) of 100 uL of JP4-039 (5 mg/kg), MCF 201-89 (10 mg/kg), or BEB55 (10 mg/kg) individually or as a mixture of all three agents in a total of 100 µl volume. The drugs were all dissolved in a mixture of ethanol and cremophor EL (1:1), and diluted 1:8 with water. Diluent alone was ineffective as a mitigator or protector. The mice were monitored for survival.
Statistical Analysis
Data were summarized as mean ± SD. The two-sided two-sample t test was used to perform a statistical analysis comparing slopes of in vitro survival curves in different experimental groups. The two-sided log-rank test was used to analyze the in vivo data for at least 15 mice in each group.
RESULTS
Mitochondrial Targeting of Nitroxide 4-AT or NOS Inhibitor AMT with Peptide by Hemigramacidin (GS) Peptide Isostere Increases Radioprotection
The strategy of targeting the nitroxide 4-AT and the NOS inhibitor AMT by conjugation to an alkene peptide isostere fragment derived from the membrane-active antibiotic GS was tested first. Treatment of 32D cl 3 cells with GS-nitroxide JP4-039 or the GS-NOS-I, MCF201-89, after irradiation showed significantly increased cell survival over the control group of untreated cells (p=0.0022, p=0.0045 respectively) compared to TEMPOL and AMT respectively (p=0.0109, p=0.0073 respectively) (Table 1).
Table 1.
Radiation damage mitigation of 32D cl3 murine hematopoietic cells in vitro by JP4-039, MCF201-89, BEB 55 individually or in combination
| Treatment | D0(Gy) | ñ |
|---|---|---|
| Control | 1.4 ± 0.3 | 1.6 ± 0.4 |
| AMT | 1.0 ± 0.1 | 10.5 ± 3.2¥ |
| MCF 201-89 | 1.0 ± 0.1 | 7.3 ± 1.9¥ |
| Control | 1.4 ± 0.3 | 1.3 ± 0.1 |
| TEMPOL | 1.3 ± 0.1 | 5.23 ± 0.8* |
| JP4-039 | 1.2 ± 0.1 | 4.55 ± 0.5* |
| Control | 1.3 ± 0.1 | 1.1 ± 0.1 |
| JP4-039 | 1.3 ± 0.1 | 5.0 ± 0.4** |
| BEB 55 | 1.3 ± 0.1 | 3.6 ± 0.6** |
| JP4-039 + BEB 55 | 1.1 ± 0.5 | 6.6 ± 1.7** |
| Control | 1.4 ± 0.1 | 1.5 ± 0.4 |
| JP4-039 | 1.4 ± 0.1 | 4.3 ± 0.7† |
| MCF 201-89 | 1.3 ± 0.1 | 4.4 ± 0.7† |
| JP4-049 + MCF201-89 | 1.2 ± 0.1 | 4.7 ± 1.3† |
| Control | 1.3 ± 0.1 | 1.0 ± 0.1 |
| BEB 55 | 1.1 ± 0.1 | 11.5 ± 2.5§ |
| MCF 201-89 | 1.1 ± 0.1 | 15.3 ± 3.7§ |
| BEB 55 + MCF201-89 | 1.2 ± 0.1 | 9.1 ± 3.2§ |
| Control | 1.5 ± 0.1 | 1.4 ± 0.2 |
| JP4-039 | 1.3 ± 0.1 | 5.1 ± 1.0‡ |
| BEB 55 | 1.3 ± 0.1 | 4.4 ± 0.9‡ |
| MCF 201-89 | 1.4 ± 0.1 | 4.0 ± 0.3‡ |
| JP4-049 + BEB 55 + MCF 201-89 | 1.3 ± 0.1 | 4.3 ± 1.1‡ |
Drugs were added individually or in combinations at 10 µg/ml to 32D cl 3 cells shortly after irradiation and was plotted in quadruplets in clonagenic survival and assays. Colonies of ≥ 50 cells were scored at day 7 as described in Materials and Methods.
(p=0.0073, p=0.0045),
(p=0.0109, p=0.0022),
(p=0.0109, p=0.0097, p=0.0403, respectively),
(p<0.0001, =0.0006, p=0.0017, respectively),
(p = 0.0105, p = 0.0147, p = 0.0407 respectively),
(p = 0.0021, p = 0.0038, p = 0.0011, p = 0.0073 respectively).
Radiation Mitigation of 32 D cl3, KM101 and IB3 cells by Mitochondria-Targeted Drugs
Treatment of 32 D cl 3 cells with JP4-039, MCF201-89 or BEB55 after irradiation, increased survival significantly, compared to the control group of untreated cells (p=0.0021, p=0.0011, p=0.0038 respectively) (Table 1). When drugs were administered as mixtures of JP4-039/MCF201-89, JP4-039/BEB55, MCF201-89/BEB55 or JP4-039/MCF201-89/BEB55, 32D cell survival was also increased compared to control cells (p=0.0403, p=0.0017, p=0.0407, p=0.0073 respectively) (Table 1). The DRF of 1.58±0.20, 2.10±0.33 and 2.05±0.93 were obtained for 32D cl 3 treated with JP4-039, MCF201-89 and BEB55 respectively.
Treatment of KM101 (Table 2) with JP4-039, MCF201-89, or BEB55 individually increased cell survival significantly over the control group of untreated cells (p=0.0007, p=0.0235, p=0.0044 respectively). The combination of two or all 3 compounds did not further increase cell survival (Table 2). The DRF of 1.28±0.07, 1.44±0.13 and 1.39±0.08 were obtained for KM101 treated with JP4-039, MCF201-89 and BEB55 respectively.
Table 2.
Radiation damage mitigation of human bone marrow stromal (KM101) and human bronchial epithelial (lB3) cells in vitro by JP4-039, MCF201-89, BEB 55 individually or in combination
| KM 101 cells | IB3 cells | |||
|---|---|---|---|---|
| Treatment | D0(Gy) | ñ | D0(Gy) | ñ |
| Control | 1.3 ± 0.1 | 2.6 ± 0.2 | 1.7 + 0.2 | 1.9 ± 0.3 |
| JP4-039 | 1.2 ± 0.1 | 6.1 ± 0.1† | 1.6 ± 0.1 | 5.7 ± 1.1‡ |
| BEB 55 | 1.1 ± 0.1 | 5.7 ± 0.5† | 1.5 ± 0.1 | 5.9 ± 0.5‡ |
| MCF 201-89 | 1.1 ± 0.1 | 6.9 ± 1.0† | 1.4 ± 0.1 | 9.1 ± 2.9‡ |
| JP4-039 + BEB 55 | 1.3 ± 0.1 | 3.2 ± 0.3* | 1.5 ± 0.1 | 7.0 + 0.2‡ |
| JP4-049 + MCF201-89 | 1.2 ± 0.1 | 2.8 ± 0.5* | 1.5 ± 0.1 | 7.1 ± 1.8‡ |
| BEB 55 + MCF 201-89 | 1.3 ± 0.1 | 2.5 ± 1.0* | 1.6 ± 0.1 | 4.9 ± 0.9‡ |
| JP4-049 + BEB 55 + MCF 201-89 | 1.2 ± 0.0 | 2.4 ± 0.0* | 1.6 ± 0.2 | 7.3 ± 0.7‡ |
Each drug was added at 10 µg/ml immediately after irradiation. Cells were plated in quadruplets in a clonogenic survival curve assay and colonies of > 50 cells counted at day 7 as described in Materials and Methods.
(p=0.0007, p=0.0044, p=0.0235, respectively),
(Not significant, p>0.05),
(p = 0.0193, p = 0.0017, p = 0.0452, p = 0.0002, p=0.0302, p=0.0259, p=0.0019, respectively).
Treatment of IB3 cells with JP4-039, MCF201-89, or BEB55 individually increased cell survival significantly over the control cells (p=0.0193, p=0.0452, p=0.0017 respectively) (Table 2). Treatment of IB3 cells with drug mixtures of JP4-039/MCF201-89, JP4-039/BEB55, MCF201-89/BEB55 or JP4-039/MCF201-89/BEB55, increased cell survival significantly (p=0.0302, p=0.0002, p=0.0259, p=0.0019 respectively) (Table 2). The DRF of 1.26 ± 0.13, 1.33 ± 0.03 and 1.27 ± 0.04 were obtained for IB3 treated with JP4-039, MCF201-89 and BEB55 respectively.
Clonogenic survival curves for all in vitro studies corresponding to data presented in Table 1 – Table 2 are shown in online supplemental data as Figures I – VI (Table 1) and Figures VII – X (Table 2).
Radiation Damage Mitigation by BEB55 is p53-dependent
We next sought to confirm that the mechanism of action of BEB55 involved its known p53/mdm2/mdm4 binding and not another, p53-independent, mechanism. Treatment of either p53+/+ or p53−/− cells with JP4-039 significantly increased cell survival (p=0.0396, p=0.0007, respectively). Treatment with MCF201-89 also increased survival of both p53+/+ and p53−/− (p=0.0071, p=0.0188 respectively). In contrast, BEB55 increased survival of irradiated p53+/+, which was not observed for p53−/− cells (p=0.0045, p=0.37, respectively) (Table 3). Clonogenic survival curves corresponding to data presented in Table 3 are shown in online supplemental material (Figures XI – XIII).
Table 3.
Radiation Damage Mitigation by BEB 55 is p53-dependent
| P53 +/+ cells | p53 −/− cells | |||
|---|---|---|---|---|
| Treatment | D0(Gy) | ñ | D0(Gy) | ñ |
| Control | 2.7 ± 0.5 | 2.5 ± 0.8 | 1.7 ± 0.2 | 4.5 ± 1.2 |
| BEB 55 | 1.6 ± 0.1 | 12.5 ± 2.1† | 1.8 ± 0.1 | 5.7 ± 0.6* |
| Control | 1.6 ± 0.2 | 8.0 ± 1.4 | 1.6 ± 0.1 | 10.2 ± 1.1 |
| JP4-039 | 1.6 ± 0.2 | 14.7 ± 0.9† | 1.3 ± 0.1 | 23.1 ± 1.8‡ |
| Control | 1.1 ± 0.2 | 8.3 ± 1.0 | 1.1 ± 0.1 | 11.5 ± 1.5 |
| MCF 201-89 | 1.2 ± 0.2 | 21.7 ± 0.5† | 1.0 ± 0.1 | 25.2 ± 1.1‡ |
Each drug was added at 10 µg/ml immediately after irradiation. Cells were plated in quadruplates in clonogenic survival curve assays and colonies of ≥ 5 × cells screened at day 7 as described in Materials and Methods.
(p=0.0045, p=0.0396, p=0.0071 respectively),
(p =0.0007, p=0.0188, respectively),
( Not significant p=0.3746).
In Vivo Radiation Mitigation by Mitochondrial Targeted Drugs
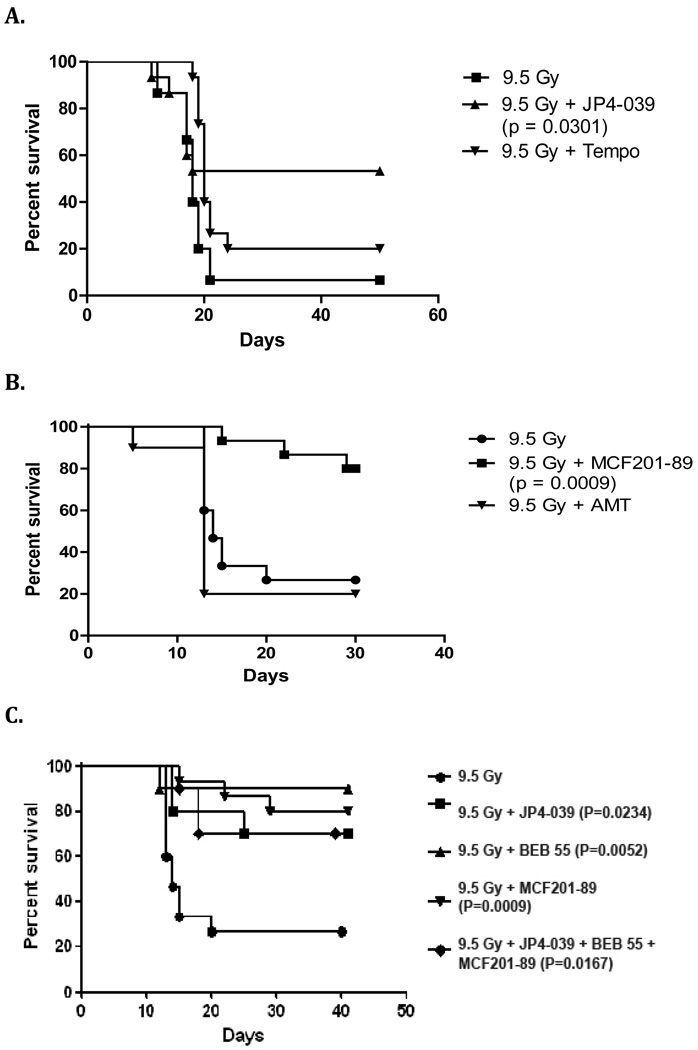
C57BL/6NHsd female mice treated after irradiation with JP4-039 compared to Tempol, or MCF201-89 compared to AMT, showed improved survival. In vivo studies showed that GS-nitroxide JP4-039 and GS-AMT conjugate MCF201-89 were both effective radiation mitigatorscompared to the control group of untreated mice (p=0.0301, p=0.0009 respectively) (Figure 3 A–B). Thus, the GS targeting strategy was effective in vivo.
Figure 3. Gramicidin S targeting strategy is effective in vivo.
C57BL/6NHsd female mice received intraperitoneal injections: (A) C57BL/6NHsd female mice received JP4-039 or TEMPOL after 9.5 Gy whole body irradiation (n = 15 mice/group) (p=0.0301 for JP4-039 vs. irradiation control). (B) MCF201-89 or AMT after 9.5 Gy TBI (n=15 mice/group) (p= 0.0009 for MCF201-89 vs. irradiation control). Mice treated with JP4-039, MCF201-89, BEB55 or a combination of all three agents showed significantly increased survival compared to the control group of irradiated mice (p=0.0234, p=0.0009, p=0.0052, p=0.0167 respectively) (Figure 3C).
JP4-039, MCF 201-89 and BEB55 delivered after TBI conferred a significant increase in survival compared to the control irradiated mice (p=0.0234, p=0.0009, p=0.0052 respectively) (Figure 3 C).
Timed Response and Dose Response Optimization for GS-Nitroxide JP4-039, GS-NOS Inhibitor MCF201-89, and P53/mdm2/mdm4 Inhibitor BEB55
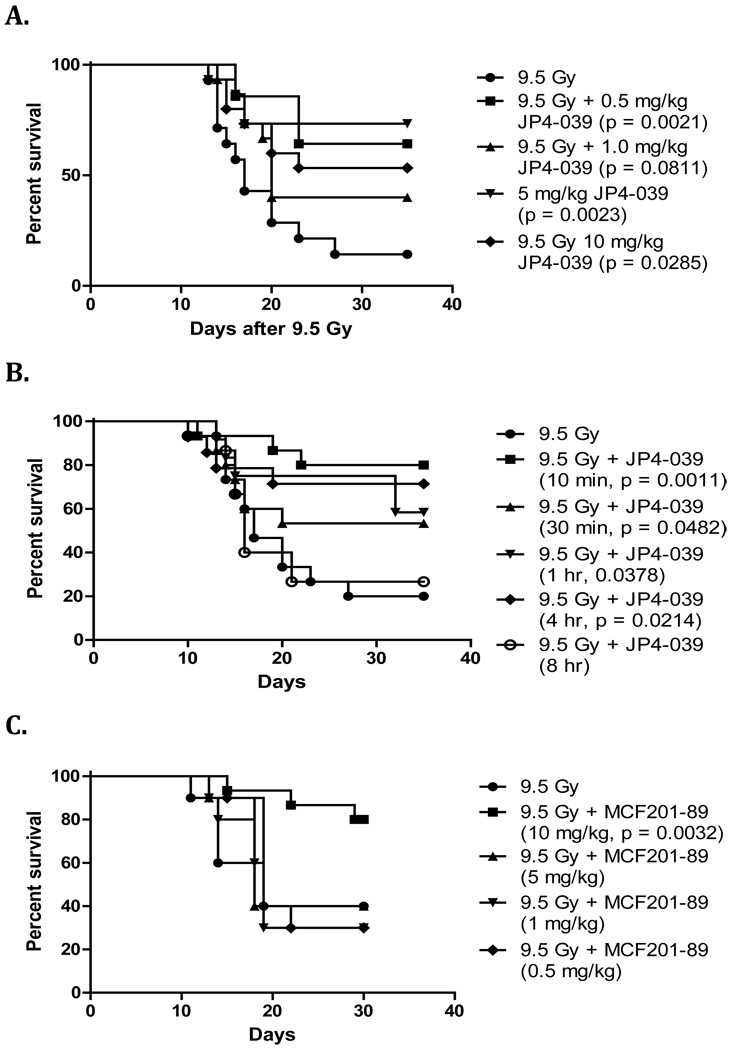
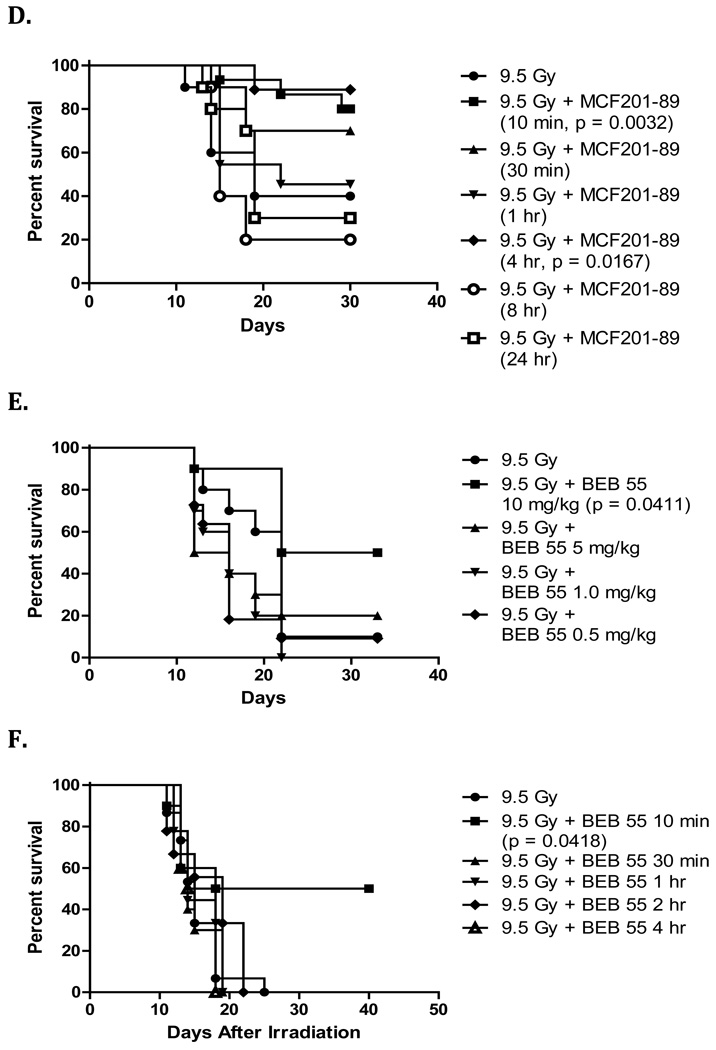
Experiments were performed to determine the optimum in vivo dose and optimal time of delivery for each of the three drugs. Groups of mice were given 1 mg/kg, 5 mg/kg, or 10 mg/kg of JP4-039, MCF201-89 or BEB55 via intraperitoneal injection after 9.5 Gy TBI, and were monitored for survival. The doses of 5 mg/kg, 10 mg/kg and 10 mg/kg were most effective for JP4-039, MCF 201-89 and BEB55, respectively (Figure 4 A, C, E).
Figure 4. Dose dependent response and time of delivery for radiation mitigation by GS-nitroxide, GS-NOS inhibitor, and p53/mdm2/mdm4 inhibitor in vivo or drug combination.
To determine the optimum dose C57BL/6NHsd female mice (n=15 mice/group) were irradiated to 9.5 Gy TBI (LD50/30) and received intraperitoneal injections (100 uL) 10 min later at different doses (A, C & E). To optimize time of delivery, 5 mg/kg of JP4-039 (B), 10 mg/kg of MCF201-89 (D), or 10 mg/kg of BEB55 (F) were given following 9.5 Gy TBI at different times. Each drug was dissolved in a 10% cremophor el, 10% ethanol and 80% water solution. Durability of radiation mitigation effect by JP4-039, MCF 201-89 and BEB55 delivered at 5 mg/kg, 10 mg/kg and 10 mg/kg respectively showed that JP4-039 and MCF 201-89 were effective up to 4 hours after 9.5 Gy TBI, whereas BEB55 was effective at 10 minutes.
We next determined if the administration after TBI of each of the 3 drugs was still effective even if given after several hours. Groups of mice were injected with either 5mg/kg JP4-039, 10 mg/kg MCF201-89, or 10 mg/kg BEB55 at 30 min, 1 hour, 4 hours or 8 hours after 9.5 Gy TBI. Mitigation was maintained when drug administration was carried out up to 4 hours after TBI for JP4-039 and 4 hours for MCF 201-89 (Figure 4 B , D). However, BEB55 was effective only if administered immediately (ie. within 10 minutes) after 9.5 Gy TBI (Figure 4 F). Mice that received all 3 drugs also had increased survival compared to control mice p = 0.0167 (Figure 3 C). We tested clearance of JP4-039 which can be measured by ESR (electron spin resonance) and observed that levels of JP4-039 reached a peak in plasma and lung tissue within 5 – 10 minutes, and clearance from plasma was detected within 20 minutes. JP4-039 levels in lungs persisted for 100 minutes, and were similar to levels observed in bone marrow and intestine (data not shown).
DISCUSSION
The two mitochondria-targeted drugs: nitroxide JP4-039, and NOS inhibitor MCF201-89, and the p53/mdm2/mdm4 inhibitor BEB55, were effective as ionizing radiation damage mitigators for hematopoietic and epithelial cells in vitro and protected mice in vivo. The 3 drugs differ in their mechanisms of action. The nitroxide JP4-039 displays superoxide dismutase mimic capacity and broad ROS neutralization capability, and offers the advantage of a catalytic cycling between nitroxide, hydroxylamine, and nitroxonium species, so that a single molecule can be bioavailable to reduce multiple free radicals (33). The GS-nitroxide JP4-039 as well as its higher molecular weight homologue XJB-5-131 have been shown to increase the concentration of 4-AT in the mitochondria by 33–600 fold (4). TEMPOL can also be delivered to the mitochondria by linkage to the organic cationic carrier triphenyl-phosphonium in conjugation with 4-AT (14). JP4-039 provided enhanced survival of 32 D cl3 murine hematopoietic cells and KM101 human bone marrow stromal cells, and human IB3 epithelial cells.
Mitochondrial targeting of the NOS inhibitor AMT (MCF201-89) was achieved through the identical peptide alkene isostere segment used for GS-nitroxides. Whether mitochondrial NO production results from NOS1 is controversial (28, 34, 35, 39–41); however, following ionizing irradiation, the toxicity of peroxynitrite in the mitochondrial membrane has focused attention on its precursor nitric oxide as well as superoxide. Irradiation induced calcium transport across the mitochondrial membrane leads to induction of nitric oxide synthase (34). The broad specificity NOS-inhibitor present in MCF201-89 provided significant mitigation against radiation damage in epithelial and hematopoietic cell lines and was also effective in vivo.
The p53/mdm2/mdm4 inhibitor BEB55 also mitigated against radiation damage to human epithelial and both murine and human hematopoietic cell lines and increased mouse survival after TBI. The p53 binding site in mdm2 and mdm4 has been shown by NMR Spectroscopy and cocrystal structure analysis to also bind other proteins including: E2F1, HIF-la, p63, p73, VP16, C-jun, MycD, and NFKB (42). Mdm2 is a negative regulator of p53 by functioning both as an E3 ubiquitin ligase that recognizes the N-terminal trans-activation domain (TAD) of p53 and by inhibiting p53 transcription. Mdm4 (-Mdmx), structurally similar to mdm2, when produced by in vitro translation, interacts with p53 via a binding domain located in the N-terminal region of the mdm4 protein. Mdm4 reduction by RNA interference increases cellular resistance to DNA-damage-induced apoptosis in a p53-dependent manner independent of transcription (43). There may be several mechanisms of radiation mitigation by p53/mdm2/mdm4 antagonists (44); however, our results suggest that the likely mechanism of action of BEB55 is through a direct inhibition of the interaction with p53. BEB55 was an effective radiation mitigator of p53+/+ but not p53−/− cells, while both GS-nitroxide (JP4-039) and GS-NOS-I (MCF201-89) mitigated both wild type and knock out cells.
The combination of 3 drugs was toxic for KM101 cells, while each drug individually was an effective mitigator. KM101 cells were derived from human bone marrow stromal fibroblasts, that were transformed by SV40 virus (16). Further studies will be required to determine whether cell phenotype or a role of SV40 contributed to a negative interaction of the drug combination with this cell line. In addition, studies are in progress testing the efficacy of these drugs in human umbilical cord blood hematopoietic cells.
To further assess the efficacy of the 3 drugs dose reduction factor (DRF) were calculated from in vitro radiation mitigation of mice and human cells were determined. DRF of 1.6, 2.1 and 2.1 were obtained with 32D cl3 cells treated with JP4-039, MCF201-89 and BEB55 respectively. The DRF for KM101 cells was 1.3, 1.4 and 1.4 for JP4-039, MCF201-89 and BEB55 respectively; whereas for IB3 cells, the DRF was 1.3 for all 3 drugs. Purdie et al (44) reported DRF of 1.3, 2.3 and 2.9 for human kidney T-cells treated with 4mM of WR-2721 (Amifostine), 2-mercaptoethylamine (cysteamine) and WR-1065 (N-(2-mercaptoethyl)-1,3-diaminopropane) respectively at 37C and 30 minutes prior to irradiation . However, no other drugs have previously been shown to enhance cell survival following radiation exposure as demonstrated by our study. Further studies are ongoing to determine DRF for the 3 drugs in in vivo radiation mitigation.
Mice receiving a mixture of all 3 drugs following TBI showed similar but not additive or synergistic mitigation effects. Since the three drugs were given simultaneously, the present studies do not rule out the possibility that sequential or staggered administration of these drugs in groups of two or three might produce an additive or synergistic effect, nor do they rule out the possibility that local organ-specific administration in combinations of two or three drugs could provide additive or synergistic effects on a particular tissue or organ (45–50).
CONCLUSION
Mitochondrial targeting of small molecule radiation mitigators by either chemical attachment of translocation anchors, or computational chemistry based design of modulators of known mitochondrially active proteins, validate the critical significance of mitochondria in irradiation-induced cell death. These approaches also highlight the potential for development of new drugs for use in clinical radiation therapy.
Supplementary Material
Acknowledgements
This work is supported by the Radiological Society for North America (RSNA) Research & Education Foundation, NIH T32AG21885 and NIH/NIAD U19AI068021
Footnotes
Conflicts of interest: None
References
- 1.Kanai AJ, Zeidel ML, Lavelle JP, et al. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J Physiol Renal Physiol. 2002;283(6):F1304–F1312. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 2.Rubin P, Casarett GW. In: Clinical Radiation Pathology. WB Saunders., editor. Philadelphia, PA: 1968. [DOI] [PubMed] [Google Scholar]
- 3.Stone HB, Coleman C, et al. Norman, Models for evaluating agents intended for the prophylaxis, mitigation, treatment of radiation injuries Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–718. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 4.Jiang J, Belikova NA, Hoye AT, et al. A mitochodnria-targeted nitroxide/hemi-gramicidin S conjugate protects mouse embryonic cells against γ-irradiation. Int J Radiat Oncol Biol Phys. 2008;70(3):816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Kim N, Yang J, et al. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome-C. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 6.Marzo I, Brenner C, Zamzami N, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 7.Zamzami N, Susin SA, Marchetti P, et al. Mitochondrial control of nuclear apoptosis. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epperly MW, Gretton JE, Sikora CA, et al. Mitochondrial localization of superoxide dismutase is required for decreasing radiation-induced cellular damage. Radiat Res. 2003;160:568–578. doi: 10.1667/rr3081. [DOI] [PubMed] [Google Scholar]
- 9.Kagan VE, Tyurin VA, Jiang J, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radioprotective gene therapy. Current Gene Therapy. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 11.Greenberger JS, Epperly MW. Radioprotective antioxidant gene therapy: Potential mechanisms of action. Gene Therapy and Molecular Biology. 2004;8:31–44. [Google Scholar]
- 12.Greenberger JS, Epperly MW. Pleiotrophic stem cell and tissue effects of ionizing irradiation protection by MnSOD-plasmid liposome gene therapy. In: Columbus Frank., editor. Progress in Gene Therapy. Nova Science Publications; 2005. pp. 110–118. [Google Scholar]
- 13.Jiang J, Kurnikov I, Belikova NA, et al. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320(3):1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Stoyanovsky DA, Belikova NA, et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172(6):706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagan VE, Bayir A, Bayir H, et al. Mitochondria-targeted disruptors and inhibitors of cytochrome c/cardiolipin peroxidase complexes: A new strategy in anti-apoptotic drug discovery. Mol Nutr Food Res. 2009;53:104–114. doi: 10.1002/mnfr.200700402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santucci MA, FitzGerald TJ, Harigaya K, et al. Gamma-irradiation response of cocultivated bone marrow stromal cell lines of differing intrinsic radiosensitivity. Int J Radiat Oncol Biol Phys. 1990;18:1083–1092. doi: 10.1016/0360-3016(90)90444-o. [DOI] [PubMed] [Google Scholar]
- 17.Kanai A, Zabbarova I, Amoscato A, et al. Mitochondrial targeting of radioprotectants using peptidyl conjugates. Org Biomol Chem. 2007;5(2):307–309. doi: 10.1039/b613334g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kranz D, Dohmesen C, Dobbelstein M. BRCA1 and Tip60 determine the cellular response to ultraviolet irradiation through distinct pathways. J Cell Biol. 2008;182:197–213. doi: 10.1083/jcb.200712014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane DP. p53, guardian of the genome. Nature. 1992;358:15. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 20.Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- 21.Gudkov AV, Komarova EA. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun. 2005;331:726–736. doi: 10.1016/j.bbrc.2005.03.153. [DOI] [PubMed] [Google Scholar]
- 22.Girnita L, Girnita A, Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci, USA. 2003;100(14):8247–8252. doi: 10.1073/pnas.1431613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava S, Beck B, Wang W, et al. Rapid and efficient hydrophilicity tuning of p53/mem2 antagonists. J Comb Chem. 2009;11:631–639. doi: 10.1021/cc9000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopalan MS, Gupta K, Epperly MW, et al. The mitochondria-targeted nitroxide JP4-039 augments potentially lethal irradiation damage repair. In Vivo. 2009;23(5):717–726. [PMC free article] [PubMed] [Google Scholar]
- 25.Dömling A. Small Molecular Weight Protein Protein Interaction Antagonists – An Insurmountable Challenge? Curr Op Chem Biol. 2008;12:281. doi: 10.1016/j.cbpa.2008.04.603. [DOI] [PubMed] [Google Scholar]
- 26.Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem Rev. 2006;106:17. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 27.Czarna A, Beck B, Srivastava S, et al. Robust Generation of Lead Compounds for Protein-Protein Interactions by Computational and MCR Chemistry: p53-Hdm2 Antagonists Angew. Chem Intl Engl Eng. 2010 doi: 10.1002/anie.201001343. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bista M, Kowalska K, Janczyk W, et al. Robust NMR screening for lead compounds using tryptophan-containing proteins. J Am Chem Soc. 2009;131:7500–7501. doi: 10.1021/ja901863h. [DOI] [PubMed] [Google Scholar]
- 29.Popowicz GM, Czarna A, Wolf S, et al. Structures of low molecular weight inhibitors bound to MDMX and MDM2 reveal new approaches for p53-MDMX/MDM2 antagonist drug discovery. Cell Cycle. 2010;9:1104–1111. doi: 10.4161/cc.9.6.10956. [DOI] [PubMed] [Google Scholar]
- 30.Epperly MW, Bray JA, Carlos TM, et al. Biology of marrow stromal cell lines derived from long-term bone marrow cultures of Trp53-deficient mice. Radiat Res. 1999 Jul;152(1):29–40. [PubMed] [Google Scholar]
- 31.Zeitlin PL, Lu L, Rhim J, et al. A cystic fibrosis bronchial epithelium cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 32.Epperly MW, Gretton JA, DeFilippi SJ, et al. Modulation of radiation-induced cytokine elevation associated with esophagitis and esophageal stricture by manganese superoxide dismutase-plasmid/liposome (SOD2-PL) gene therapy. Radiat Res. 2001;155:2–14. doi: 10.1667/0033-7587(2001)155[0002:morice]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Hahn SM, Krishna Cm, Samuni A, et al. Identification of nitroxide radioprotectors. Radiat Res. 1992;132:87–93. [PubMed] [Google Scholar]
- 34.Kanai AJ, Pearce LL, Clemens PR, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci, USA. 2001;98(24):14126–14131. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venkatakrishnan P, Nakayasu ES, Almeida IC, et al. Absence of nitric oxide synthase in sequentially purified rat liver mitochondria. J Biol Chem. 2009;284:19843–19855. doi: 10.1074/jbc.M109.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavannya P, Katarzyna KE, Shenyan Z, et al. The physiologic aggresome mediates cellular inactivation of iNOS. Proc Natl Acad Sci, USA. 2009;106:1211–1215. doi: 10.1073/pnas.0810968106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epperly MW, Travis EL, Sikora C, et al. Magnesium superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: Modulation of irradiation-induced mRNA for IL-1, TNF-α, and TGF-β correlates with delay of organizing alveolitis/fibrosis. Biology of Blood and Marrow Transplantation. 1999;5:204–214. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- 38.Cooney R, Hynes SO, Sharif F, et al. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Therapy. 2007;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- 39.Oberleithner H, Callies C, Kusche-Vihrog K, et al. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci, USA. 2009;106:2829–2834. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martine D, Fabrice LB, Jacques H, et al. Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Molecular Biology of the Cell. 2007;18:4659–4668. doi: 10.1091/mbc.E07-05-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahfuzul HM, Koustubh P, Jesus T, et al. A connecting hinge repress the activity of endothelial nitric oxide synthase. Proc Natl Acad Sci, USA. 2007;104:9254–9259. doi: 10.1073/pnas.0700332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srivastava S, Beck B, Wang W, et al. Rapid and efficient hydrophilicity tuning of p53/mdm2 antagonists. J Comb Chem. 2009;11:631–639. doi: 10.1021/cc9000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Francesca M, Giusy D, Marsha P, et al. MDM4 (MDMX) localizes at the mitochondria and facilitates the p53-mediated intrinsic-apoptotic pathway. The EMBO Journal. 2009;28:1926–1939. doi: 10.1038/emboj.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purdie WJ. A Comparative Study of the Radioprotective Effects of Cysteamine, WR-2721, and WR-1065 in Cultured Human Cells. Radiat Res. 1979 Feb;77(2):303–311. [PubMed] [Google Scholar]
- 45.Popowicz GM, Czarna A, Wolf S, et al. Structures of low molecular weight inhibitors bound to Mdmx and Mdm2 reveal new approaches for p53-MDMX/MDM2 antagonist. Drug Discovery. doi: 10.4161/cc.9.6.10956. (In press) [DOI] [PubMed] [Google Scholar]
- 46.Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Experimental Hematology. 2007;35:96–104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Himburg HA, Daher P, Meadows SK, et al. Systemic Administration of Pleiotrophin Induces Hematopoietic Stem Cell Regeneration In Vivo. American Society of Hematology 51st annual meeting; presentation 1498. 2009 December 3; [Google Scholar]
- 48.Muramoto GG, Russell JL, Safi R, et al. Inhibition of Aldehyde Dehydrogenase Expands Hematopoietic Stem Cells with Radioprotective Capacity. Stem Cells. 2010 doi: 10.1002/stem.299. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Netzer N, Goodenbour JM, David A, et al. Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature. 2009;462(7272):522–526. doi: 10.1038/nature08576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh SN, Zhang R, Fish BL, et al. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(5):1528–1536. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.