Abstract
We report that the disruption of bidirectional signaling between ephrin-B2 and EphB receptors impairs morphogenetic cell-cell septation and closure events during development of the embryonic midline. A novel role for reverse signaling is identified in tracheoesophageal foregut septation, as animals lacking the cytoplasmic domain of ephrin-B2 present with laryngotracheoesophageal cleft (LTEC), while both EphB2/EphB3 forward signaling and ephrin-B2 reverse signaling are shown to be required for midline fusion of the palate. In a third midline event, EphB2/EphB3 are shown to mediate ventral abdominal wall closure by acting principally as ligands to stimulate ephrin-B reverse signaling. Analysis of new ephrin-B26YFΔV and ephrin-B2ΔV mutants that specifically ablate ephrin-B2 tyrosine phosphorylation- and/or PDZ domain-mediated signaling indicate there are at least two distinct phosphorylation-independent components of reverse signaling. These involve both PDZ domain interactions and a non-canonical SH2/PDZ-independent form of reverse signaling that may utilize associations with claudin family tetraspan molecules, as EphB2 and activated ephrin-B2 molecules are specifically co-localized with claudins in epithelia at the point of septation. Finally, the developmental phenotypes described here mirror common human midline birth defects found with the VACTERL association, suggesting a molecular link to bidirectional signaling through B-subclass Ephs and ephrins.
Keywords: ephrin-B2, EphB2, receptor tyrosine kinase, bidirectional signaling, claudin, VACTERL, tracheoesophageal septation, cleft palate, omphalocele
INTRODUCTION
VACTERL is used to denote a non-random association of human embryonic malformations. The defining features of VACTERL include vertebral defects (V), anorectal malformations (A), cardiac anomalies (C), tracheoesophageal septation defects (TE), renal dysplasia (R), and limb abnormalities (L) (Temtamy and Miller, 1974). Cases of VACTERL are defined as presenting with two or more of these anomalies, and most of the 1 in 5,000 live births associated with VACTERL have only diads or triads (Rittler et al., 1996). Additional congenital anomalies are also observed in VACTERL patients outside of the defining features, including hypospadias, cleft palate, neural tube defects, and omphalocele (Botto et al., 1997; Rittler et al., 1996). Evidence has built for a genetic basis behind VACTERL, however a molecular understanding of the association remains rudimentary (Aynaci et al., 1996; Nezarati and McLeod, 1999). At present, the only VACTERL association animal models are mice impaired in Shh signaling (Shh, Gli deficient animals), knockouts for Foxf1, Noggin, or Sox2, or animals treated in utero with the anthracycline antibiotic, adriamycin (Kim et al., 2001; Shaw-Smith, 2009).
The Eph receptor family represents the largest collection of transmembrane receptor tyrosine kinases, while the ephrins are their membrane-bound ligands. A-subclass ephrins are attached to the outer leaflet of the plasma membrane by a GPI-linkage, while B-subclass ephrins possess a single-pass transmembrane domain and a short cytoplasmic tail. In general, EphA receptors can promiscuously bind to A-subclass ephrins and EphB receptors can similarly bind to B-subclass ephrins, although subclass crosstalk has been documented (Bergemann et al., 1998; Davis et al., 1994; Himanen et al., 2004). As both Ephs and ephrins are membrane anchored, signaling by these molecules occurs at sites of cell-cell contact. Signal transduction between Ephs and ephrins is atypical from other receptor/ligand pairs in that the potential exists for bidirectional signaling into both the Eph and ephrin expressing cells (Henkemeyer et al., 1996; Holland et al., 1996). Signaling into cells expressing Eph receptors (forward signaling) occurs principally through activation of its intracellular tyrosine kinase catalytic domain which leads to autophosphorylation, coupling to Src homology 2 (SH2) domain proteins, and phosphorylation of downstream substrates (Kullander and Klein, 2002). Eph receptors can also form other protein-protein associations, notably through their sterile-alpha (SAM) and C-terminal PDZ-binding motifs. Conversely, signaling into cells expressing B-subclass ephrins (reverse signaling) also leads to the phosphorylation of conserved tyrosine residues on the cytoplasmic tail of the ephrin-B molecule and the subsequent docking to SH2 adaptor proteins such as Grb4/Nck-β (Cowan and Henkemeyer, 2001; Xu and Henkemeyer, 2009). B-subclass ephrins also contain a C-terminal PDZ-binding motif. The bidirectional nature of Eph/ephrin signaling complicates studies of these molecules. Therefore, to precisely elucidate the physiological role of a given Eph or ephrin it is necessary to dissect both the forward and reverse signaling contributions of that particular molecule.
Beyond their potential for bidirectional signaling, the Ephs and ephrins are further unique with respect to the targets of their signal transduction. Whereas most tyrosine kinase-mediated signaling events ultimately target the nucleus to regulate transcription, the Ephs and ephrins appear to principally exert control over cytoskeletal dynamics. This control is achieved through the activation of secondary molecules linked to cytoskeletal control, many of which ultimately regulate small Rho family GTPases (Egea and Klein, 2007; Noren and Pasquale, 2004). Given this ability to fundamentally alter the structure of the cell in response to cell-cell contact, it is not surprising that Ephs and ephrins are utilized as key regulators of cell migration/differentiation events throughout development, as typified by their well-defined physiological roles in axon pathfinding (Flanagan and Vanderhaeghen, 1998; Wilkinson, 2001), angiogenic remodeling (Cowan et al., 2004; Wang et al., 1998), and neural crest cell migration (Davy et al., 2004; Smith et al., 1997).
While the majority of studies implicate Eph-ephrin signaling in cell migration and axon pathfinding by inducing repulsion, there is some evidence to suggest these molecules may also function in the opposite manner to induce cell adhesion responses. This evidence includes the characterization of apparent cell adhesion defects in the palate, urethra, cloaca, and neural tube in Eph/ephrin mutant animals (Dravis et al., 2004; Holmberg et al., 2000; Orioli et al., 1996), Eph-ephrin mediated axon guidance events that appear to utilize attraction outcomes following axon-cell contact instead of the canonical repulsion seen most often with the Ephs and ephrins (Eberhart et al., 2004; Hindges et al., 2002), and cell-based studies indicating positive outgrowth of cytoskeletal structures in response to Eph-ephrin signaling activation instead of the more typical cytoskeletal collapse associated with these molecules (Hansen et al., 2004; Huynh-Do et al., 1999; Stein et al., 1998). Here we focus on describing new roles for these molecules that also appear consistent with cell-cell adhesion responses.
Using a variety of EphB/ephrin-B mutant alleles, we identify requirements for ephrin-B2 and EphB2 in septation and closure events at the embryonic midline in the developing foregut, palate, and ventral body wall. The LTEC, cleft palate, and omphalocele phenotypes mimic VACTERL association birth defects. Combined with previous studies reporting the involvement of the Ephs and ephrins in other VACTERL-like developmental malformations (Compagni et al., 2003; Cowan et al., 2004; Davy and Soriano, 2007; Dravis et al., 2004; Holmberg et al., 2000), we now find a near complete overlap between Eph/ephrin-associated developmental defects and the malformations of VACTERL. This leads us to propose EphB/ephrin-B signaling as a molecular basis for VACTERL association and to highlight the use of EphB/ephrin-B deficient mice for VACTERL research.
RESULTS
To characterize roles for ephrin-B2 and EphB2 in midline development, we utilized 1) ephrin-B2lacZ mice, in which the cytoplasmic domain of ephrin-B2 has been replaced by β-galactosidase (β-gal) to abolish reverse signaling while leaving intact its ligand-like ability to bind Eph receptors and activate forward signaling in the same fashion as native ephrin-B2 (Supplemental Figure 1), and 2) EphB2lacZ mice, in which the majority of the EphB2 cytoplasmic domain has been replaced by β-gal to block forward signaling by this molecule while leaving intact its ligand-like ability to bind ephrins and stimulate reverse signaling (Dravis et al., 2004; Henkemeyer et al., 1996). The ephrin-B2lacZ allele has been used successfully in the past to identify a distinct ligand-only role for ephrin-B2 in angiogenesis (Cowan et al., 2004) and axon guidance (Williams et al., 2003), as well as clear receptor roles in urorectal development (Dravis et al., 2004), anterior commissure axon guidance and cardiac development (Cowan et al., 2004), and inner ear fluid dynamics (Dravis et al., 2007). The ephrin-B2lacZ allele, which is homozygous viable throughout embryonic development, has proven to be very useful for understanding the functions of ephrin-B2 reverse signaling, given that ephrin-B2−/− null mutants are early embryonic lethal due to vascular defects caused by the loss of EphB4 forward signaling that preclude analysis of later development (Wang et al., 1998). Likewise, the EphB2lacZ allele has been used to identify ligand-only roles for EphB2 in axon guidance (Birgbauer et al., 2000; Henkemeyer et al., 1996), and receptor roles for EphB2 in axon guidance (Cowan et al., 2000; Hindges et al., 2002), vestibular development (Cowan et al., 2000), and urorectal development (Dravis et al., 2004).
Ephrin-B2 reverse signaling mediates midline septation of the foregut
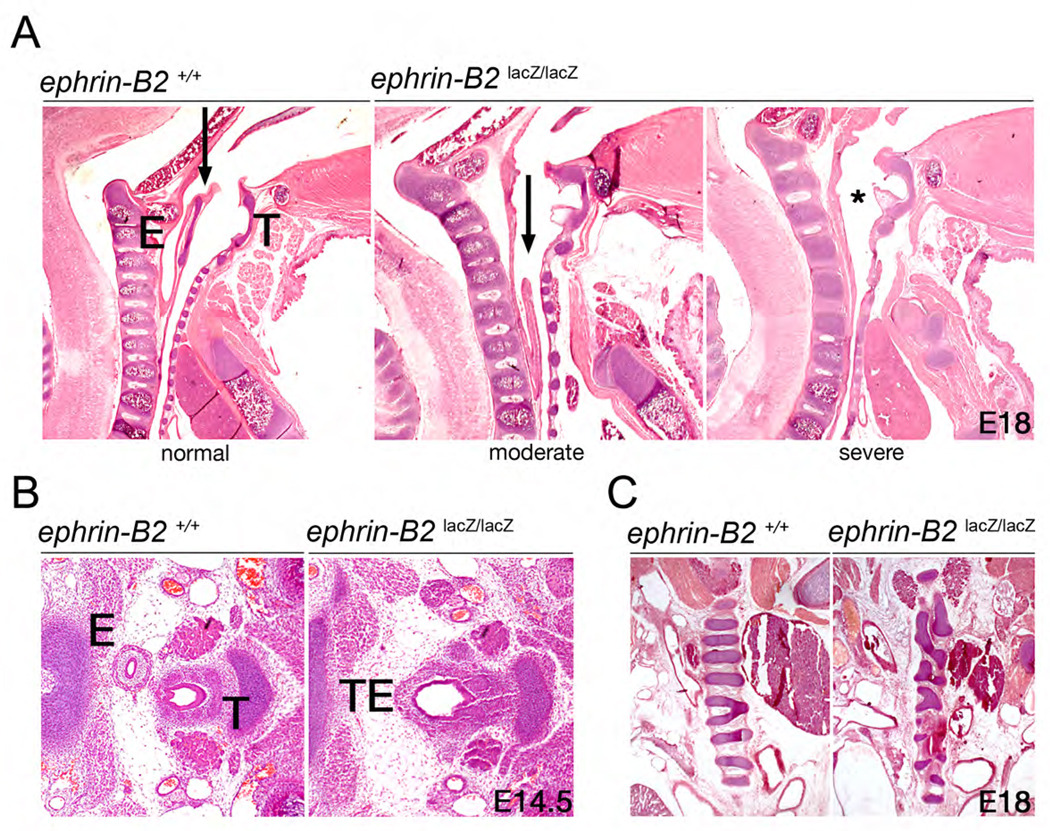
Initial studies of ephrin-B2lacZ mice indicated a loss of ephrin-B2 reverse signaling leads to neonatal lethality, with defects in cardiac development and midline adhesion of the urethra and anorectum (Cowan et al., 2004; Dravis et al., 2004). Because of the urorectal/hindgut defects, ephrin-B2lacZ embryos were also examined to determine if septation of the foregut was similarly affected. This revealed that 47% (n=15) of late stage embryonic day 18 (E18) ephrin-B2lacZ/lacZ mutants exhibited defects in tracheoesophageal (TE) septation as evidenced by common, unseptated foregut. The defects observed in the ephrin-B2lacZ/lacZ embryos appeared with a phenotypic range of moderate, in which a septum was visible but had failed to extend to the rostral apex, to severe, in which no septum was detected, and only unseptated foregut was present rostral to the bronchi (Fig 1A). Sections taken of earlier E14.5 embryos showed the same defect in foregut septation; distinct trachea and esophagus were visible in the wild-type (WT) embryo, while an ephrin-B2lacZ/lacZ littermate showed only unseptated foregut (Fig 1B). The failure of foregut septation in ephrin-B2lacZ/lacZ mutants results in morphological abnormalities in the unseptated trachea, as visualized by the appearance of disorganized cartilage rings in the mutants (Fig 1C).
Figure 1.
Failed midline septation of the foregut in ephrin-B2lacZ mutants. (A) H&E stained sagittal sections from E18 WT or ephrin-B2lacZ/lacZ littermates. In the WT (left), a septum is discernable (arrow), separating the esophagus (E) from the trachea (T). Defects in septation in the mutants were classified as: moderate, where a septum is visible but failed to fully extend rostrally (arrow, middle); or severe, where no septum formed (*, right). (B) H&E stained transverse sections from E14.5 WT and ephrin-B2lacZ/lacZ littermates. In the WT (left), septation occurred to produce distinct esophageal (E) and tracheal (T) endoderm. Equivalent section of a mutant (right) revealed an unseptated foregut (TE). (C) H&E stained coronal sections of E18 WT and ephrin-B2lacZ/lacZ littermate embryos. While a normal banding of the cartilage rings surrounding the trachea are observed in the WT (left), mutants with failed tracheoesophageal septation present with disorganized cartilage bands (right).
We next examined ephrin-B2lacZ embryos at earlier stages of development, and found that homozygotes collected at E10.5 also showed defective TE septation. While ephrin-B2lacZ/+ embryos appeared normal and showed a common rostral foregut that septated into separate tracheal and esophageal endoderm caudally (Fig 2A), ephrin-B2lacZ/lacZ littermates showed no signs of TE septation all the way down to the terminal caudal point of the foregut, despite otherwise normally patterned foregut endoderm (Fig 2B).
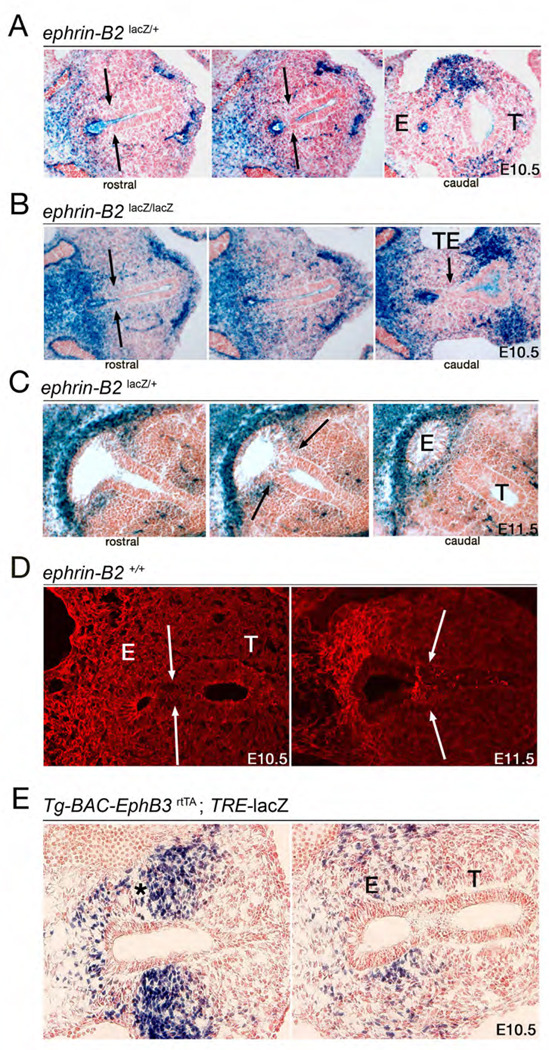
Figure 2.
Ephrin-B2 and EphB3 expression during foregut septation. (A, B) Serial X-gal stained transverse sections from E10.5 ephrin-B2lacZ/+ and ephrin-B2lacZ/lacZ littermates. Normal septation in the ephrin-B2lacZ/+ embryo proceeds from common foregut (left), to septating foregut (middle), to septated foregut showing separated esophagus (E) and trachea (T) (right). Septation of the foregut in the homozygous mutant failed to occur (left), caudal to which only unseptated foregut remains (middle). A fistula (TE) between esophagus and the emerging bronchi is present at the very caudal end of the foregut (right). X-gal stain shows expression of the ephrin-B2-β-gal fusion protein (blue) in the endoderm of the foregut at septation, with higher levels of expression detected at the future esophageal pole of the foregut. Ephrin-B2 is also found in the mesenchyme surrounding the future esophagus. (C) Serial X-gal stained sections from an E11.5 ephrin-B2lacZ/+ embryo. Similar to E10.5, ephrin-B2 is highly expressed in the mesenchyme and epithelia associated with future esophageal endoderm. Ephrin-B2 is also present on the endoderm and flanking mesenchyme at the point of septation (arrows). (D) Coronal sections capturing foregut septation from WT embryo stained at E10.5 and E11.5 with a pan-ephrin-B antibody. Ephrin-B is present in the mesenchyme associated with the future esophageal endoderm and in the endoderm of the foregut. (E) BluO-gal stained coronal sections from a Tg-BAC-EphB3-rtTA; TRE-lacZ embryo treated with dox to visualize EphB3 expression (blue). Like ephrin-B2, EphB3 is highly expressed in the mesenchyme flanking the future point of septation (*, left), where lateral folds will invaginate into the foregut and meet at the midline as seen in an adjacent caudal section (right).
The discovery of TE fistula in ephrin-B2lacZ mutants suggests that reverse signaling through the cytoplasmic domain of ephrin-B2 is necessary for proper septation of the foregut. However, the ephrin-B2lacZ allele is not able to answer which Eph receptors are involved in activating these signals. To address this, Eph receptor compound null animals were generated, as these genes can show redundant functions. However, analysis of EphB2;EphB3, EphB2;EphB3;EphA4, and EphB1;EphB2;EphB3;EphA4 compound knockouts did not reveal any defects in TE septation. This suggests at least one additional Eph receptor (i.e. EphB4 or EphB6) may be paired with ephrin-B2 in foregut septation.
X-gal stains on foregut sections from ephrin-B2lacZ embryos were carried out to document expression of the ephrin-B2-β-gal fusion protein, which provides a high signal-to-noise ratio reporter of protein expression (Fig 2). The data showed ephrin-B2 is expressed along the length of the foregut endoderm, and appears to be most highly expressed in the epithelia of the foregut destined to become esophagus as well as in the mesenchyme surrounding the esophagus. Ephrin-B2 is notably present at the site of TE septation in ephrin-B2lacZ/+ specimens or where TE septation should be occurring in ephrin-B2lacZ/lacZ mutants (Fig 2, Supplemental Fig 2A). Indirect immunofluorescence (IF) using a pan-ephrin-B antibody on WT embryos shows a similar expression profile as the X-gal stains for the ephrin-B2-β-gal fusion protein (Fig 2D).
While the Eph receptors involved in foregut septation are unknown, we reasoned that EphB3 was likely involved based on its role in cell adhesion events during hindgut septation (Dravis et al., 2004). Unfortunately, conventional methods to examine EphB3 expression by mRNA in situ hybridization or immunohistochemistry have not provided satisfactory results, so a BAC transgenic animal was generated that expresses the reverse tetracycline transactivator, rtTA2S-M2 (Urlinger et al., 2000), under control of EphB3 promoter sequences (Villasenor et al., in preparation). Tg-BAC-EphB3-rtTA transgenic mice were crossed to a TRE-lacZ reporter line (Ludwig et al., 2004) and dox-induced embryos hemizygous for both transgenes were collected and stained with BluO-gal to reveal β-gal expressing cells. This showed that EphB3 is expressed in the mesenchymal cells coming into contact with epithelial cells at the site of TE septation (Fig 2E). BluO-gal stains on foregut tissue from EphB2lacZ/+ animals indicated expression of EphB2 on epithelia at the point of septation (Supplemental Fig 2B). The expression data strongly indicates EphB2 and EphB3 likely participate in foregut septation.
EphB:ephrin-B bidirectional signaling in midline formation of the palate
EphB2;EphB3 compound null mice exhibit a cleft palate phenotype that is not observed in EphB2 or EphB3 single mutants (Orioli et al., 1996; Risley et al., 2009). To determine if EphB2 is acting as a receptor to transduce forward signals or as a ligand to activate reverse signals important in palate development, both EphB2− null and EphB2lacZ Cterminal truncation mutants were utilized. Histological analysis at E18.5 revealed that 41 % of EphB2lacZ/lacZ;EphB3−/− and 15% of EphB2−/−EphB3−/− compound mutants exhibited severe cleft palates (Table 1). Finding cleft palates with the EphB2lacZ allele indicates that EphB forward signaling is important in development of this midline structure. The increase in penetrance to 41% further suggests involvement of more than EphB2 and EphB3 in palate development, as the dominant negative effect seen here with the EphB2lacZ allele is best explained by the ability of the intracellular truncated EphB2-β-gal fusion protein to disrupt forward signaling of other co-expressed Eph receptors (Cowan et al., 2000; Hindges et al., 2002).
Table 1.
Incidence of cleft palate in EphB2;EphB3 compound mutants
| EphB2 mutation | background | B2/B2;B3/B3 | B2/+;B3/B3 | +/+;B3/B3 |
|---|---|---|---|---|
| EphB2− | CD1 | 27 (4) | 32 (1) | NA |
| EphB21acZ | CD1 | 29 (12) | 17 (2) | NA |
EphB2−/−;EphB3−/− or (EphB2lacZ/lacZ; EphB3−/−) males were intercrossed with EphB2−/+; EphB3−/− females (or EphB2lacZ/+;EphB3−/−) and offspring collected at E18.5. Animals with cleft palate are in parentheses.
To determine if ephrin-B2 is involved in palate formation, embryos carrying the ephrin-B2lacZ allele were examined. Histological analysis revealed that 26% of ephrin-B2lacZ/lacZ homozygotes and 7% of ephrin-B2lacZ/+ heterozygotes showed a severe cleft palate (Table 2, Fig 3A). This indicates that ephrin-B2 is involved in palate fusion and that reverse signaling through this molecule is important for this process, which is consistent with the conclusion from recent in vitro analyses of Eph-ephrin signaling in palatal shelf fusion (San Miguel et al., 2011). Coupled with the aforementioned EphB2;EphB3 results, the data indicate both forward signaling through EphB receptors and reverse signaling through ephrin-B2 are important for midline closure of the palate. This involvement of bidirectional signaling through both EphB and ephrin-B mirrors a similar requirement for both forward and reverse signaling in urorectal/hindgut midline development (Dravis et al., 2004).
Table 2.
Incidence of cleft palate in ephrin-B2lacZ mutants
| background | ephrin-B21acZ/lacZ | ephrin-B21acZ/+ | ephrin-B2+/+ |
|---|---|---|---|
| 129/CD1 | 35 (9) | 59 (4) | >40 (0) |
Ephrin-B2lacZ/+ heterozygotes were intercrossed and offspring collected at E18.5. Animals with cleft palate are in parentheses.
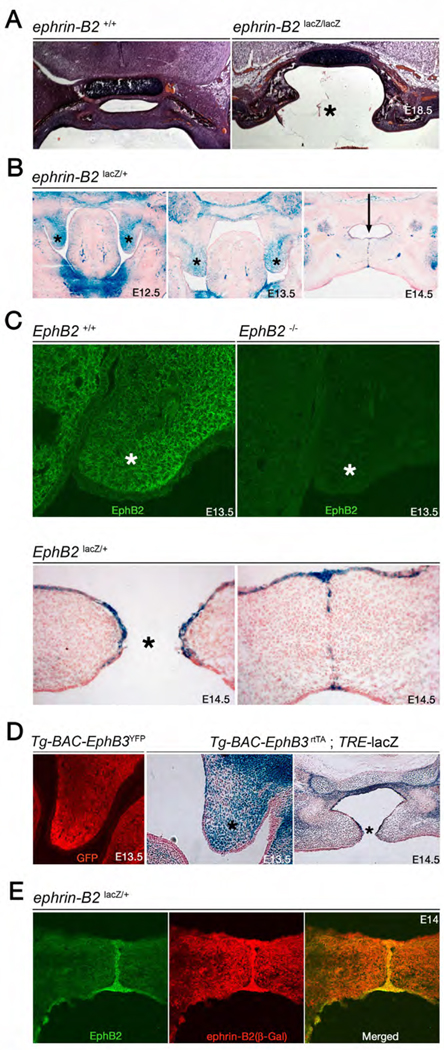
Figure 3.
Cleft palate in ephrin-B2lacZ mutants. (A) H&E stained coronal sections of WT and ephrin-B2lacZ/lacZ E18 littermates. The ephrin-B2lacZ homozygote presents with a cleft palate (*). (B) X-gal stained coronal sections from ephrin-B2lacZ/+ embryos at E12.5, E13.5, and E14.5 detect expression of the ephrin-B2-β-gal fusion protein (blue). Ephrin-B2 is expressed in the mesenchyme and leading epithelia of the palatal shelves at E12.5 and E13.5 (*, left and middle) before palatal shelf closure. After adhesion, ephrin-B2 expression is strongest in the midline epithelial seam (arrow, right). (C) Coronal sections at E13.5 from WT and EphB2−/− embryos treated with anti-EphB2 antibody (top). EphB2 is expressed in the mesenchyme of the palatal shelf in the WT (*, left), while no signal is detected in the mutant control. Coronal sections from an E14.5 EphB2lacZ/+ embryo stained with X-gal to show expression of the EphB2-β-gal fusion protein (bottom) reveal EphB2 becomes specifically expressed in the epithelia of the palatal shelves, both immediately preceding (left) and at the site of adhesion (right). (D) Coronal section from an E13.5 Tg-BAC-EphB3-YFP embryo treated with anti-GFP antibody shows specific expression of EphB3 in the mesenchyme of the palatal shelf (left). X-gal stained coronal sections from E13.5 and E14.5 Tg-BAC-EphB3-rtTA; TRE-lacZ embryos treated with dox show EphB3 is initially highly expressed in the mesenchyme of the palatal shelf at E13.5 (*, middle) but then becomes more preferentially expressed in the leading epithelia as midline adhesion becomes imminent (*, right). The expression of EphB3 is very similar to EphB2 and ephrin-B2. (E) Coronal section from an E14 ephrin-B2lacZ/+ embryo treated with anti-EphB2 (left) and anti-β-gal (middle) antibodies. The merged image (right) shows EphB2 and ephrin-B2-β-gal are co-expressed on the midline epithelial seam where adhesion has occurred.
The expression profiles of ephrin-B2 and EphB2 were examined during palatal development. X-gal stains of ephrin-B2lacZ/+ embryos revealed expression of ephrin-B2 throughout the mesenchyme of the palatal shelf, as well as in the leading epithelia before adhesion and fusion take place (Fig 3B). Later, when adhesion has taken place, ephrin-B2 is clearly visible on the multilayer epithelial seam, as well as in the mesenchyme flanking the midline site of adhesion. IF of palatal shelves with an antibody against EphB2 revealed EphB2 expression in the mesenchyme and leading epithelia of the palatal shelf at E13.5 (Fig 3C). No signal was detected in control EphB2−/− tissue (Fig 3C). X-gal stains of EphB2lacZ/+ animals further showed that as palatal shelf closure nears and as it occurs EphB2 expression becomes more restricted to the leading epithelia where adhesion takes place (Fig 3C).
To investigate EphB3 expression, a BAC transgenic line was generated that expresses eYFP under EphB3 promoter sequences, termed Tg-BAC-EphB3-YFP. IF for GFP to detect expression of eYFP showed that EphB3 is expressed at the leading mesenchyme of the palatal shelves (Fig 3D). EphB3 expression was further examined in X-gal stains of palatal shelves from dox treated embryos hemizygous for the Tg-BAC-EphB3-rtTA and TRE-lacZ transgenes. This also showed EphB3 expression in the mesenchyme of the palatal shelves at E13.5 and that this expression shifts from the mesenchyme to epithelia as palatal shelf closure nears at E14.5 (Fig 3D). To summarize this expression data, remarkably the profiles for EphB2, EphB3, and ephrin-B2 appear nearly identical to each other during formation of the palate.
Based on the individual expression studies taken for ephrin-B2 and the EphB receptors, we speculated that these molecules were likely co-expressed at the site of cell-cell adhesion as the palatal shelves meet and fuse at the midline. To address this, double IF analysis on ephrin-B2lacZ/+ embryos using antibodies against EphB2 and β-gal were performed. This revealed that EphB2 and ephrin-B2 are co-expressed on the midline epithelia where palatal shelf closure occurs (Fig 3E). Interestingly, this mimics the previous finding of co-expression in adhering midline epithelial cells at the site of urorectal/hindgut septation (Dravis et al., 2004).
EphB2/EphB3 activate reverse signaling for midline closure of the ventral body wall
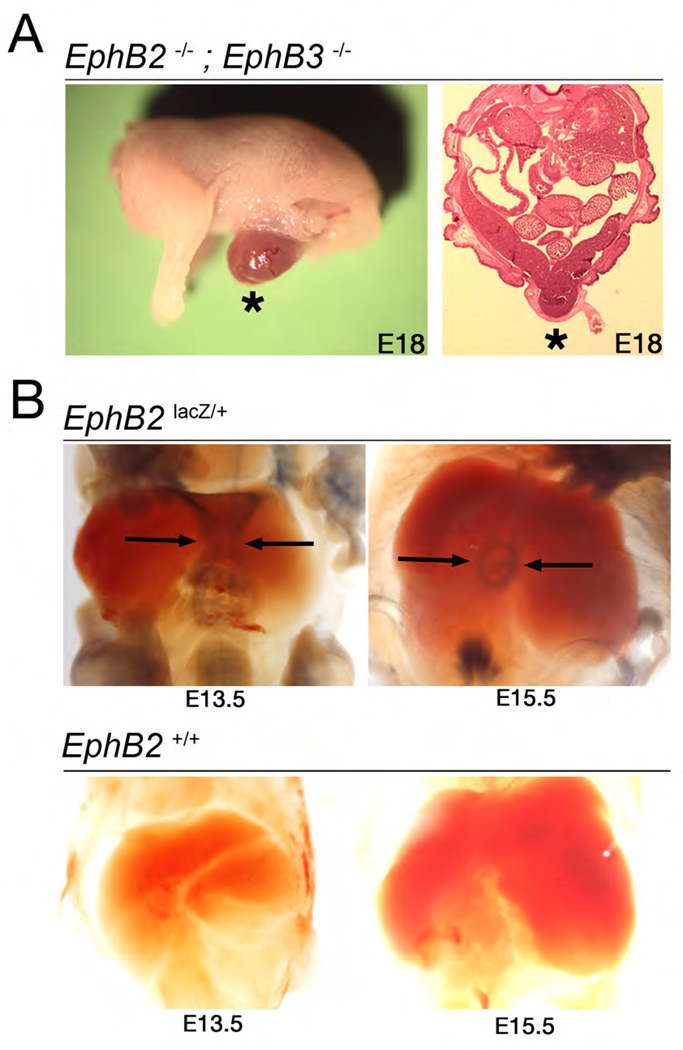
While analyzing palate and foregut defects in the EphB receptor mutant mice, it was noticed that 40% of EphB2−/−;EphB3−/− compound nulls exhibited a failure in midline closure of the abdominal wall (Fig 4A, Table 3), as briefly reported before (Orioli et al., 1996). The ventral midline defect in these mice resembles the birth defect omphalocele, in which visceral organs are herniated. This defect appears to be dependent on the genetic background of the mouse, as omphalocele was detected in the inbred 129 strain, but not in mice of a CD1 background. To determine where EphB2 is expressed during ventral midline closure of the abdominal wall, BluO-gal stains on whole-mounted EphB2lacZ/+ embryos were performed (Fig 4B). Expression of EphB2 was detected at E13.5 in cells at the ventral midline where abdominal closure takes place and at E15.5 was localized to the terminal closure spot of the umbilical ring. To determine if forward signaling through EphB2 participates in ventral body wall closure, the penetrance of this phenotype was investigated using the EphB2lacZ allele, revealing that only 9% of EphB2lacZ/lacZ;EphB3−/− animals exhibited omphalocele (Table 3). Given this data, and taking into account the reports that ephrin-B1 knockouts also have incompletely-penetrant omphalocele (Compagni et al., 2003; Davy et al., 2004), it appears that EphB2 and EphB3 act primarily as ligands to stimulate ephrin-B1 reverse signaling during midline closure of the ventral body wall.
Figure 4.
Failed closure of the ventral abdominal wall in EphB2; EphB3 compound null embryos. (A) Whole-mount image of an E18 EphB2−/−;EphB3−/− compound null shows herniated visceral organs (*) due to failed abdominal body wall closure (left). Transverse H&E stained section of an EphB2−/−;EphB3−/− E18 embryo similarly shows failed midline closure of the ventral body wall and herniated visceral organs (*, right). (B) Whole-mount BluO-gal stained EphB2lacZ/+ and EphB2+/+ embryos at E13.5 and E15.5 to detect expression of the EphB2-β-gal fusion protein. EphB2 is expressed at the ventral midline at E13.5 (arrows, top left) and at leading edges of the closing umbilical ring at E15.5 (arrows, top right). No BluO-gal activity is detected in the WT embryos that do not express the EphB2-β-gal fusion protein (bottom).
Table 3.
Incidence of omphalocele in EphB2;EphB3 compound mutants
| EphB2 mutation | background | B2/B2;B3/B3 | B2/+;B3/B3 | +/+;B3/B3 |
|---|---|---|---|---|
| EphB2− | 129 | 38 (15) | 63 (2) | 42 (0) |
| EphB21acZ | 129 | 11 (1) | 21 (0) | 14 (0) |
EphB2−/+;EphB3−/− (or EphB2lacZ/+; EphB3−/−) males were intercrossed with EphB2−/+; EphB3−/− females (or EphB21acZ/+;EphB3−/−) and offspring collected at E18.5. Animals with defective ventral body wall closure are in parentheses.
Ephrin reverse signaling is activated at sites of septation
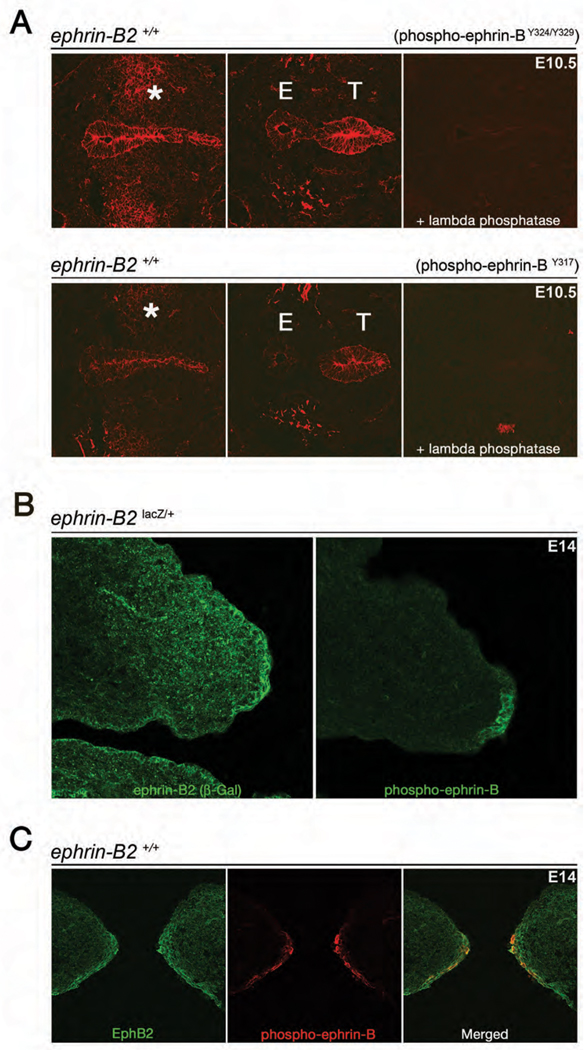
Eph-ephrin bidirectional signaling occurs at sites of cell-cell contact, which leads to clustering of the molecules into tetramers and higher order aggregates, along with tyrosine phosphorylation of their intracellular domains. We therefore sought to address where ephrin-B reverse signaling was activated during these midline events by using two different antibodies that recognize phosphorylated tyrosine residues within the conserved ephrin-B cytoplasmic domain. IF on sections of septating foregut at E10.5 showed that while some ephrin-B is tyrosine phosphorylated in the mesenchyme flanking the point of septation, the most striking tyrosine phosphorylation of ephrin-B is detected along the septating endoderm (Fig 5A). Adjacent sections treated with lambda phosphatase confirmed the phospho-specific activity of the antibodies. This data localizes ephrin-B tyrosine phosphorylation to midline septation in the foregut.
Figure 5.
Ephrin-B is tyrosine phosphorylated at the point of midline septation and closure in the foregut and palate. (A) Coronal sections from E10.5 WT embryos stained with an antibody that recognizes specific phosphorylation of tyrosines 324 and 329 (top) or of tyrosine 317 (bottom) on the conserved cytoplasmic tail of ephrin-B molecules to detect reverse signaling activity in vivo. Both antibodies detect tyrosine phosphorylated ephrin-B on the endoderm before (left) and during septation (middle). Activated ephrin-B also appears in the mesenchyme flanking the site of septation (*). Adjacent sections pre-treated with λ-phosphatase confirm antibody specificity (right). (B) Adjacent coronal sections from an E14 ephrin-B2lacZ/+ embryo treated with anti-β-gal (left) or anti-phospho-ephrin-BY324/Y329 (right) antibodies. While ephrin-B2 is expressed in both the mesenchyme and leading epithelia of the palatal shelf (left), it is only the ephrin in the leading epithelia that is tyrosine phosphorylated (right). (C) Coronal section from a WT E14 embryo treated with anti-EphB2 (left) and anti-phospho-ephrin-BY324/329 (middle) antibodies. EphB2 is co-expressed with tyrosine-phosphorylated ephrin-B in leading epithelia of the palatal shelves.
Adjacent palatal shelf sections from an E14 ephrin-B2lacZ/+ embryo were similarly probed with an antibody against β-gal to detect ephrin-B2 and phospho-ephrin-B antibodies to determine where ephrin-B2 was phosphorylated. While the β-gal antibody labeled the leading epithelia of the palatal shelf as well as the flanking mesoderm, the phospho-ephrin antibodies indicated that only ephrin-B2 in the leading epithelia of the palatal shelf was tyrosine phosphorylated (Fig 5B). Double IF with EphB2 and phospho-ephrin antibodies co-localized EphB2 with tyrosine phosphorylated ephrin-B2 (Fig 5C). This data importantly localizes ephrin-B2 reverse signaling to epithelia at the point of cell adhesion and closure in the developing palate.
Ephrin-B2 PDZ domain interactions contribute to midline septation
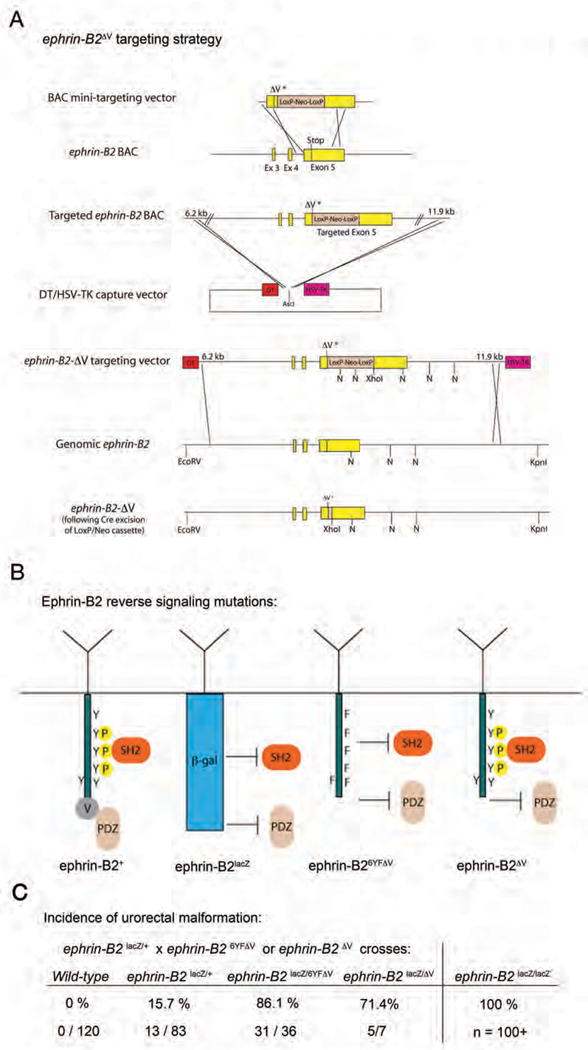
Having characterized roles for ephrin-B2 reverse signaling in midline septation, we next sought to determine which signaling avenues of the ephrin-B2 cytoplasmic tail are utilized for these morphogenetic events. To address this, two new ephrin-B2 alleles were created, ephrin-B2ΔV (Fig 6, Supp. Fig 2) and ephrin-B26YFΔV (Thakar et al., in preparation). In ephrin-B2ΔV mice, the codon for the C-terminal valine residue of ephrin-B2 was deleted to prevent the interaction of ephrin-B2 with PDZ domain-containing proteins. In ephrin-B26YFΔV mice, the six tyrosines of the ephrin-B2 cytoplasmic domain were changed to phenylalanine to prevent tyrosine phosphorylation of ephrin-B2 and the same C-terminal valine was deleted, resulting in the loss of interactions with both SH2 and PDZ domain-containing proteins (Fig 6B). The cell surface localization of these molecules and their inability to interact with PDZ domain-containing proteins have been demonstrated (Thakar et al., in preparation; Makinen et al., 2005), while proper expression of ephrin-B2ΔV was confirmed by immunoblot analysis (Supplemental Fig 3).
Figure 6.
New ephrin-B2 reverse signaling point mutant mice identify separate tyrosine phosphorylation-independent components of ephrin-B reverse signaling at the midline. (A) Targeting strategy for the generation of the ephrin-B2ΔV allele. (N= NcoI). (B) Summary of the expected consequences on signaling for the ephrin-B2lacZephrin-B26yfΔv and ephrin-B2ΔV alleles. (C) Incidence of urorectal malformation in male progeny following crosses between ephrin-B2lacZ/+ males and either ephrin-B26YFV/+ or ephrin-B2ΔV/+ females, compared to the 100% incidence found in ephrin-B2lacZ/lacZ animals.
Interestingly, neither ephrin-B2ΔV/ΔV nor ephrin-B26YFΔV/6YFΔV mutant mice present with defects in urorectal, tracheoesophageal, or palatal development. Remarkably, while 100% of ephrin-B2lacZ/lacZ animals die within hours of birth due to VACTERL defects, ephrin-B2ΔV/ΔV and ephrin-B26YFΔV/6YFΔV mice can be recovered as adults, although many die before adulthood, presumably due to lung and lymphatic defects (Makinen et al., 2005; Wilkinson et al, 2008). This indicates that tyrosine phosphorylation of ephrin-B2 and the transduction of ephrin-B2 reverse signaling through SH2 and PDZ domain interactions are not necessary for the development and septation of these midline structures.
A role for ephrin-B2 reverse signaling through PDZ domain interactions in midline development did become apparent however when ephrin-B2lacZ/+ and ephrin-B26YFΔV/+ mice were crossed to generate ephrin-B2lacZ/6YFΔV mutant animals (Fig 6C). The incidence of hypospadias was significantly greater in ephrin-B2lacZ/6YFΔV mutants (86%) compared to that seen in ephrin-B2lacZ/+ littermates (16%) (P<0.0001,***). A similar increase in the incidence of hypospadias was found in parallel crosses generating ephrin-B2lacZ/ΔV mutants (71%) (P=0.0031,**). Because both ephrin-B2lacZ/6YFΔV and ephrin-B2lacZ/ΔV mice present with increased hypospadias, it appears that PDZ domain interactions through the C-terminal valine represent an important component of ephrin-B2 reverse signaling in midline closure.
EphB2 forward signaling in midline septation does not require receptor tyrosine kinase activity or PDZ domain interactions
Having also characterized roles for EphB2 forward signaling in palate and urorectal development, we similarly attempted to identify what elements of EphB2 forward signaling might be involved at the midline by analyzing recently generated EphB2 forward signaling mutant animals. These mice include EphB2VEV mutants, in which the codons for the terminal three amino acids, valine-glutamate-valine (VEV), were deleted to abolish interactions between EphB2 and PDZ domain-containing proteins, EphB2K661R mutants, in which the lysine residue at amino acid 661 was mutated to arginine to abolish the tyrosine kinase catalytic activity of the EphB2 receptor, and EphB2KVEV mutants, which combine both mutations to simultaneously lose PDZ domain interactions as well as catalytic tyrosine kinase activity (Genander et al., 2009). No urorectal malformation was found in any of the EphB2VEV/VEV (n=107), EphB2K661R/K661R (n=102), or EphB2KVEV/KVEV (n=202) mutant mice in an EphB3−/− background. Further, all compound mutants were recovered as adults at expected Mendelian ratios, indicating no neonatal lethality, and thus no cleft palate phenotype. The data indicates EphB2 forward signaling in these midline structures does not require the phosphorylation of secondary molecules by the receptor tyrosine kinase domain or the interaction of EphB2 with PDZ domain-containing proteins.
Ephrin-B2 reverse signaling through claudin at the midline
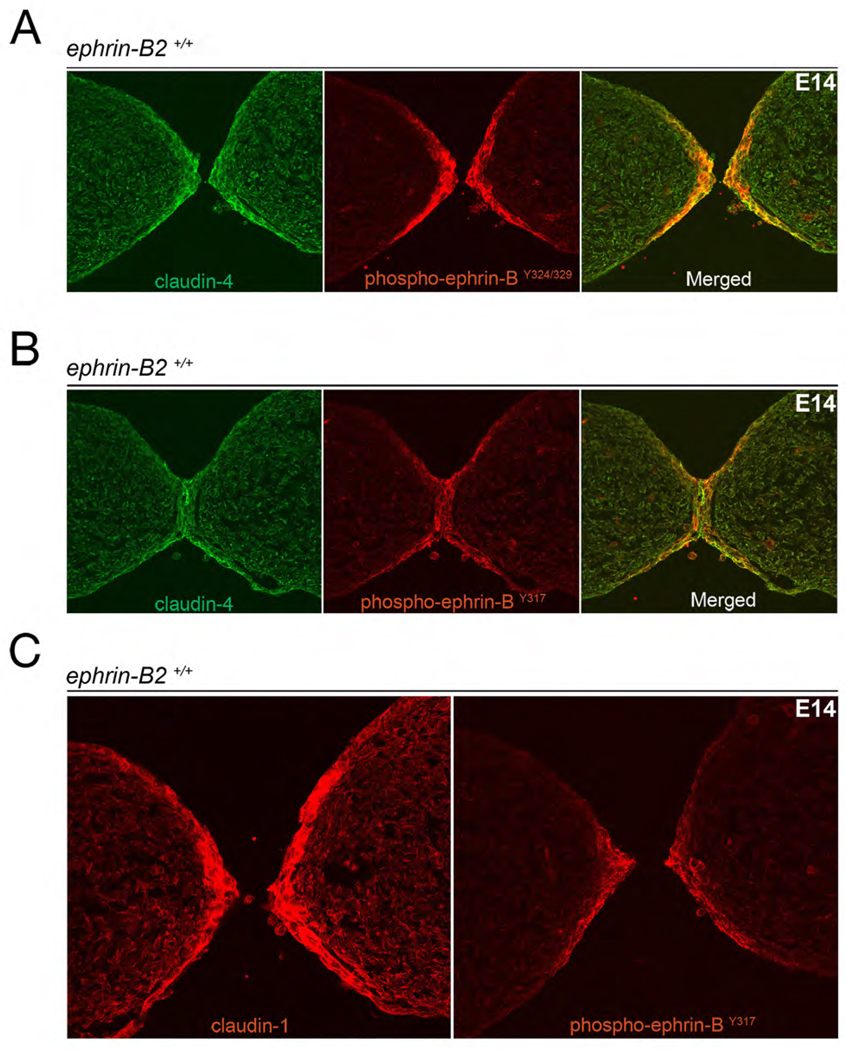
That ephrin-B2ΔV and ephrin-B26YFΔV mice do not phenocopy ephrin-B2lacZ mice suggests: 1) there is more to ephrin-B2 reverse signaling than just SH2 domain interactions through phosphorylated tyrosines and PDZ domain interactions through the C-terminus, and 2) these non-canonical signaling avenue(s) are critical elements of the reverse signal employed for midline septation or closure. B-subclass ephrins have been shown to interact with the claudins, a class of tetraspan proteins known to directly mediate cell-cell adhesion (Tanaka et al., 2005). To examine a potential role for the claudins in ephrin-B2 reverse signaling, the expression of claudin-1 and claudin-4 (the two claudin molecules shown to associate with ephrin-B) was investigated at the point of adhesion in the palate. Strikingly, IF shows that both claudin-1 and claudin-4 are highly expressed in epithelia directly before and right at midline cell-cell adhesion in the palate of WT embryos (Fig 7). This expression mimics the profile of tyrosine-phosphorylated ephrin-B, and indeed double IF shows that both claudin and activated ephrin co-localize in adherent epithelia. This data supports a tyrosine phosphorylation-independent role for ephrin-B2 reverse signaling involving the claudins in midline septation.
Figure 7.
Claudin-1 and claudin-4 are co-expressed with activated ephrin-B and EphB2 at the point of midline adhesion in the palate. (A) Coronal section from a WT E14 embryo treated with anti-claudin-4 (green) and phospho-ephrin-BY324/Y329 (red) antibodies reveal both molecules are expressed in adherent epithelia of the palatal shelves. (B) Coronal section from a WT E14 embryo treated with anti-claudin-4 (green) and phospho-ephrin-BY317 (red) reveals the same co-expression after adhesion has been initiated. (C) Adjacent sections from a WT E14 embryo treated with anti-claudin-1 (left) and phospho-ephrin-BY317 (right) reveal claudin-1 is also present in the adherent palatal epithelia with tyrosine phosphorylated ephrin-B.
DISCUSSION
The data presented here show that ephrin-B2lacZ/lacZ homozygotes present with LTEC and cleft palate phenotypes, indicating novel roles for ephrin-B2 reverse signaling in foregut septation and palatal shelf closure. Moreover, we have shown that EphB2lacZ/lacZ;EphB3−/− animals also present with cleft palate, indicating a role for EphB forward signaling in midline fusion of the palate as well. The roles identified here for both ephrin-B2 reverse signaling and EphB2 forward signaling in midline formation of the palate are remarkably similar to what we previously reported for the tubularization of the urethra and septation of the hindgut/cloaca into distinct urogenital and anorectal compartments (Dravis et al., 2004). We also report here that EphB2−/−; EphB3−/−compound null animals, but less so forward signaling deficient EphB2lacZ/lacZ; EphB3−/− animals present with omphalocele. This indicates that closure of the ventral abdominal wall occurs through EphB2/B3 acting primarily as ligands to stimulate ephrin-B reverse signaling. Surprisingly, deconstruction of the ephrin-B2 reverse signal and the EphB2 forward signal in these midline structures using select point mutant mice indicates that these bidirectional signals do not appear to require tyrosine kinase phosphorylation events. Instead, the ephrin-B2 reverse signal can be broken down into distinct tyrosine phosphorylation-independent elements, one of which involves PDZ domain interactions, and the other we propose involves interaction with claudin family tetraspan molecules.
Fidelity of the lacZ alleles
A question to address in considering the data is the reliability of lacZ alleles that result in the synthesis of C-terminally truncated proteins that lack their cytoplasmic domains and covalently attach β-gal. This has been controversial given that similar animal models have produced divergent claims about the physiological roles of forward and reverse signaling events (Adams et al., 2001; Cowan et al., 2004). Therefore, we would like to point out that: 1) the ephrin-B2- β gal fusion protein encoded by the ephrin-B2lacZ allele localizes properly to the cell surface and clusters into higher order oligomers, unlike other ephrin-B2 unconjugated C-terminal cytoplasmic truncations which become trapped in the trans-Golgi network and do not traffic to the plasma membrane (Cowan et al., 2004), and can properly activate forward signaling through EphB receptors (Supplemental Figure 1); 2) the success of the lacZ alleles to properly signal non-cell autonomously has been demonstrated in vivo now several times over, as the ephrin-B3lacZ/lacZ mutant does not present with locomotor defects in the spinal cord associated with the ephrin-B3−/− protein null (Yokoyama et al., 2001), the ephrin-B2lacZ/lacZ mutant does not result in early embryonic lethality due to vascular angiogenic failures associated with either ephrin-B2−/− protein null or ephrin-B2 unconjugated C-terminal truncation mice (Adams et al., 2001; Cowan et al., 2004), and EphB2lacZ/lacZ mutants do not exhibit axon pathfinding defects associated with EphB2−/− protein nulls (Birgbauer et al., 2000; Cowan et al., 2004; Henkemeyer et al., 1996); 3) the lacZ alleles do not hyperactivate forward signaling, as genetic assays have shown (Dravis et al., 2004), and as is suggested by the fact that EphB2F620D mice with hyperactive EphB2 forward signals (Holmberg et al., 2006) do not present with any midline defects (data not shown); and 4) the cell autonomous defects seen with the lacZ alleles have also been found in traditional germline knockout animals for EphB and as well as the targeted mutations of ephrin-B26YFΔV and ephrin-B2ΔV now, further confirming the midline defects characterized in lacZ mice are not the result of hypermorphic or neomorphic activity.
Midline EphB:ephrin-B bidirectional signaling operates independently of tyrosine phosphorylation
One of the most interesting findings in our data is that ephrin-B26YFΔV/6YFΔV mice do not phenocopy ephrin-B2lacZ/lacZ mice, which is surprising because both alleles block canonical signaling structures of the ephrin-B cytoplasmic domain: phosphorylated tyrosines that serve as a substrate for SH2 domain docking and a C-terminal valine that interacts with PDZ domains. This indicates that signaling through these two elements are not necessary in the midline septation events we have linked to ephrin-B reverse signaling. However, by combining the ephrin-B26YFΔV and ephrin-B2ΔV alleles with ephrin-B2lacZ, to produce ephrin-B2lacZ/6YFΔV and ephrin-B2lacZ/ΔV mice, and comparing those animals to ephrin-B2lacZ/+ animals (Fig 6C), it becomes apparent that PDZ domain interactions through the C-terminus of ephrin-B2 do contribute to these midline events. The disconnect between the ephrin-B2lacZ and ephrin-B26YFΔV or ephrin-B2ΔV alleles suggests there are alternate avenues of reverse signaling through ephrin-B that are independent of SH2/PDZ domain interactions, but nonetheless still necessary for proper midline development.
Other groups have identified potential non-canonical signaling avenues that may be of relevance here. These include the discovery that the ephrin-B cytoplasmic tail is serine phosphorylated, that a P-P-Q-S-P motif on the cytoplasmic tail can mediate SH3 domain interactions, that Dishevelled can constitutively interact with the ephrin-B cytoplasmic tail in Xenopus, and that ephrin-Bs can interact with claudins (Essmann et al., 2008; Segura et al., 2007; Tanaka et al., 2005; Tanaka et al., 2003). The identification of the SH3 binding site is particularly interesting, as it could explain why our genetic studies indicate no apparent role for ephrin-B2 tyrosine phosphorylation in midline septation, even though our expression data clearly demonstrates that ephrin-B is tyrosine phosphorylated precisely at the adhesion sites where septation takes place. Reverse signaling might still occur in the absence of ephrin-B tyrosine phosphorylation with our ephrin-B26YFΔV mice, if a key adaptor protein like Grb4 could simply find itself recruited to the ephrin cytoplasmic tail through one of its three SH3 domains, instead of its SH2 domain, to produce similar physiological outcome at the midline. The ephrin-B SH3 and SH2 domain binding motifs might therefore possess some redundancy that masks their importance in midline signaling.
However, given the nature of the defects in midline septation and closure that are characterized in our mice here, it is the interaction between the ephrins and claudins that we find of particular interest. The claudins have been characterized to directly mediate adhesion between cells through high affinity interactions involving their extracellular loops, and the interaction between ephrin-B and claudin has been shown to modulate this epithelial adhesion, albeit in regulating paracellular flow (Tanaka et al., 2005) . This suggests that the interaction between ephrin-B2 and claudins might regulate the adhesiveness of epithelia coming into contact at the midline, and indeed we find that both claudin-1 and claudin-4 are highly co-localized with ephrin-B2 and EphB2 on the cell surface of adhering epithelial cells in the palate. Of note, claudin expression does not appear restricted to its more canonical tight junction localization, which visualize as distinct puncta, but instead appears to be expressed across the breadth of the epithelial cell surface. Based on this expression, we strongly suspect that ephrin-claudin interactions represent a tyrosine phosphorylation-independent portion of ephrin-B reverse signaling in these midline septation events, perhaps in mediating the actual cell-cell adhesion of these lateral structures.
On the other side of the bidirectional signal, it is also interesting that forward signaling through the EphB2 receptor appears to require neither the tyrosine kinase catalytic activity of the receptor nor the C-terminal PDZ-binding motif in these midline structures. This is less surprising than our findings with reverse signaling, however. Even without tyrosine kinase activity, EphB2 can still be phosphorylated on critical tyrosine residues by intracellular molecules such a Abl, Src family kinases, or co-expressed Eph receptors in much the same way the kinase-dead Eph receptor EphB6 activates forward signaling (Freywald et al., 2002; Vindis et al., 2003; Yu et al., 2001). Further, EphB2 is a large molecule with numerous protein interaction sites that were untouched by our targeted mutations (notably including the juxtamembrane tyrosines and SAM domain), which could play important roles in the forward signaling element involved in midline development.
EphB/ephrin-B signaling in midline cell-cell adhesion
Our genetic analyses indicate critical roles for ephrin-B reverse signaling in multiple septation events at the embryonic midline. What then is the ephrin-B reverse signal doing in these structures? Our analysis of where ephrin-B reverse signaling is localized by utilizing IF is very useful here, as it demonstrates that both ephrin-B tyrosine phosphorylation (representing activated ephrin-B) and claudin specifically localize to the leading epithelia at the sites where cell adhesion initiates septation and closure. In addition, EphB2 and EphB3 are also expressed and likely actively signaling in the same midline epithelia. These epithelial cells are not necessarily proliferative or migratory; their principal function is to adhere to adjacent epithelia at the point of septation. When we couple the failure of midline structures to properly adhere in forward and reverse signaling deficient animals with this expression data localizing EphB, ephrin-B, and the adhesion regulatory molecule claudin to sites of cell adhesion, it appears that these bidirectional signals might be responsible for producing a cell adhesion outcome in these midline septation events. This is an exciting hypothesis that will need to be addressed through future analyses of the behavior of these cells in vivo and in cell culture assays.
How then might the Ephs and ephrins produce a cell-cell adhesion response? This continues to be a confounding question, trying to determine how in one instance these molecules can provoke a cell repulsion response and in others lead to cell adhesion. An emerging answer is that Eph-ephrin reverse signaling produces a cell adhesion response the same way it produces a cell repulsion response—through modulating the balance of activity in the Rho family GTPases Rho, Rac, and Cdc42. Both forward and reverse signal transduction function to regulate these Rho-family GTPases, and subtle shifts in the relative activity of these molecules (through differential activation or antagonistic crosstalk) can produce different cytoskeletal behavior consistent with either cell adhesion or cell repulsion outcomes (Burridge and Doughman, 2006; Jaffe and Hall, 2005).
The link between ephrin reverse signaling and Rho family GTPases is potentially interesting given that the activation of these molecules has been linked to the production of filopodia-like extensions, which facilitate midline adhesion in Drosophila dorsal closure, a paradigm for the epithelial adhesion events discussed here (Jacinto et al., 2000). This presents the intriguing hypothesis that ephrin-B reverse signaling might become activated in these adherent epithelia, producing activated combinations of Rho/Rac/Cdc42 that then elicit filopodia-like extensions in these cells; these filopodia then extend across the midline to establish initial sites of contact between adjacent cells that rapidly mature into adherens junctions to zip these midline structures closed. Eph-ephrin regulation of filopodia generation and motility has previously been shown to be important in synaptic development, particularly for the generation of dendritic spines, suggesting some potential functional redundancy in these very different structures (Klein, 2009). For the PDZ-dependent aspect of midline ephrin signaling identified in our new ephrin-B2ΔV mice, this might involve the documented interaction of ephrin-B with PAR-3, which targets Rho family molecules through its complex with PAR-6 and aPKC (Lin et al., 1999; Nishimura et al., 2005). For the tyrosine phosphorylation- and PDZ-independent signaling avenues suggested by the mice, this might involve Grb4 docking via the ephrin-B SH3 domain or the association of ephrin-B with Dishevelled, both of which are linked to Rho family GTPase activity (Segura et al., 2007; Tanaka et al., 2003).
It is less clear how ephrin and claudin might work together to mediate midline adhesion. This interaction is not thought to require the cytoplasmic tail of ephrin, so it is possible that differential clustering of WT ephrin-B2 and the ephrin-B2-β-gal fusion protein might somehow alter the respective interactions of the ephrin with claudin, resulting in compromised midline adhesion in ephrin-B2lacZ animals. Future biochemical analyses must aim to address the nature of this ephrin-claudin interaction, how it might function to control cell adhesion, and how this process may fail in our mutant animals.
Our data differs with other studies proposing how Eph-ephrin signaling can produce cell adhesion. It has been suggested that cell adhesion is a more passive outcome that takes place through the inhibition of canonical repulsive Eph-ephrin signaling, either through cis-inhibition on co-expressing cells (Carvalho et al., 2006; Yin et al., 2004), or through inhibitory splice variants (Holmberg et al., 2000). Our work demonstrates that midline epithelia are actively transducing reverse signals, suggesting that B-subclass ephrins are eliciting an active adhesion response, not just inhibiting cell repulsion to permit adhesion. It has also been suggested that the Ephs and ephrins are themselves adhesion molecules, and cell repulsion occurs when the interaction of these cell-surface receptors is broken, either by endocytosis or by a protease (Marston et al., 2003; Zimmer et al., 2003). This model is not consistent with our observations because the ectodomains of both Eph and ephrin in the lacZ truncation alleles are left intact and are able to traffic to the plasma membrane, where they should be able to still perform as non-signaling cell adhesion molecules. And yet, as the data shows, mice with these truncated alleles present with the same, if not more severe, adhesion defects as the null mutants. This further supports an active signaling role for the Ephs and ephrins in promoting cell adhesion.
EphB/ephrin-B signaling and the VACTERL association
VACTERL refers to an association of embryonic malformations comprised of vertebral (V), anorectal (A), cardiac (C), tracheoesophageal (TE), renal (R), and limb (L) abnormalities, that often presents with additional anomalies such as hypospadias, cleft palate, neural tube defects, and omphalocele. Here we find that animals with EphB/ephrin-B signaling ablations present with LTEC, cleft palate, and omphalocele, which represent one of the defining features of VACTERL as well as two associated anomalies. This data extends upon previous studies demonstrating that Eph/ephrin signaling disruptions cause anorectal malformations and hypospadias (Dravis et al., 2004), cardiac defects (Cowan et al., 2004), limb and skeletal abnormalities (Compagni et al., 2003), and neural tube defects (Holmberg et al., 2000). We have also noticed a distended kidney phenotype in ephrin-B2lacZ mice, due to a defect in ureter-bladder integration (unpublished data), suggesting links to renal development as well. This striking overlap between EphB/ephrin-B malformations and VACTERL association (Table 4) leads us to propose Eph/ephrin signaling as a molecular basis for the association. This extends our knowledge of VACTERL from morphogens like Shh to cytoskeletal regulators like the Ephs and ephrins, which are likely directly responsible for guiding the cell-cell interactions, movements, and adhesion events that are disrupted in affected individuals.
Table 4.
| VACTERL Association Defects |
Eph/ephrin involved |
Defect | References |
|---|---|---|---|
| Vertebral | ephrin-B1 | Asymmetric rib attachment | (Compagni et al., 2003) |
| ephrin-B2 | Abnormal somite patterning | (Davy and Soriano, 2007) | |
| Anorectal | EphB2,EphB3 ephrin-B2 | Failed cloacal septation leading to persistent cloaca with GI fistula | (Dravis et al., 2004) |
| Cardiac | ephrin-B2 | Enlarged cardiac valves | (Cowan et al., 2004) |
| Tracheo-Esophageal | ephrin-B2 | Tracheoesophageal fistula with esophageal atresia | (this manuscript) |
| Renal | ephrin-B2 | Hydronephrosis due to failed ureter-bladder integration | (unpublished data) |
| Limb | ephrin-B1 | polydactyly | (Compagni et al., 2003) (Davy et al., 2004) |
| Peripheral VACTERL Defects |
Eph/ephrin involved |
References |
|---|---|---|
| Hypospadias | EphB2,EphB3 ephrin-B2 | (Dravis et al., 2004) |
| Cleft Palate | EphB2,EphB3 ephrin-B1 ephrin-B2 | (Orioli et al., 1996) (Compagni et al., 2003) (Davy et al., 2004) (this manuscript) |
| Omphalocele | EphB2,EphB3 ephrin-B1 | (Compagni et al., 2003) (Orioli et al., 1996) (this manuscript) |
| Neural Tube Closure | EphA7 ephrin-A5 | (Holmberg at al., 2000) |
METHODS
Animals
EphB2 (Henkemeyer et al., 1996), EphB3 (Orioli et al., 1996), EphB2VEV, EphB2K661R and EphB2KVEV (Genander et al., 2009), ephrin-B26YFΔV (Thakar et al., submitted), and ephrin-B2lacZ (Dravis et al., 2004) mice have been described. The EphB2 and EphB3 mutations have been maintained on an inbred 129 background and have also been backcrossed to the CD1 strain. All ephrin-B2 mutants were maintained on a mixed 129/CD1 background. Induction of the tet-on system was achieved by supplying pregnant females with drinking water containing 0.5 mg/ml doxycycline and 5% sucrose for 16–24 hours. Statistical comparisons utilized the two-tail Fischer’s extract test.
Generation of Tg-BAC-EphB3-YFP and ephrin-B2ΔV animals
Tg-BAC-EphB3-YFP mice contain a destabilized variant of eYFP knocked into the ORF of BAC EphB3 sequences. Transgenic mice were generated from BAC clone RP23-213M14, a 208 kb BAC from a C57BL/6J background containing 42 kb upstream of the EphB3 start codon, that was purchased from the BACPAC resources center at the Children’s Hospital Oakland. D2eYFP-1 (Clontech) was targeted into the EphB3 ORF in RP23-213M14 using bacterial homologous recombination (http://web.ncifcrf.gov/research/brb/recombineeringInformation.aspx). Targeting was confirmed by PCR and restriction digests. Pronuclear injection was performed by Transgenic Core facilities at the UTSW Department of Developmental Biology. To construct the targeting vector for ephrin-B2ΔV, RP23-328F4 was purchased from the same BACPAC resources center. This BAC contains ephrin-B2 exon 5, which has the sequences coding for the cytoplasmic tail. Through bacterial homologous recombination, the ephrin-B2 exon 5 was targeted to delete the GTC codon for valine at the ephrin-B2 C-terminus and insert a LoxP-flanked NeoR cassette 27 bp downstream of the stop codon. Insertion of the LoxP site results in a 53 bp deletion of 3′ non-coding sequence downstream of the stop codon, GACCTGC.…GGACTGC. Bacterial homologous recombination was then performed on the modified BAC with a capture vector containing 5′ Diphtheria toxin and 3′ HSV-tk selection markers to produce a targeting vector with a 6.1 kb 5′ homology arm and an 11.9 kb 3′ homology arm. The targeting vector was linearized through AscI digestion and electroporated into ES cells, with cell lines screened through Southern blot analysis with 5′ and 3′ external probes to detect proper homologous recombination. Blastocyst injection was performed with two of the positive cell lines to generate chimeric mice through the UTSW Transgenic Core Facility, and germline transmission was confirmed. The LoxP-flanked cassette was removed with a germline Krox20-Cre line. Protein expression was confirmed by immunoblotting protein lysates from E12.5 embryos with an antibody recognizing the N-terminus of ephrin-B2 (Novus Biologicals)
Immunofluorescence
Immunofluorescence was performed as described (Dravis et al., 2004). As a phosphotyrosine control sections were treated with λ-protein phosphatase (NEB) for 45' prior to primary application. Primary antibodies used: goat anti-EphB2 (R&D Systems), rabbit anti-β-Gal (Cappel), rabbit anti-phospho-ephrin-B (Cell Signaling Technology), rabbit anti-phospho-ephrin-B [Tyr298] recognizing mouse Y317 (Novus Biologicals), rabbit anti-ephrin-B1 (C-18, Santa Cruz), rabbit anti-GFP (Molecular Probes), rabbit anti-claudin-1 (Invitrogen) and Alexa Fluor 488-conjugated claudin-4 (Invitrogen). Secondary antibodies were Cy2 donkey anti-goat, Cy3 donkey anti-rabbit, and Cy2 donkey anti-rabbit (Jackson ImmunoResearch). Images were visualized on a Zeiss 510 LSM confocal microscope.
Histochemical β-gal staining
X-gal and BluO-gal stains were performed as described (Dravis et al., 2004) and counterstained with nuclear fast red. Whole-mount embryos were dehydrated through sequential ethanol washes and cleared in methyl salicylate.
EphB2 tyrosine phosphorylation detection
Protein lysates from the head of E14.5 embryos were dissociated mechanically in lysis buffer (30 mM Hepes pH 7.5, 150 mM NaCl, 10% glycerol, 1.5% Triton-X 100, 1 mM EGTA, 10 mM sodium pyrophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 1 mM PMSF, and protease inhibitor cocktail), immunoprecipitated with an antibody against EphB2 (R&D Systems), separated by SDS-PAGE, and immunoblotted with antibodies against EphB2 or phosphotyrosine (Upstate Biotechnology).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Jennifer Shay, Tracey Bowdler, and Jan La for their histological and genotyping assistance in the laboratory. C.D. was supported by the Division of Cell and Molecular Biology Training Program (T32 GM08203). This work was funded in part by the NIH (R01 EY017434 and 2R01 MH66332).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Aynaci FM, Celep F, Karaguzel A, Baki A, Yildiran A. A case of VATER association associated with 9qh+ Genet Couns. 1996;7:321–322. [PubMed] [Google Scholar]
- Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG. Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene. 1998;16:471–480. doi: 10.1038/sj.onc.1201557. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Cowan CA, Sretavan DW, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- Botto LD, Khoury MJ, Mastroiacovo P, Castilla EE, Moore CA, Skjaerven R, Mutchinick OM, Borman B, Cocchi G, Czeizel AE, Goujard J, Irgens LM, Lancaster PA, Martinez-Frias ML, Merlob P, Ruusinen A, Stoll C, Sumiyoshi Y. The spectrum of congenital anomalies of the VATER association: an international study. Am J Med Genet. 1997;71:8–15. doi: 10.1002/(sici)1096-8628(19970711)71:1<8::aid-ajmg2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Burridge K, Doughman R. Front and back by Rho and Rac. Nat Cell Biol. 2006;8:781–782. doi: 10.1038/ncb0806-781. [DOI] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Bianchi LM, Henkemeyer M, Fritzsch B. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Wu T, Chumley MJ, Yokoyama N, Wei S, Wu DK, Marcus DC, Henkemeyer M. EphB2 and ephrin-B2 regulate the ionic homeostasis of vestibular endolymph. Hear Res. 2007;223:93–104. doi: 10.1016/j.heares.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Barr J, O'Connell S, Flagg A, Swartz ME, Cramer KS, Tosney KW, Pasquale EB, Krull CE. Ephrin-A5 exerts positive or inhibitory effects on distinct subsets of EphA4-positive motor neurons. J Neurosci. 2004;24:1070–1078. doi: 10.1523/JNEUROSCI.4719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007 doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Essmann CL, Martinez E, Geiger JC, Zimmer M, Traut MH, Stein V, Klein R, Acker-Palmer A. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci. 2008;11:1035–1043. doi: 10.1038/nn.2171. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Freywald A, Sharfe N, Roifman CM. The kinase-null EphB6 receptor undergoes transphosphorylation in a complex with EphB1. J Biol Chem. 2002;277:3823–3828. doi: 10.1074/jbc.M108011200. [DOI] [PubMed] [Google Scholar]
- Genander M, Halford MM, Xu NJ, Eriksson M, Yu Z, Qiu Z, Martling A, Greicius G, Thakar S, Catchpole T, Chumley MJ, Zdunek S, Wang C, Holm T, Goff SP, Pettersson S, Pestell RG, Henkemeyer M, Frisen J. Dissociation of EphB2 signaling pathways mediating progenitor cell proliferation and tumor suppression. Cell. 2009;139:679–692. doi: 10.1016/j.cell.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O'Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35:475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, Silvany RE, Henkemeyer M, Frisen J. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Huynh-Do U, Stein E, Lane AA, Liu H, Cerretti DP, Daniel TO. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5beta1 integrins. Embo J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/s0960-9822(00)00796-x. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim P, Hui CC. The VACTERL association: lessons from the Sonic hedgehog pathway. Clin Genet. 2001;59:306–315. doi: 10.1034/j.1399-0004.2001.590503.x. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J Biol Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Schlierf B, Schardt A, Nave KA, Wegner M. Sox10-rtTA mouse line for tetracycline-inducible expression of transgenes in neural crest cells and oligodendrocytes. Genesis. 2004;40:171–175. doi: 10.1002/gene.20083. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Nezarati MM, McLeod DR. VACTERL manifestations in two generations of a family. Am J Med Genet. 1999;82:40–42. [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. Embo J. 1996;15:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- Risley M, Garrod D, Henkemeyer M, McLean W. EphB2 and EphB3 forward signalling are required for palate development. Mech Dev. 2009;126:230–239. doi: 10.1016/j.mod.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med Genet. 1996;63:529–536. doi: 10.1002/(SICI)1096-8628(19960628)63:4<529::AID-AJMG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- San Miguel S, Serrano MJ, Sachar A, Henkemeyer M, Svoboda KK, Benson MD. Ephrin reverse signaling controls palate fusion via a PI3 kinase-dependent mechanism. Dev Dyn. 2011;240:357–364. doi: 10.1002/dvdy.22546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Shaw-Smith C. Genetic factors in esophageal atresia, tracheo-esophageal fistula and the VACTERL association: roles for FOXF1 and the 16q24.1 FOX transcription factor gene cluster, and review of the literature. Eur J Med Genet. 2009;53:6–13. doi: 10.1016/j.ejmg.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, Daniel TO. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kamata R, Sakai R. Phosphorylation of ephrin-B1 via the interaction with claudin following cell-cell contact formation. Embo J. 2005;24:3700–3711. doi: 10.1038/sj.emboj.7600831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor and ephrin mediates cell repulsion. Embo J. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temtamy SA, Miller JD. Extending the scope of the VATER association: definition of the VATER syndrome. J Pediatr. 1974;85:345–349. doi: 10.1016/s0022-3476(74)80113-7. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C, Cerretti DP, Daniel TO, Huynh-Do U. EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis. J Cell Biol. 2003;162:661–671. doi: 10.1083/jcb.200302073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Wilkinson GA, Schittny JC, Reinhardt DP, Klein R. Role for ephrinB2 in postnatal lung alveolar development and elastic matrix integrity. Dev Dyn. 2008;237:2220–2234. doi: 10.1002/dvdy.21643. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39:919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci Res. 2004;48:285–296. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Yu HH, Zisch AH, Dodelet VC, Pasquale EB. Multiple signaling interactions of Abl and Arg kinases with the EphB2 receptor. Oncogene. 2001;20:3995–4006. doi: 10.1038/sj.onc.1204524. [DOI] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.