Abstract
Purpose
This phase II study was designed to determine the objective response rate and 6-month progression free survival of adult patients with recurrent supratentorial anaplastic glioma when treated with the immune modulator, polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose (poly-ICLC).
Methods and Materials
This was an open-labeled, single arm phase II study. Patients were treated with poly-ICLC alone. Patients may have had treatment for no more than two prior relapses. Treatment with poly-ICLC continued until tumor progression.
Results
55 patients were enrolled in the study. 10 were ineligible after central review of pathology. 11% of patients (5 of 45) had a radiographic response. Time to progression was known for 39 patients and 6 remain on treatment. The estimated 6-month progression free survival was 24%. The median survival time was 43 weeks.
Conclusions
Poly-ICLC was well tolerated, but there was no improvement in 6-month progression free survival compared to historical database nor was there an encouraging objective radiographic response rate. Based on this study, poly-ICLC does not improve 6moPFS in patients with recurrent anaplastic gliomas but may be worth further study in combination with agents such as temozolomide.
Keywords: anaplastic glioma, radiation therapy, adjuvant therapy, poly-ICLC
INTRODUCTION
Anaplastic gliomas (AG), otherwise known as World Health Organization (WHO) grade III gliomas, include anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and anaplastic mixed oligoastrocytoma (AMO). These tumors are challenging to treat and associated with a high degree of morbidity and mortality. Due in part to the rarity of anaplastic gliomas, there are few studies that specifically address their treatment at diagnosis or recurrence. Instead, most prior studies combined both WHO grades III and IV and treatment conclusions are extrapolated or inferred from the treatment of “high-grade” or “malignant” glioma rather than explicitly from the treatment of grade III gliomas [1, 2].
Based on the randomized data available, the post surgical treatment and treatment at recurrence of AG remains unsatisfactory. Post resection radiation therapy increases median survival for patients and there appears only to be a modest benefit with the use of nitrosourea based, adjuvant chemotherapy [3–7]. Chemotherapy is commonly prescribed to patients with recurrent AGs and chief among the agents used in this setting is temozolomide (TMZ) although to date there are no phase III data demonstrating the superiority of TMZ over other cytotoxic agents [8]. Because of these imprecise and limited results, continuous efforts are ongoing to develop more novel, effective agents or combinations of agents that may improve overall survival or prolong time to progression for patients with AG. One such novel agent and one that modulates the immune system is polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose (poly-ICLC).
Poly-ICLC is a double-stranded RNA (dsRNA) previously utilized as an interferon inducer and immune modulating agent at high doses (up to 300mcg/kg IV) in clinical cancer trials. These trials were based on preclinical evidence that poly-ICLC possessed anti-neoplastic activity, including in glioma cell lines. In clinical studies the anti-tumor effect was thought due to induction of interferon and an interferon-independent generation of a pervasive immune enhancing effect which involved an increased antibody response to antigen and activation of natural killer cells, T-cells, macrophages, and cytokines [9–14]. These original trials showed varied results with considerable toxicity and the use of poly-ICLC was by and large discarded when interferons became available via recombinant DNA technology [15–20]. However, ensuing studies demonstrated that low dose (10–50mcg/kg) poly-ICLC resulted in less toxicity and a broader host defense stimulation, including activation of myeloid dendritic cells via Toll-like receptor 3 (TLR3) as well as induction of a mix of interferons, cytokines, and chemokines. The host defense stimulation also resulted in an antiviral and antiproliferative effect mediated by activation of interferon-inducible dsRNA-dependent enzyme systems which regulate such cell functions as protein synthesis, proliferation, and apoptosis [15–22].
The enzyme systems thought to be activated by poly-ICLC are 2′5′oligoadenylate sythetase (OAS), protein kinase R (PKR), the RIG-1 helicase, and melanoma differentiation associated gene-5 (MDA5) [23, 24]. The immunostimulatory and marked vaccine adjuvant actions of dsRNAs have come into focus in more recent preclinical and clinical studies demonstrating the mix of interferons, cytokines and chemokines that they induce and the critical immune role of pattern recognition receptors such as the toll-like receptors (TLR). In particular, TLR3, which responds to dsRNAs such as poly-ICLC, is found on astrocytes, respiratory epithelium and myeloid dendritic cells, which in turn mediate a Th1 cellular immune response that appears more suited to antiviral and antineoplastic action. When presented along with antigen, poly-ICLC markedly enhances both induction of tumor-specific cytotoxic lymphocytes (CTL) as well as targeting and infiltration of those CTL into gliomas. Enhanced targeting appears related to induction of chemokine expression at the tumor site as well as to the expression of TLR3 on astrocytes [25].
A pilot study of poly-ICLC in patients with newly diagnosed or recurrent GBM or AA, treated 38 patients (11 newly diagnosed AAs, 18 newly diagnosed GBMs, with the remaining 9 patients either recurrent AA or GBM) with poly-ICLC at 10–50 mcg/kg, administered intramuscularly one to three times weekly[24]. Twenty of 38 patients (10 of 11 newly diagnosed AAs) also received at least one cycle of concurrent CCNU at 120mg/m2 once every six weeks including 10 of 11 newly diagnosed patients with AA. 66% of patients (including all AAs) receiving at least twice-weekly poly-ICLC showed regression or stabilization of gadolinium enhancing tumor volume on MRI for at least 6 months. Only 2 of the 11 newly diagnosed AA patients subsequently showed tumor progression while on poly-ICLC. These same 11 patients with newly diagnosed AAs had a median progression-free survival of 77 months from diagnosis and a median survival of 102 months which compares favorably to previously published studies [2, 6, 26]. Fundamentally, this pilot study demonstrated the safety and tolerability of long-term, low-dose intramuscularly administered poly-ICLC in patients with malignant glioma at a potentially beneficial dose range of 20 mcg/kg administered two to three times weekly. This pilot study also provided encouraging results for both AA and GBM though it was too small and the patients too heterogeneous to provide a reliable evidence of efficacy.
Based on this background information, we designed this phase II trial with the dual primary objectives of 1) determining whether poly-ICLC can produce a significant, objective radiographic response rate and 2) improve the 6-month progression free survival in patients with recurrent anaplastic glioma. The tolerance and toxicity of low-dose poly-ICLC has been described in previous studies but a secondary objective of this trial was to determine the toxicity of poly-ICLC in this patient population.
MATERIALS AND METHODS
Patient Eligibility
Patients who were at least 18 years of age with a histologically confirmed, anaplastic glioma (AA, AO, or AMO) were eligible. Pathological material was centrally reviewed and met criteria to be classified as World Health Organization grade III glioma. Patients were also eligible if the original histology was low grade glioma and a subsequent histological diagnosis of an anaplastic glioma was made. Additionally, patients must have shown unequivocal evidence for tumor recurrence by MRI or CT scan performed within 14 days prior to registration and on a steroid dosage that had been stable for at least 5 days.
Eligible patients must have had prior radiation therapy and must have an interval of greater than or equal to 4 weeks from the completion of radiation therapy to study entry. Patients with prior therapy that included interstitial brachytherapy or stereotactic radiosurgery required confirmation of true progressive disease rather than radiation necrosis based upon either PET or Thallium scanning, MR spectroscopy or surgical documentation of disease.
Eligible patients may have had treatment for no more than 2 prior relapses. Relapse was defined as progression following initial therapy (i.e. radiation +/− chemotherapy if that was used as initial therapy). The intent therefore was that patients may have had up to 3 prior therapies (initial therapy and treatment for 2 relapses). For patients who had prior therapy for a low-grade glioma, the surgical diagnosis of WHO grade III glioma was considered the first relapse.
Patients were required to have a Karnofsky Performance Score (KPS) status of ≥ 60 and an estimated survival time of greater than 8 weeks. Patients were also required to have appropriate hematological, renal, and hepatic status. No patients were pregnant or nursing. All patients were willing to practice birth control during and for two months after treatment. Each patient had recovered from the effects of surgery before entry into the study. All patients or their designated surrogates signed a consent form approved by the participating institution’s review board.
Study Design
This was a single arm, open-labeled, phase II study. All patients were evaluated for toxicity (including those later judged ineligible), radiographic response to therapy, 6-month progression free survival, and survival. A combination of standard neurological examination and neuro-imaging was used to define overall response or progression [27]. Pathology was centrally reviewed. Clinical and radiological assessments of disease status were performed every 8 weeks. Imaging was assessed by the treating physician starting 8 weeks following therapy initiation and at 8 week intervals thereafter. Patients achieving 6 months of progression free survival or deemed to have a partial or complete response on imaging were centrally reviewed.
Transient enlargement of contrast enhancing tumor with subsequent shrinkage has been reported during poly-ICLC treatment. Because of this possibility of “pseudo-progression,” the treating physician and patient had the option of continuing treatment if a patient had progressive disease by conventional definition; however, for the sake of statistical analysis the official progression date was defined as the initial MRI which demonstrated an enlargement of contrast enhancing disease. If patients continued on poly-ICLC on the premise of pseudo-progression the protocol further defined imaging criteria for radiological progression under which the patient was definitively discontinued from treatment (see Table 1 for criteria).
Table 1.
Radiological Criteria for Unacceptable Progression:
Transient enlargement of enhancing disease with subsequent shrinkage has been reported during poly-ICLC treatment. Because of this, if the patient has progressive disease by the conventional definition (≥ 25% increase in the sum of products of all measurable lesions over the smallest sum observed using the same techniques as baseline or appearance of any new lesion/site) but does not have unacceptable progression by the definitions below, the treating physician and patient have the options of continuing poly-ICLC treatment on this protocol or of discontinuing treatment.
|
Immunotherapy
Poly-ICLC was given at a dose of 20 mcg/kg three times weekly by intramuscular injection in 4 week cycles. The days of administration were at least 2 days apart (i.e., usually a Monday-Wednesday-Friday schedule). Poly-ICLC could be administered at any time of day, but it was recommended that each patient choose a consistent time for administration. The initial dose of poly-ICLC was administered in the presence of a physician or nurse who monitored the patient for at least 30 minutes after injection, including a determination of blood pressure, heart rate, and respiratory rate before and after injection. Patients were trained on the proper method for storing, preparing, and administering poly-ICLC and administered their own doses thereafter. Acetaminophen was used to treat fever or flu-like side effects of poly-ICLC. Patients were pretreated with acetaminophen as warranted by side effects at the discretion of the local investigator. Such pretreatment was recorded in the treatment diary. For fever, arthralgias, or myalgias of grade 2 or greater, poly-ICLC was discontinued for at least one dose. When symptoms returned to grade 0, poly-ICLC was resumed at 50% of the original dose. If further dosing was well tolerated, the original dose was subsequently re-instituted at the discretion of the investigator. All dose changes were recorded in the treatment diary. Corticosteroids were used in the smallest dose to control symptoms of cerebral edema, mass effect, and fatigue, and were discontinued if possible. Anti-seizure medications were used as indicated and patients were not stratified based on the use of enzyme-inducing anti-epileptic drugs.
Statistical Methods and Considerations
This study was initially designed with a primary endpoint of 6 month progression free survival (6moPFS). The results were to be compared with appropriately matched historical controls. The historical values were from a database of 150 recurrent AG patients enrolled in 8 previous phase II studies (in which none of the treatments were considered particularly effective). The proportion of AG patients remaining alive and free from progression at 6 months in the historical data was 31% [28]. The current trial was designed to have a greater than 90% probability of declaring failure if the true 6moPFS was 30% and over 90% probability of success if the true success rate were 50%.
The study was to utilize a 2-stage design. At the end of the first stage (22 patients) the study did not meet the PFS success rate required to continue. However some objective responses were observed (see Figure 1 for an example) and because poly-ICLC was very well-tolerated, it was decided to continue the trial to the original maximum target enrollment of 46 patients to better estimate this response rate. When this protocol change was implemented the primary focus of the analysis was changed to one of estimation. Response rates with exact confidence intervals were calculated. Kaplan Meier curves were used to estimate PFS and survival measured from time of study registration.
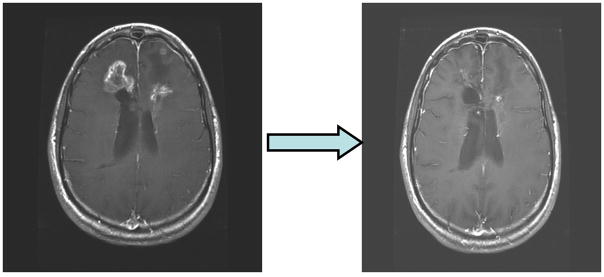
Figure 1.
An example of radiographic response. The axial T1-post contrast image to the left is the patient’s on-study MRI and the axial T1 post-contrast image to the right is after 4 cycles of poly-ICLC indicating partial response to therapy.
RESULTS
From 7/14/03 to 12/19/05 55 patients were enrolled in the study. The study was intended to have 46 patients. Additional patients were added because on central pathology review 9 of these patients had glioblastoma. Following closure of the study it was determined that 1 additional patient was ineligible; this patient had more than 3 prior treatments for relapse. Toxicity was recorded for those patients who were deemed ineligible and did not differ from eligible patients. Patient characteristics for the 45 eligible patients are shown in Table 2.
Table 2.
Patient Characteristics (n=45)
| Age, years | |
| Median | 43 |
| Range | 25–70 |
| Sex | |
| Male | 22 |
| Female | 23 |
| KPS | |
| Median | 90 |
| Range | 60–100 |
| Histology | |
| AA | 32 |
| AO | 10 |
| AMO | 3 |
| Race | |
| White | 40 |
| Asian | 2 |
| Black | 2 |
| Other | 1 |
| Imaging Response | |
| Partial Response | 5 |
| No Change | 18 |
| Progression | 22 |
Poly-ICLC was well-tolerated with little toxicity. There were the expected mild to moderate toxicities related to fatigue and the most common toxicity encountered was mild, temporary soreness at the injection site. Of all the events deemed to be possibly, probably, or definitely related to the therapy, most are grade 2 or less. No patients discontinued poly-ICLC because of toxicity. The relatively few cases of grade 3 adverse events are shown in Table 3. At the time of this analysis, 32 of 45 patients had died and 6 patients remain on treatment (see Table 4). Response to therapy as assessed on imaging and progression free survival data was known for all 45 eligible patients.
Table 3.
Grade #3 Toxicities using National Cancer Institute Common Toxicity Grading System 3.0
| Adverse events | # patients | Comment |
|---|---|---|
| DYSPNEA | 1 | Possibly related |
| ELEV GPT | 4 | 3 cases possibly related and 1 case probably related |
| HYPOXIA | 1 | Possibly related |
| LEUKOPENIA | 2 | Possibly related |
| MUSCLE WEAKNESS | 1 | Possibly related |
| SODIUM SERUM-HIGH | 1 | Possibly related |
| TREMORS | 2 | Possibly related |
Table 4.
Patient Characteristics for those still on treatment (n=6)
| Age/Gender | KPS | Histology | Imaging response | Treatment start date | Prior Chemo | Tumor Location | |
|---|---|---|---|---|---|---|---|
| 1 | 52 F | 100 | AO | PR | 3/23/2004 | TMZ | LF |
| 2 | 59 M | 90 | MA | NC | 6/10/2005 | TMZ | RT |
| 3 | 41 F | 100 | AA | NC | 7/20/2005 | TMZ | LF |
| 4 | 28 M | 90 | AA | NC | 7/25/2005 | TMZ | LF |
| 5 | 36 M | 80 | AO | PR | 8/2/2005 | TMZ, BCNU | BiF |
| 6 | 50 F | 90 | AA | NC | 12/19/2005 | TMZ | RF |
PR=partial response, NC= no change; AO= anaplastic oligodendroglioma; AA= anaplastic astrocytoma; MA =mixed oligoastrocytoma. LF= left frontal; RT= right temporal; biF= bifrontal; RF= right frontal.
Out of 45 patients, 5 had partial radiographic responses, 18 had stable disease, and 22 had progressive disease as their best response for an estimated objective response rate of 11% (95% c.i. 0.04 – 0.24). 11 of 45 patients remained progression free at 6 months thus the estimated 6-month progression free survival was 24%. The median survival time is 43 weeks (95% c.i. 27.4–106 weeks). Survival time was known for all 45 patients and 13 were censored as they were alive at last contact.
Despite the concern that transient enlargement of contrast enhancing tumor with subsequent shrinkage had been seen during poly-ICLC treatment, no patients on this study were continued on poly-ICLC past the initial MRI which indicated enlargement of contrast enhancing areas thought to be tumor growth.
DISCUSSION
Given the possible activity in AG, we treated patients with multiply recurrent AG with poly-ICLC in the hope that this treatment would elicit positive radiographic responses and improve 6moPFS while having minimal toxicity. Poly-ICLC was very well-tolerated. Fatigue, myalgia and pain at the injection site were the main adverse events. These events were not substantially different from the toxicities seen in the pilot trial which used poly-ICLC in high-grade glioma patients; nor were these events different from a recent trial in which newly diagnosed GBM patients were treated with poly-ICLC (see accompanying manuscript). No patients went off-study because of toxicity.
The 6moPFS for this study was 24% which is similar to the 6moPFS of 31% from a study performed by Wong et al. which pooled the results of 8 consecutive, negative phase II trials in patients with recurrent grade III astrocytomas [28]. A recent NABTC publication, which evaluated the importance of PFS as an end point in glioma studies, analyzed over 100 recurrent AG patients treated on phase II studies and found a 6moPFS of 47% for patients treated with TMZ versus 17% for similar patients treated as part of trials which did not include TMZ [29]. A study by Yung and colleagues gave TMZ to patients with AA or AMO, but at first relapse only, which resulted in a 6moPFS of 46% [8]. The difference between these studies is that Yung et al only enrolled patients at first relapse while the Wong et al and NABTC studies, similar to the present study, involved patients who had multiple relapses. As it is widely held that results for patients with multiple failures (more than 2 prior surgeries or chemotherapies) generally result in poorer outcome it is not unexpected that the Yung et al. study yielded superior results. This point raises the interesting possibility that the use of poly-ICLC at initial diagnosis or first recurrence for patients with AG may produce improved results which more closely resemble the results of the pilot study of poly-ICLC in which patients with newly diagnosed AA had a PFS of 77months. An equally important point to consider is that the pilot study also allowed the use of combination CCNU chemotherapy in 10 of 11 newly diagnosed patients with AA which likely influenced results. Given the more favorable toxicity profile of TMZ compared with CCNU it would be of interest to study the combination of TMZ and poly-ICLC in patients with newly diagnosed AG or at first relapse. In fact, the NABTT (New Approaches to Brain Tumor Therapy) Consortium has an ongoing Phase II trial of RT plus TMZ followed by adjuvant TMZ and poly-ICLC in patients with newly diagnosed GBM (seehttp://www.nabtt.org/protocols/poly.htm). A similar trial in AG patients would be of interest.
The radiographic response of 11% in this study is not a particularly favorable result. However, there is no accepted benchmark for comparison for radiographic response. 11% may not be encouraging as an indication of further study but objective radiographic response is rare in brain tumor studies and is an imprecise method of estimating efficacy especially when one considers that of the 6 patients who remain on treatment only 2 had a PR. Moreover, despite the possibility of transient enlargement of enhancing tumor caused by poly-ICLC, no patients on this study were continued on poly-ICLC past the initial MRI which indicated enlargement of contrast enhancing area; thus it is possible that some patients were prematurely discontinued from treatment. Also, most of the patients on this trial did not have surgical debulking prior to enrollment which arguably may make them even more susceptible to the possible inflammatory actions of poly-ICLC. This insight should be taken into consideration with the design of future immunotherapy in glioma patients. At a minimum, this difficulty also raises the question of whether PFS based on subjective interpretation of anatomic imaging should be used as an endpoint in evaluating outcome for such immunotherapies used in this patient population.
The aforementioned NABTC publication, which evaluated the importance of PFS as an end point, demonstrated a median survival of 52 weeks for recurrent AG patients treated with TMZ and 36 weeks for those not treated with TMZ. The median survival for this study is a comparable 43 weeks. The median survival in the Yung et al. study was 59 weeks which is superior to 43 weeks but derives itself from a patient population treated only at first relapse. Again, treatment at initial diagnosis or even first relapse with poly-ICLC, possibly in combination with TMZ, may render better results.
Limitations of this single-arm phase II study design include that it accrued a relatively small and heterogeneous patient population both in terms of number of relapses and differing histologies (AA may behave differently than AO or AMO). Another limitation is the potential for selection bias (young median age, high functional status) that may render more positive results; however, these variables were accounted for in the statistical analysis.
CONCLUSION
We report the results of a novel agent poly-ICLC for patients with recurrent AG. The therapy was relatively well tolerated, with the expected toxicities of fatigue, myalgia, and pain at the injection site. A radiographic response of 11% and 6moPFS of 24% are not encouraging of further study of single agent poly-ICLC in patients with multiply recurrent AG. However, based on the positive results of the original poly-ICLC pilot study in newly diagnosed AA and the potential efficacy of TMZ in this patient population, the combined use of poly-ICLC with TMZ presents interesting and relatively non-toxic possibilities for future investigations.
Acknowledgments
We would like to thank Oncovir Inc. for their support of this study.
References
- 1.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, et al. Anaplastic astrocytoma in adults. Crit Rev Oncol Hematol. 2007;63(1):72–80. doi: 10.1016/j.critrevonc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Walker MD, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303(23):1323–9. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- 4.Walker MD, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 5.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 6.Prados MD, et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: A retrospective review of radiation therapy oncology group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol. 1999;17(11):3389–95. doi: 10.1200/JCO.1999.17.11.3389. [DOI] [PubMed] [Google Scholar]
- 7.Levin VA, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18(2):321–4. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- 8.Yung WK, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17(9):2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 9.Droller MJ. Immunotherapy of metastatic renal cell carcinoma with polyinosinic-polycytidylic acid. J Urol. 1987;137(2):202–6. doi: 10.1016/s0022-5347(17)43953-x. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins MJ, Levin M, Borden EC. An Eastern Cooperative Oncology Group phase I–II pilot study of polyriboinosinic-polyribocytidylic acid poly-L-lysine complex in patients with metastatic malignant melanoma. J Biol Response Mod. 1985;4(6):664–8. [PubMed] [Google Scholar]
- 11.Krown SE, et al. Phase I trials of poly(I,C) complexes in advanced cancer. J Biol Response Mod. 1985;4(6):640–9. [PubMed] [Google Scholar]
- 12.Nakamura O, et al. Phase I. –II trials of poly(ICLC) in malignant brain tumor patients. J Interferon Res. 1982;2(1):1–4. doi: 10.1089/jir.1982.2.1. [DOI] [PubMed] [Google Scholar]
- 13.Rettenmaier MA, Berman ML, DiSaia PJ. Treatment of advanced ovarian cancer with polyinosinic-polycytidylic lysine carboxymethylcellulose (poly(ICLC] Gynecol Oncol. 1986;24(3):359–61. doi: 10.1016/0090-8258(86)90313-6. [DOI] [PubMed] [Google Scholar]
- 14.Theriault RL, et al. Evaluation of polyinosinic-polycytidylic and poly-L-lysine in metastatic breast cancer. Cancer Treat Rep. 1986;70(11):1341–2. [PubMed] [Google Scholar]
- 15.Talmadge JE, et al. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J Immunol. 1985;135(4):2483–91. [PubMed] [Google Scholar]
- 16.Ewel CH, et al. Polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose in combination with interleukin 2 in patients with cancer: clinical and immunological effects. Cancer Res. 1992;52(11):3005–10. [PubMed] [Google Scholar]
- 17.Black KL, et al. Inflammatory leukocytes associated with increased immunosuppression by glioblastoma. J Neurosurg. 1992;77(1):120–6. doi: 10.3171/jns.1992.77.1.0120. [DOI] [PubMed] [Google Scholar]
- 18.Talmadge JE, et al. Immunomodulatory effects in mice of polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose. Cancer Res. 1985;45(3):1058–65. [PubMed] [Google Scholar]
- 19.Levy HB. Historical overview of the use of polynucleotides in cancer. J Biol Response Mod. 1985;4(5):475–80. [PubMed] [Google Scholar]
- 20.Levy HB, Levine AS. Antitumor effects of interferon and poly ICLC, and their possible utility as anti-neoplastic agents in man. Tex Rep Biol Med. 1981;41:653–62. [PubMed] [Google Scholar]
- 21.Matsumoto M, Seya T. TLR3: Interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008 doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, et al. Double-stranded RNA mediates interferon regulatory factor 3 activation and interleukin-6 production by engaging Toll-like receptor 3 in human brain astrocytes. Immunology. 2008 doi: 10.1111/j.1365-2567.2007.02799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balachandran S, Barber GN. PKR in innate immunity, cancer, and viral oncolysis. Methods Mol Biol. 2007;383:277–301. doi: 10.1007/978-1-59745-335-6_18. [DOI] [PubMed] [Google Scholar]
- 24.Salazar AM, et al. Long-term treatment of malignant gliomas with intramuscularly administered polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose: an open pilot study. Neurosurgery. 1996;38(6):1096–103. discussion 1103–4. [PubMed] [Google Scholar]
- 25.Zhu X, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin VA, et al. Radiation therapy and bromodeoxyuridine chemotherapy followed by procarbazine, lomustine, and vincristine for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 1995;32(1):75–83. doi: 10.1016/0360-3016(94)00488-7. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald DR, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 28.Wong ET, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 29.Lamborn KR, et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162–70. doi: 10.1215/15228517-2007-062. [DOI] [PMC free article] [PubMed] [Google Scholar]