Abstract
A wealth of data from the last fifty years documents the potency of early life experiences including maternal care on developing offspring. A majority of this research has focused on the developing stress axis and stress-sensitive behaviors in hopes of identifying factors impacting resilience and risk-sensitivity. The power of early life experience to shape later development is profound and has the potential to increase fitness of individuals for their environments. Current findings in a rat maternal care paradigm highlight the complex and dynamic relation between early experiences and a variety of outcomes. In this review we propose adaptive hypotheses for alternate maternal strategies and resulting offspring phenotypes, and ways to distinguish between these hypotheses. We also provide evidence underscoring the critical role of context in interpreting the adaptive significance of early experiences. If our goal is to identify risk-factors relevant to humans, we must better explore the role of the social and physical environment in our basic animal models.
Keywords: maternal behavior, maternal effects, stress, adaptation, fitness, licking, grooming
Early-life experience during sensitive periods has the potential to shape developmental trajectories and has profound impacts on physiology and behavior. Maternal care is one potent source of variability in the early social environment that affects multiple outcomes in mammalian development, from stress reactivity to sexual behavior. Aspects of maternal care and offspring characteristics have been studied extensively in rodents, primates, and humans (Cameron et al., 2008; Denenberg et al., 1962; Fleming et al., 2002; Francis et al., 1999; Harlow and Zimmermann, 1959; Levine et al., 1957). To the extent that variation in maternal care across species is sensitive to external cues, it may provide an opportunity for offspring physiology and behavior to be tuned or calibrated for the expected environment. To understand the relevance of variation in maternal behavior in the rat maternal care paradigm, we explore the potential adaptive significance of behavioral variation for dams, and of resulting phenotypic outcomes for offspring. Understanding the range of outcomes affected by early experience and their adaptive significance, if any, will have diverse implications that may ultimately inform human interventions. In order to allow for such broad translational impacts, we need to better understand the role of context—including but not limited to genetic, developmental, social, ecological, and psychological context—in shaping development of physiology and behavior.
Recent research focused on how naturally occurring variations in rat maternal care impact developing offspring has permeated and influenced a wide variety of disciplines. Many of these disciplines require the translation and interpretation of basic rodent laboratory findings into the human realm. Not surprisingly, critical details and caveats relevant to the animal findings often get ‘lost in translation’. The inherent plasticity of the stress-axis to perturbations and experiences that occur during early postnatal development including the quality and quantity of parental care provided the offspring is undisputed across a variety of rodent and primate models (Coplan et al., 2006; Liu et al., 1997; Suomi, 1991). Contention exists when trying to identify which particular components of the early-life environments are ‘most salient’ to later offspring phenotypes and when attempting to ‘valence’ early-experiences as ‘good’ or ‘bad’ without adequate attention to the context in which the data is both acquired and assessed. For example, one of the most common interpretations or assumptions extracted from the animal literature is that ‘stressful’ experiences early in development will always negatively compromise a given phenotype: as we discuss in this review, this is not the case. Clearly we must do a better job of effectively translating findings from animal models to humans. In a recent review, Loman and Gunnar suggest that this will require ‘a conceptual model that is general enough to apply across species, but specific enough to guide empirical investigation…’ to inform better design and interpretation of both animal model research and human research (Loman and Gunnar, 2010).
In this paper we highlight details and caveats from animal model data on early-life maternal care—primarily for the laboratory rat. We propose that attention to the adaptive significance of maternal behavior is applicable across species in a way that specific maternal behaviors may not be, and we provide a framework for evaluating adaptive hypotheses about maternal care in rats. Finally we discuss how social, environmental, psychological and physiological context are key to evaluating the adaptive significance of maternal behavior across model systems. Ultimately we wish to promote better integration and interpretation of animal and human models that study maternal care.
1. Selected approaches to the study of maternal care
A brief synopsis of the origins of commonly used primate and rodent models of early-life maternal care may be of use to those who primarily work with human data or are new to the field.
1.1 From humans to rodents and back: an historical perspective
From the inception of research on maternal care-giving, animal research has inspired the development of human theories and vice versa. Perhaps the most influential theory of child social development is attachment theory, developed by psychiatrist and psychoanalyst John Bowlby. Bowlby’s primary interest was in the bond formed between a child and its caregiver. His theoretical framework was heavily inspired by the methods used by early ethologists, such as Tinbergen, Lorenz and Hinde, to study animal behavior (Van Der Horst et al., 2007). This approach was dramatically different from the psychoanalytic perspective Bowlby had been trained in.
Bowlby described his seminal findings on mother-infant attachment in a report for the WHO published in 1951 titled Maternal Care and Mental Health (Bowlby, 1951). These findings directly inspired the primate work pioneered by Harry Harlow at the University of Wisconsin-Madison in the 1950s-1960s. Harlow and his trainees employed separation procedures during various early developmental stages to directly assess which components of maternal care-giving were critical in the formation of the quality of bond described by Bowlby in humans. Harlow’s later worked expanded these separation periods in young rhesus monkeys to include extended periods of total isolation (Harlow, 1965). These earliest primate models are the antecedents to much of the current work exploring the earliest social experiences of the developing macaque (see Stevens et al., 2009 for an excellent review/overview of current primate models). This work also set the stage for consideration of the role of peer interactions and other important aspects of the early social environment besides maternal care (e.g. Harlow, 1969, Branchi, 2009).
Recent work exploring the effects of natural variations in maternal care in rats on developing offspring is an extension of the neonatal separation/handling paradigm pioneered by Seymour Levine (and employed by many others) in the 1950’s and 1960’s. While Bowlby’s work in human development and attachment was influenced by animal behavior and ethology, Levine’s earliest rodent experiments were inspired by human psychoanalytic theories. Levine wanted to create a rodent model that would allow him to causally test the psychoanalytic hypothesis that trauma early in life induced later neuroticism (Hutt, 1974). The traumatic event for Levine’s young rats was a brief electric shock administered daily for the first few weeks of postnatal life. The results from this early study were unexpected, and central to the creation of the rodent neonatal ‘handling’ model that has been in existence for almost half a century. Contrary to the hypothesis that rat pups experiencing aversive shock early in life would exhibit ‘compromised’ phenotypes later, rats in the daily shock condition as well as in one of the control conditions (those simply exposed to shock chambers) had similar phenotypes. Moreover, rats from these two experimental conditions had significantly different behavioral and neuroendocrine profiles when compared to unmanipulated/unhandled control animals (Levine, 1957; Levine 1962). Animals subjected to the ‘stress’ condition or the ‘handling’ condition as infants, when tested as adults, exhibited less ‘emotionality’ in response to an acute stress challenge and demonstrated more efficient neuroendocrine responses.
These early findings serve as a reminder that the common, implicit assumption that stressful events early in life inevitably lead to poor outcomes or phenotypes is simply wrong. Infantile stimulation early in the life of a mammal commonly results in beneficial effects later in life, particularly with respect to stress-sensitive measures (Levine, 1957; Levine, 1962; Lyons & Parker, 2007; Lyons et al, 2009; Macri & Wurbel, 2006; Parker et al., 2005).
Levine’s work highlights that i) brief separations of rat pups from their dams, independent of the manipulation imposed, served to ‘increase’ maternal care and attention received upon being reunited (Smotherman, 1983) and ii) the neuroendocrine stress-axis in the developing rat was not a ‘fixed’ process, but rather, was sensitive to perturbations experienced in the life of the young animal. The research of Victor Denenberg (another pioneer in this field), using these same models, served to highlight another critical aspect of developmental processes in the rat—that the effects of various maternal experiences may be non-genomically transmitted to the next generation (Denenberg and Whimbey, 1963). These early findings clearly underscore the power of early life experiences to influence the developing rat pup both within and across generations.
1.2 Natural variations in rat maternal care and stress-reactivity
The pioneering work of Levine and Denenberg laid the groundwork for current research exploring how natural variations in maternal care influence the stress-sensitivity of the young rat, including work in Michael Meaney’s laboratory at McGill University in the 1990’s. This research helped elucidate a pathway by which variations in maternal care in the lab rat are associated with the development of differences in behavioral and endocrine responses to stress in the offspring. Naturally occurring variations in maternal licking—the largest source of pup tactile stimulation—are associated with the development of individual differences in the hypothalamic-pituitary-adrenal (HPA) endocrine axis and behavioral responses to stress in the offspring. A central component of the neuroendocrine response to stress is the synthesis and release of glucocorticoids from the adrenal glands in response to a signaling cascade originating from the hypothalamus. Glucocorticoids travel throughout the body to regulate and promote physiological arousal in response to stress; they also feedback to the brain in a negative-feedback manner to inhibit further activation of the stress-axis (Jacobson and Sapolsky, 1991). As adults, offspring of high-licking mothers are behaviorally less fearful and exhibit a more modest HPA response to stress than offspring of low-licking mothers (Liu et al., 1997). Adult offspring reared in a high-licking maternal environment also demonstrate enhanced glucocorticoid feedback sensitivity when compared to offspring reared by low-licking dams (Liu et al., 1997) This finding suggests that offspring reared in the high-maternal condition possess more tightly regulated stress-axes. Glucocorticoid receptors (GR) in the hippocampus are central to the negative-feedback regulation of the HPA axis (Sapolsky et al., 1984, 1986). Consistent with the stress measures described above, differential maternal care also influences the level of expression of GRs in the hippocampus. Offspring of high-licking mothers express significantly more GR in all regions of the hippocampus relative to offspring of low-licking mothers (Liu et al., 1997).
The simple procedure of rat pup cross-fostering at birth is sufficient to reverse the phenotypes described above. Rat pups born to a high-licking mother but reared by a low-licking mother exhibit stress reactivity as adults that are indistinguishable from that of offspring born to and reared by a low-licking mother. Conversely, offspring born to a low-licking mother but reared in a high-licking and grooming maternal environment exhibit stress-reactivity profiles of offspring born to and reared by a high-licking mother (Francis et al., 1999). These collective finding suggests that the quality of maternal care provided to offspring early in life is directly involved in the developmental programming and calibration of the HPA axis.
Recent work from this group has focused on molecular processes that mediate the transduction of the ‘lived experience’ into a biological signal. Maternal licking/grooming appears to influence glucocorticoid receptor density in the hippocampus at least in part by differential methylation of the GR promoter in the days after birth (Weaver et al. 2004a,b). This discovery has broadened our understanding of the potential for epigenetic mechanisms such as DNA methylation to allow for the biological encoding of experience in ways that affect long-term gene expression (see Meaney, 2010 for a review of findings to date).
2. Stress in context
Two oversimplifications typically result from the rodent findings reviewed here: (i) stressful early experiences inevitably lead to dysregulated stress-reactivity profiles, and (ii) increases in stress-reactivity are “bad.” As we have discussed with the work of Levine, some early life “stressors” are actually stimulating, and lead to lower reactivity later in life. This phenomenon has been described as early-life stress ‘inoculation’ and is discussed in greater depth by Parker, Lyons, and others in this issue.
The second oversimplification of the current findings in rodent maternal care is particularly pervasive: it is intuitive to assign positive value to high maternal care because more care sounds good and greater stress-reactivity sounds bad. Stress responses have been associated with behavioral and health problems in humans—including depression, cardiovascular disease, HIV progression, and tumor development in cancer (Cohen et al., 2007)—but it is also clear that organisms depend on stress responses for survival (Sapolsky, 2004; McEwen, 2008). The HPA stress response is often considered to be adaptive in the short term as a response to environmental conditions (‘allostasis’) but it exacts a physiological toll over the long term (‘allostatic load’) (e.g. McEwen and Gianaros, 2010). Whether heightened stress-reactivity and other outcomes of maternal care are of particular value to an organism will depend on the circumstances in which organisms operate. The assessment of the adaptive value of developmental programming can only be evaluated in a given context.
Few studies have explored whether the behavioral and physiological consequences of low levels of maternal care-giving, or variation in stress-reactivity may be adaptive in some circumstances, though some speculate that this might be the case. We discuss the available rat literature on this topic in section 4. In the primate realm, early maternal deprivation increases threat responses (Suomi, 2006), which may be of survival benefit in threatening environments. This same maternal deprivation also leads to altered responses to rewarding stimuli (Nelson et al., 2009), potentially altering goal directed behavior, and risk for addiction.
In humans, Pollak and colleagues demonstrated that maltreated children show different neural responsivity to angry faces and have a lower detection threshold for discrimination of anger from other facial expressions, particularly in the amygdala. The amydala appears to be a locus in the brain that serves as an automatic threat detector (Öhman, 2005). Hyper-arousal of this region may be adaptive for these children in their anticipated environments (Pollak et al., 2000; Pollak and Kistler, 2002). Enhancements of amygdala responses to angry faces were also found in children reporting low parental socioeconomic status (Gianaros et al., 2008).
Boyce and colleagues have speculated that stress reactivity may be conceptualized as greater susceptibility to environmental influences, leading to greater responsivity to stimulating environments and interventions (Blair, 2002; Boyce and Ellis, 2005; Ellis et al., 2005; Velderman et al. 2006,). A recent study found that children with high levels of stress-reactivity (measured by respiratory sinus arrhythmia in response to challenges) had the highest levels of prosocial behavior and school engagement and the greatest improvement under conditions of decreasing adversity (Obradović et al., 2010).
The above studies are an important beginning, but paint an incomplete picture of the value (if any) of reactivity, and few were designed to specifically assess this. More work is needed to determine when and if altered regulation of stress responsivity is an adaptation to or a maladaptive consequence of stress. By replacing the morally valenced term “good” with the more neutral term “adaptive”, we can attempt to better evaluate the impacts of maternal care on offspring in the rat model, and to consider the significance of the outcomes of maternal care for multiple traits in multiple environments.
3. Towards the adaptive significance of natural variation in maternal care: theoretical framework
Adaptation is defined differently by discipline, from heritable traits that confer a selective reproductive advantage, to habituation to environmental conditions. Here we consider multiple timescales of responses to the environment that have the potential to confer fitness benefits.
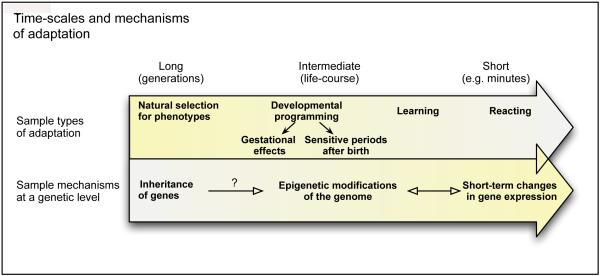
In his classic paper on the aims and methods of Ethology, Tinbergen distinguished between types of questions that behavioral biologists study, including mechanisms underlying behavior, evolutionary history, developmental origins, and adaptive significance (Tinbergen, 1963). The distinction between “how” (proximate) and “why” (ultimate) questions is often made by discipline; behavioral ecologists traditionally assess the adaptive significance of species-typical behaviors that have evolved over generations (the “why” perspective), while physiologists focus on molecular, cellular, and network processes that may contribute to behaviors (the “how” perspective). Proximate and ultimate causes can both be assessed at each time-scale of adaptation described below (Figure 1). We first consider mechanisms by which experience may play a role in adaptation over multiple time-scales, and then generate hypotheses that distinguish between different adaptive outcomes of maternal care.
Figure 1.
Time-scales of adaptation and sample genetic mechanisms. Organisms adapt to their environments in both temporary and long-term ways; adaptation involves processes including regulation across these time-scales with different regulatory mechanisms allowing for distinct temporal patterns of regulation.
3.1 The role of experience in adaptation (proximate mechanisms)
Experience becomes embedded in biology across multiple time-scales from evolutionary to momentary. Over generations, mutations accumulate and natural selection acts on populations to produce a pool of individuals that exhibit distinct genotypes including heritable adaptations (Fig 1, left). Genetic differences accumulate between and within species where they contribute to physical appearance, disease risk, and behavioral tendencies. Some diseases have been traced to single genes, and many more gene-disease associations have been found. As sequencing technology has allowed more extensive surveys of sequence variation and human characteristics, however, most traits with genetic components appear to have complex correlates (Shastry, 2002; Botstein and Risch, 2003), and many genetic correlates have only weak power to explain differences between individuals. Combined with the assessment that the human genome only has about 20-25,000 protein-coding genes by recent estimates (IHGSC, 2004)—far fewer than expected—the idea that genotype determines phenotype is giving way to the understanding that “DNA is not destiny”, and that phenotypes are moderated by both the internal and external environment (e.g. Waters, 2006; Pigliucci, 2010).
From moment to moment, the physical and social environment we live in impacts our thoughts, behaviors, and the regulation of gene expression (Figure 1, right). While not “genetic” in the sense that DNA sequence is altered, many short term reactions nevertheless often involve changes in gene expression.
Between relatively immutable genetic differences (accrued over an evolutionary timescale) and temporary responses to environmental conditions (which occur within milliseconds), individuals are shaped by stable changes in gene expression that persist over the life-course (figure 1, center). Some of these changes in gene activity may be regulated by epigenetic mechanisms. The term “epigenetic” has been defined in different ways. Conrad Waddington coined the term in 1942 to describe the study of “interactions between genes and their products which bring phenotype into being.” (Waddington 1968; Jablonka and Lamb 2002). This 1942 definition pre-dated the elucidation of the structure of DNA and the specific mechanisms of such interactions. In its present usage, the term can be applied broadly to developmental changes in cellular and sometimes organismal phenotype that don’t alter the sequence of DNA, or to specific described mechanisms such as DNA methylation and histone acetylation (Jablonka and Raz, 2009). We limit our use of epigenetic to phenomena for which such mechanisms have been described. Epigenetic changes can be inherited from cell to cell and in some cases from organism to organism (reviewed in Jablonka and Raz, 2009). Such mechanisms may be particularly important during organism ontogeny, for example the silencing of developmentally active genes involved in cellular differentiation once those processes are complete (Hershko et al., 2003, Laurent et al., 2010; Spivakov and Fisher, 2007). Changes in DNA methylation in response to early postnatal experience were first observed in response to variation in maternal care (Weaver et al., 2004a,b). Since then methylation changes have been detected in response to memory formation (Miller and Sweatt, 2007), and different methylation profiles in the GR promoter have been detected in human suicide victims with childhood experience of abuse (McGowan et al., 2009). As a mechanism of adaptation, epigenetic modifications may span multiple time-scales, capable of influencing complex traits across generations (Rando and Verstrepen, 2007; Jablonka and Lamb, 2005) and in some cases responsive to experience on even short time-scales (Miller and Sweatt, 2007). The existence of regulatory processes of varying time-scales and sensitivities allows for complex storage of environmental information in the long and short term (c.f. cascade models, Fusi et al., 2005).
Without changing the sequence of genes, experience during sensitive periods may greatly increase the possible outcomes of a particular genome. Variations in DNA sequence are important, but may be a less influential factor than anticipated, particularly for behavioral traits. Furthermore, organisms may even be selected for their capacity to exhibit diverse behavioral responses (Baldwin, 1896). Genes are modulated by their environment (Gene x Environment interactions); reversing the primacy of these factors (Environment x Genes) may help frame the concept and highlight that environmental influences may alter the activity and role of genes (Francis, 2009).
3.2 Adaptive hypotheses
The majority of research on rat maternal care has focused on proximate mechanisms of variation in stress-reactivity, but a tantalizing premise underlying this work is that variation in phenotype increases fitness whether offspring are from high or low LG litters because it tunes offspring for their environment. In order to evaluate whether this is the case, we must consider the fitness of the resulting phenotypes in the context of the organism’s environment.
From an evolutionary perspective, fitness is typically assessed in terms of reproductive success, particularly over the multiple rounds of reproduction (lifetime reproductive success) and sometimes including reproduction of kin (inclusive fitness). This emphasis on reproduction as a bottom line means that behavioral traits such as stress-reactivity are evaluated in terms of their potential impacts on reproduction. As encapsulated by ecologist Elizabeth Lacey, “an adaptive environmentally induced parental effect increases the probability of reproductive success of an offspring phenotype relative to others in a population.” (Lacey, 1998) There exists a broad-ranging body of research on parental effects on offspring fitness, with several studies demonstrating maternal effects that improve offspring fitness, and others failing to identify advantages to offspring (Mousseau and Fox, 1998). Our goal is to apply a similar framework to the study of the adaptive significance of rat maternal behavior.
Assessment of the fitness of high and low LG phenotypes in a natural setting would be difficult – direct maternal behavior is difficult to observe in a truly natural setting, and the reproductive success of dams across litters is an important factor for assessing the outcomes of maternal investment. Nonetheless we can compare predictions from different adaptive hypotheses, and envisage how experimental progress on these questions can be made.
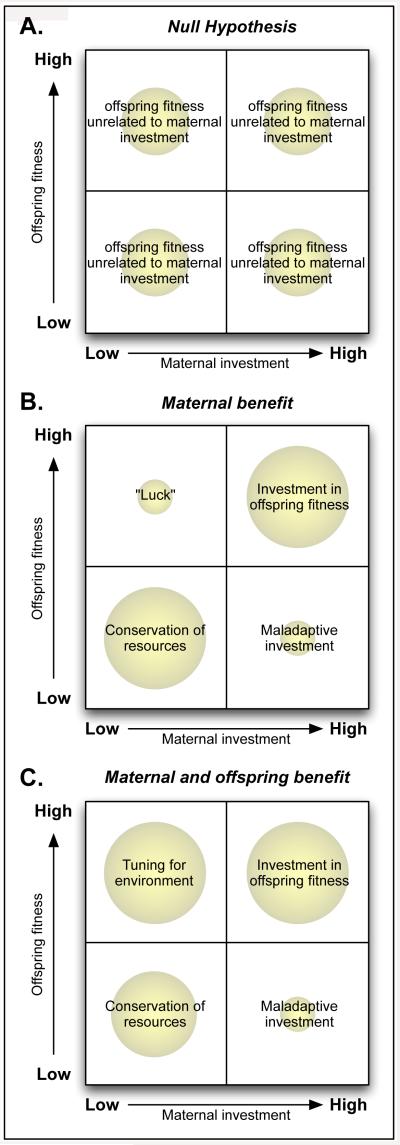
By considering only the extremes of offspring fitness and maternal behavior, we have constructed possible scenarios for each of three hypotheses described below: no fitness benefits of variation in maternal care, fitness benefits to the mother alone, and fitness benefits for the offspring (and indirectly the mother) (figure 2). The frequency of individual offspring representing each combination of maternal care and fitness (indicated by the size of the circles in figure 2) may then inform our understanding of the past fitness of behavior in different scenarios and support different hypotheses (adapted from Reeve and Sherman, 1993).
Figure 2.
Schema of maternal strategy, offspring fitness, and expected phenotype abundance. If variation in maternal care is not adaptive, one would offspring fitness to be unrelated to maternal investment (panel A). To the extent that maternal behavior is adaptive, high maternal investment in offspring should be associated with an increase in offspring fitness to offset time and energy costs (panel B, C upper right); higher investment associated with lower offspring fitness (lower right) does not represent an optimal reproductive strategy. Low maternal investment under conditions of relative environmental adversity may be advantageous despite the potential for low fitness of the offspring (panel B, lower left) because the mother may increase her odds of survival to future reproduction. In contrast, if offspring of females providing low levels of maternal care are routinely of high fitness for their local environment, this may represent tuning (panel C, upper left).
3.2.1 Null hypothesis: no fitness benefits of variation
The first (null) hypothesis is that there are no detectable fitness benefits from variation in maternal care: variation appears to benefit neither mother nor offspring, in which case it is not, per se, an adaptation. Evolution does not produce optimal phenotypes: non-adaptive traits exist, and maladaptive traits can persist at least temporarily through genetic or developmental linkage with other traits that confer advantages (e.g. Gould and Lewontin, 1979). At its extremes, the null hypothesis is obviously false: without maternal care, mammalian offspring do not survive. Conversely, with total time investment in maternal care, mothers would not meet their own basic needs for nutrition. It is more plausible that minor variations in maternal care (for example, of the order seen in laboratory rats) are without adaptive significance for mother or offspring despite variation in offspring phenotype.
If the null hypothesis is correct, maternal investment should be unrelated to offspring fitness and it would be as likely to be a high fitness offspring of a low LG mother as of a high LG mother. In this scenario, offspring of high and low fitness would result at roughly equal frequency from high and low maternal care (figure 2A).
3.2.2 Maternal investment hypothesis: low maternal care benefits the mother but not offspring
Because care of young takes time and energy, it may be of benefit to the mother to minimize offspring care under circumstances in which such care jeopardizes the mother’s chance of survival to reproduce again, especially if offspring are unlikely to survive. Current and future reproduction can be in conflict with one another (Trivers 1972; Trivers 1974), and many species abandon offspring in select circumstances and/or limit reproduction to favorable conditions (e.g. Beery et al., 2007; Olsson, 1997; Winkler, 1991). Organisms may also increase investment when future reproduction is less certain (e.g. Heubel et al., 2008). Maternal behaviors in the rat appear to be responsive to environmental conditions; female rats exposed to prenatal stress exhibit reduced maternal behaviors such as offspring grooming (Champagne and Meaney, 2006), nest attendance, and arched-back nursing (Smith et al., 2004). One might reasonably hypothesize that limiting maternal care benefits the mother by conserving maternal resources, but that current young suffer from this limited investment with reduced fitness. This hypothesis is represented by figure 2B; when maternal investment is high, offspring fitness is assumed to be high. When maternal investment is low, offspring fitness would be low. High maternal investment would rarely be followed by low offspring fitness (maladaptive investment), and only occasionally would low maternal investment result in highly fit offspring (luck).
3.2.3 Tuning hypothesis: both high and low maternal care increase offspring fitness in different circumstances
Variation in maternal care may be adaptive for the offspring in addition to the mother if resulting offspring characteristics are particularly well adapted for the sort of environment in which they will exist. For instance, if the mother spends extensive time foraging for food and provides less maternal care as a result, it may indicate an environment in which greater vigilance and effort will be required by the developing offspring. A key distinguishing characteristic of this hypothesis is that the reproductive fitness of offspring receiving low and high levels of maternal care should differ in different environmental circumstances or contexts, and offspring receiving relatively low maternal care should be relatively more fit in the environments that favor low maternal care (figure 2C “tuning” circle, upper left).
4. Current evidence in the rat maternal behavior paradigm
The “tuning” hypothesis—that both high and low levels of maternal care prepare offspring for their anticipated environment (figure 2C)—is often suggested, but experimental evidence supporting this proposal is scarce, both in the realm of offspring stress-reactivity, and other phenotypic outcomes of early-life maternal care received. Preliminary evidence for this hypothesis in rats has come from a few circumstances under which low LG offspring appear to have more adaptive traits (described below), but the best evidence for the this hypothesis would be identification of contexts that influence whether low or high LG offspring have better outcomes (and greater fitness).
4.1 Stress reactivity and tuning in laboratory studies
Stress-related behaviors known to associate with low maternal LG include decreased exploration in behavioral testing arenas (Caldji et al., 1998; Francis et al., 1999), increased startle responses and fear-induced defensive behaviors (Menard et al., 2004), and enhanced fear learning (Champagne et al., 2008). If fearful behavior is uniformly detrimental to fitness, this would support the hypothesis that low levels of maternal grooming produce poorer outcomes for offspring (“maternal investment hypothesis” section 3.2.2). Conversely, these traits might be adaptive in less rich environments that may lead to decreased maternal care (“tuning hypothesis” section 3.2.3)—an argument that parallels the hypothesis that increased reactivity to angry faces in children subjected to abuse may be useful in their future environments. Distinguishing evidence for the tuning hypothesis depends on identification of fitness benefits of being low LG offspring, and of variable fitness in different environments. So far this has been demonstrated and reported in select circumstances: offspring of low and high LG rat mothers showed enhanced learning under high and low stress conditions respectively (Champagne et al., 2008). Barha et al. (2007) found that female offspring of low LG rat dams had superior working memory compared to female high LG offspring (and males from high or low LG) even in conditions of no stress, however female low LG offspring also had superior reference memory following a stressor so this evidence suggests a benefit to low LG offspring but not tuning for a specific environment.
4.2 Non-stress outcomes of maternal care for offspring
Variations in rat maternal care were initially related to consequences for the developing HPA axis, but perturbation of the stress response system is not the sole physiological target. For example, females born to high LG dams display higher levels of maternal behavior, allowing for the intergenerational transmission of maternal behavior and stress-reactivity phenotypes (Francis et al. 1999). This behavioral change is accompanied by variation in the density and gene expression of several receptors in the brain (Caldji et al. 1998; Caldji et al. 2003; Champagne et al. 2003a; Champagne et al. 2004; Champagne et al. 2008; Francis et al. 2002a). Additional recent studies have documented effects of natural variation in maternal care on cognition (Liu et al., 2000), feeding behavior (Hancock et al., 2005), play behavior and aggression (Parent and Meaney, 2008), spatial memory (Barha et al., 2007), pain sensitivity (Walker et al., 2008), and sexual behavior (Cameron et al., 2008a,b; Sakhai et al., 2008).
The impact of maternal care on sexual behavior is of particular interest because of its direct relevance to reproductive fitness. Dams distinguish pup sex based on hormonal cues (Moore, 1982) and lick the anogenital region of male pups more frequently than that of female pups (Moore and Morelli, 1979); this process aids in the sexual differentiation of the nervous system and development of male sexual behavior (Kurian et al. 2010; Lenz and Sengelaub 2009; McCarthy et al. 1997; Moore, 1992). While anogenital licking is particularly directed at males and has important repercussions for their reproductive competence (Moore, 1992), female offspring of high LG dams also experience higher LG relative to female offspring of low LG dams (Champagne et al., 2003b). Females offspring exposed to high levels of simulated grooming also demonstrate masculinized profiles of ER-alpha promoter methylation and gene expression (Kurian et al., 2010).
Females born to high LG mothers display less sexually receptive behavior, receive fewer ejaculations, release fewer oocytes when ovulating, and are less likely to become pregnant (Cameron et al. 2008a; Sakhai et al., 2008; Uriarte et al., 2007). They may go through puberty later, and cross-fostering demonstrates these differences are at least in part due to variation in post-natal care (Cameron et al., 2008b). A study performed under semi-natural conditions at UC Berkeley’s Field Station for the Study of Behavior, Ecology, and Reproduction found similar results, with only females from the lowest quartile of maternal LG distribution (characterized under standard laboratory housing conditions) successfully rebreeding at the field station (Margerum and Francis, 2006). These findings could be interpreted as a general fitness benefit of low LG, or of a benefit under specific environmental contexts. For example, adverse environmental conditions can speed sexual maturity and promote earlier reproduction (reviewed in Meaney, 2007).
An important caveat to consider in evaluating the impact of maternal grooming of offspring is that costs and benefits may differ by sex. From a translational perspective, rat maternal licking has often been considered a proxy for general care-giving. However, from the example described above, it may be just as appropriate to view high LG as a specific mechanism of masculinizing male offspring not extensible to species that don’t groom their young.
The current body of evidence strongly suggests that maternal care impacts offspring characteristics with some gender specificity. Male offspring of high LG dams demonstrate enhanced exploration, and female offspring of low LG mothers experience increased reproductive fitness under some circumstances. Only one study demonstrates presumed fitness benefits of both high and low LG under different environmental circumstances within the same sex (Champagne et al., 2008); a challenge for future research on the adaptive significance of maternal care will be to identify contexts in which high and low LG offspring exhibit fitness differences and to observe whether or not both upbringings are adaptive in specific circumstances.
Nearly all studies which explore the effects of natural variation in maternal care on stress reactivity have been conducted solely on male offspring (Barha et al. 2007, Francis 1999a, and reproductive studies are exceptions); lack of consideration of females has serious repercussions for our understanding of biological mechanisms (Zucker and Beery, 2010, Beery and Zucker 2010), but inclusion of females will be particularly important for the interpretation of rat maternal behavior. It could potentially be the case that high LG males are typically of high fitness while low LG females are typically of higher fitness. Ultimately we may replace our understanding of high and low quality maternal care with a more nuanced view of how maternal care alters multiple phenotypic attributes including body weight, sexual behavior, stress reactivity, and parenting style. Expansion of the known set of offspring characteristics for each sex will enhance our understanding of how these outcomes may relate to one another and whether their functional consequences for offspring are adaptive.
4.3 Developmental Programming of Biological Sensitivity to Context; Inflammation as a biomarker
Very recent work from our laboratory (Saxton et al. in prep) has begun to explore the effects of early life experience and later social position on levels of inflammatory markers (e.g. IL-6) in both rats and humans (inflammatory cytokine levels predict a wide range of disease outcomes). Human participants reported family homeownership during their childhood and current social status. In rats, quality of maternal care received was recorded along with a later measure of social status (rats were housed in groups of four; social status was assessed via competition for resources). In rats and humans plasma IL-6 was assessed in adulthood. In both humans and rats, we identified an interaction effect; early social experience moderated the effect of adult social status on IL-6 production. Rats and humans who experienced low levels of maternal care (rats) or low childhood socioeconomic status (humans) represented both the highest and lowest levels of IL-6 in adulthood, depending on their social status as young adults. Adversity early in life may not have a monotonically negative effect on adult health, but may alter biological sensitivity to later social experiences. These new data support the Boyce and Ellis theory that early life experiences can prime biological reactivity to later environments.
5. Conclusion and future directions
The study of human maternal care in the post-Bowlby era of attachment theory began with the acknowledgement that rather than a single ‘best’ attachment strategy for infants in all environments, some flexibility allows them to respond to particular social and environmental conditions they face (Hrdy, 1999). So too, in the rodent literature, is the understanding shifting from the idea that high LG offspring are “best off” to the idea that differences between high and low LG offspring may be part of strategies for success in different environments. The framework of adaptation will be key to understanding the significance of different developmental trajectories for offspring.
Organisms come with genetic background, developmental history (including maternal care experienced), social status, age, sex, and reproductive status, and they operate in a particular physical environment, just to name a few variables. We have outlined specific adaptive hypotheses concerning the significance of variation in rat maternal care behavior for the mother and her offspring; tests of the fitness of the outcomes of maternal care will necessarily require attention to both the ontogeny of organisms and the physical and social environments in which these outcomes are evaluated. Evaluation of the tuning hypothesis for naturally occurring variation in maternal care in rats (section 3.2.3) will specifically require variation of experimental circumstances and the demonstration that low and high LG offspring having greater fitness in these different contexts. Providing evidence of the latter is a key challenge for researchers proposing that maternal behavior is a means of preparing offspring for different environments in any species.
Thus far, research on natural variation in maternal care in rats has incorporated a few contexts including standard laboratory housing versus enriched housing (Bredy et al. 2003; Bredy et al. 2004; Champagne and Meaney, 2007; Francis et al., 2002b), and conditions of high versus low stress or stress hormone exposure (e.g. Bagot et al., 2009; Barha et al., 2007; Champagne et al., 2008). From human population level data we know that relative social status is the most powerful known risk factor for mental and physical health outcomes. To begin to address this potent risk factor our laboratory is currently exploring the impacts of relative social status on the behavior and physiology of developing laboratory rats. Additional comparisons of male and female offspring will be key for distinguishing whether maternal grooming is important in a male-specific manner for masculinization of the nervous system. Many laboratory phenomena discovered in rodents have either not been validated in field settings or are not present in the field (Wolff, 2003); we should be cautious in our extensions of laboratory findings outside of their initial contexts, but we should also attempt to bring additional context into the laboratory. While many adaptations will be species-specific, looking for common mechanisms across species will also aid in the identification of robust mechanisms and ones we are interested in for human health (Pryce, 2008).
Adaptation is a concept that transcends species, and may be a heuristic for translational research to investigators across multiple disciplines. However, from an evolutionary perspective, fitness and adaptedness are assessed in terms of reproductive success, with the best assessment being lifetime reproductive fitness of a trait in its natural setting. This standard is difficult even for field biologists to achieve, and not easy to apply to laboratory rodents or humans. One strategy may be to replace reproductive fitness with intermediate fitness endpoints such as changes in cognition, behavior, and health. This would also make findings more relevant to human researchers who may be more concerned with the impacts of early upbringing and rearing on an individual’s quality of life or longevity and less concerned with the quantity of offspring reared and whether or not they reproduce.
While attempts to translate the significance of maternal care in the rat along with some of the basic biological mechanisms have given us substantial insights into human development, we wish to underscore the ease of overinterpretation and misinterpretation of findings from basic rodent models. In particular, it becomes difficult to ensure that findings are interpreted appropriately when there is broad appeal for a paradigm. Early rodent work focusing on how early life experiences influence emerging phenotypes in developing offspring had a discrete academic appeal in disciplines such as neuroendocrinology, behavioral neuroscience, and developmental psychobiology. These related fields share an interest in the study of individual differences in behavior and work at similar levels of analyses, making interpretation and extension of research findings between these areas relatively straightforward. More recent work focused on exploring the molecular and epigenetic processes that mediate the effects of maternal care on offspring has attracted a significantly broader audience. This is accompanied by an increased risk that interpretation and application of the data may occur without full understanding of the context and limitations of the primary data.
Translation may ultimately be best accomplished by inspiration: many fundamental biological mechanisms are shared between species, but the contexts that induce these mechanisms and the relative advantages of their outcomes may differ. A universal message is that early-life experience of maternal care profoundly affects biology and subsequent behavior in mammals, and that environmental context is key to determination of the adaptive benefits of variation in traits.
Acknowledgements
We are grateful to Kaja LeWinn for feedback on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joëls M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Baldwin JM. A new factor in evolution. American naturalist. 1896;30:441–451. [Google Scholar]
- Barha CK, Pawluski JL, Galea LA. Maternal care affects male and female offspring working memory and stress reactivity. Physiol. Behav. 2007;92:939–50. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Beery AK, Trumbull JJ, Tsao JM, Costantini RM, Zucker I. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc. R. Soc. Lond. B. Biol. Sci. 2007;274:281–6. doi: 10.1098/rspb.2006.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex Bias in Neuroscience and Biomedical Research. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. Am Psychol. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat. Genet. 2003;33(Suppl):228–37. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]; Bowlby J. Maternal care and mental health. Bull World Health Organ. 1951;3:355–533. [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–6. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur. J. Neurosci. 2004;20:1355–62. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–9. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm. Behav. 2008a;54:178–84. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE. 2008b;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003a;144:4720–4. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 2003b;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 2004;24:4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol. Psychiatry. 2006;59:1227–35. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 2007;121:1353–63. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joëls M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28:6037–45. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Smith EL, Altemus M, Mathew SJ, Perera T, Kral JG, Gorman JM, Owens MJ, Nemeroff CB, Rosenblum LA. Maternal-infant response to variable foraging demand in nonhuman primates: effects of timing of stressor on cerebrospinal fluid corticotropin-releasing factor and circulating glucocorticoid concentrations. Ann. N.Y. Acad. Sci. 2006;1071:525–533. doi: 10.1196/annals.1364.057. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, Stephens MW. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 1962;33:65–71. doi: 10.1111/j.1467-8624.1962.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Whimbey AE. Behavior of Adult Rats Is Modified by the Experiences Their Mothers Had as Infants. Science. 1963;142:1192–1193. doi: 10.1126/science.142.3596.1192. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17:303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol. Biochem. Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J. Neuroendocrinol. 2002a;14:349–53. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002b;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD. Conceptualizing child health disparities: a role for developmental neurogenomics. Pediatrics. 2009;124(Suppl 3):S196–202. doi: 10.1542/peds.2009-1100G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusi S, Drew PJ, Abbott LF. Cascade models of synaptically stored memories. Neuron. 2005;45:599–611. doi: 10.1016/j.neuron.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc. Cogn. Affect. Neurosci. 2008;3:91–6. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1979;205:581–98. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Hancock SD, Menard JL, Olmstead MC. Variations in maternal care influence vulnerability to stress-induced binge eating in female rats. Physiol. Behav. 2005;85:430–9. doi: 10.1016/j.physbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. Agemate or peer affectional system. In: Lehrman DS, Hinde RA, Shaw E, editors. Advances in the Study of Behavior. Academic press; New York: 1969. [Google Scholar]
- Hershko AY, Kafri T, Fainsod A, Razin A. Methylation of HoxA5 and HoxB5 and its relevance to expression during mouse development. Gene. 2003;302:65–72. doi: 10.1016/s0378111902010910. [DOI] [PubMed] [Google Scholar]
- Heubel KU, Lindström K, Kokko H. Females increase current reproductive effort when future access to males is uncertain. Biol. Lett. 2008;4:224–7. doi: 10.1098/rsbl.2007.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy SB. Mother nature: a history of mothers, infants, and natural selection. Pantheon Books; New York: 1999. [DOI] [PubMed] [Google Scholar]
- Hutt SJ. Biological Aspects of Early Development. Paedagogica Europaea. 1974;9:18–32. [Google Scholar]
- Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann N Y Acad Sci. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ. Evolution in four dimensions: genetic, epigenetic, behavioral, and symbolic variation in the history of life. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009;84:131–76. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey EP. What is an adaptive environmentally induced parental effect? In: Mousseau TA, Fox CW, editors. Maternal effects as adaptations. Oxford University Press; New York: 1998. pp. 54–66. [Google Scholar]
- Laurent L, Wong E, Li G, Huynh T, Tsirigos A, Ong CT, Low HM, Kin Sung KW, Rigoutsos I, Loring J. Dynamic changes in the human methylome during differentiation. Genome Research. 2010;20:320–331. doi: 10.1101/gr.101907.109. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal care effects on SNB motoneuron development: the mediating role of sensory afferent distribution and activity. Dev. Neurobiol. 2009;69:603–15. doi: 10.1002/dneu.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Morton A, Lewis GW. Infantile Experience and the Maturation of the Pituitary Adrenal Axis. Science. 1957;126:1347. doi: 10.1126/science.126.3287.1347. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-Free Corticosteroid Response to Electric Shock in Rats Stimulated in Infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nature Neuroscience. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews. 2010;34:867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front. Behav. Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Wurbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Margerum LA, Francis DD. Are naturally-occuring differences in maternal licking and grooming profiles consistent across different environmental conditions? Soc. Neurosci. Abstr. Online. 2006 Program No. 562.2/GG4. [Google Scholar]
- McCarthy MM, Besmer HR, Jacobs SC, Keidan GM, Gibbs RB. Influence of maternal grooming, sex and age on Fos immunoreactivity in the preoptic area of neonatal rats: implications for sexual differentiation. Dev. Neurosci. 1997;19:488–96. doi: 10.1159/000111246. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N.Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Environmental programming of phenotypic diversity in female reproductive strategies. Adv. Genet. 2007;59:173–215. doi: 10.1016/S0065-2660(07)59007-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene×environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Menard JL, Champagne DL, Meaney MJ. Variations of maternal care differentially influence ‘fear’ reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–69. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J. Comp. Physiol. Psychol. 1979;93:677–84. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal behavior of rats is affected by hormonal condition of pups. J. Comp. Physiol. Psychol. 1982;96:123–9. doi: 10.1037/h0077866. [DOI] [PubMed] [Google Scholar]
- Moore CL. The role of maternal stimulation in the development of sexual behavior and its neural basis. Ann. N. Y. Acad. Sci. 1992;662:160–77. doi: 10.1111/j.1749-6632.1992.tb22859.x. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. Maternal effects as adaptations. Oxford University Press; New York: 1998. [Google Scholar]
- Nelson EE, Herman KN, Barrett CE, Noble PL, Wojteczko K, Chisholm K, Delaney D, Ernst M, Fox NA, Suomi SJ, Winslow JT, Pine DS. Adverse rearing experiences enhance responding to both aversive and rewarding stimuli in juvenile rhesus monkeys. Biol. Psychiatry. 2009;66:702–4. doi: 10.1016/j.biopsych.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological Sensitivity to Context: The Interactive Effects of Stress Reactivity and Family Adversity on Socioemotional Behavior and School Readiness. Child Dev. 2010;81:270–89. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendo. 2005;30(10):953–8. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Olsson O. Clutch abandonment: a state-dependent decision in king penguins. Journal of Avian Biology. 1997;28:264–7. [Google Scholar]
- Parent CI, Meaney MJ. The influence of natural variations in maternal care on play fighting in the rat. Dev. Psychobiol. 2008;50:767–76. doi: 10.1002/dev.20342. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biological Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. Genotype-phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:557–66. doi: 10.1098/rstb.2009.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 2000;36:679–88. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9072–6. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res. Brain Res. Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rando OJ, Verstrepen KJ. Timescales of genetic and epigenetic inheritance. Cell. 2007;128:655–68. doi: 10.1016/j.cell.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Reeve HK, Sherman PW. Adaptation and the goals of evolutionary research. The Quarterly Review of Biology. 1993;68:1–32. [Google Scholar]
- Sakhai S, Kriegsfeld LJ, Francis DD. Are naturally-occuring differences in maternal licking and grooming profiles consistent across different environmental conditions? Soc. Neurosci. Abstr. Online. 2008 Program No. 866.19/NN15. [Google Scholar]
- Sapolsky RM. Why zebras don’t get ulcers. Holt Paperbacks; New York: 2004. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–292. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Shastry BS. SNP alleles in human disease and evolution. J. Hum. Genet. 2002;47:561–6. doi: 10.1007/s100380200086. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–44. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Mother-infant interaction and the modulation of pituitary-adrenal activity in rat pups after early stimulation. Dev. Psychobiol. 1983;16:169–176. doi: 10.1002/dev.420160303. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:114–27. doi: 10.1097/CHI.0b013e318193064c. [DOI] [PubMed] [Google Scholar]
- Suomi S. Early stress and adult emotional reactivity in rhesus monkeys; Ciba Found. Symp.; 1991; pp. 171–183. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann. N. Y. Acad. Sci. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Animal Biology-Leiden. 1963;55:297–322. reprinted 2005. [Google Scholar]
- Uriarte N, Breigeiron MK, Benetti F, Rosa XF, Lucion AB. Effects of maternal care on the development, emotionality, and reproductive functions in male and female rats. Dev. Psychobiol. 2007;49:451–62. doi: 10.1002/dev.20241. [DOI] [PubMed] [Google Scholar]
- Van Der Horst F, Van Der Veer R, Van Ijzendoorn M. John Bowlby and ethology: An annotated interview with Robert Hinde. Attachment & Human Development. 2007;9:321–335. doi: 10.1080/14616730601149809. [DOI] [PubMed] [Google Scholar]
- Velderman MK, Bakermans-Kranenburg MJ, Juffer F, van IMH. Effects of attachment-based interventions on maternal sensitivity and infant attachment: differential susceptibility of highly reactive infants. J Fam Psychol. 2006;20:266–274. doi: 10.1037/0893-3200.20.2.266. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The basic ideas of biology. In: Waddington CH, editor. Towards a theoretical biology. Edinburgh Univ. Press; Edinburgh: 1968. pp. 1–31. [Google Scholar]
- Walker CD, Xu Z, Rochford J, Johnston CC. Naturally occurring variations in maternal care modulate the effects of repeated neonatal pain on behavioral sensitivity to thermal pain in the adult offspring. Pain. 2008;140:167–76. doi: 10.1016/j.pain.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Watters E. DNA is not destiny. Discover Magazine. 2006;11 2006. [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004a;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann. N.Y. Acad. Sci. 2004b;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Winkler DW. Parental investment decision rules in tree swallows: parental defense, abandonment, and the so-called Concorde Fallacy. Behavioral Ecology. 1991;2:133. [Google Scholar]
- Wolff JO. Laboratory studies with rodents: Facts or artifacts? Bioscience. 2003;53:421–7. [Google Scholar]
- Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]