Abstract
TRP channels participate in many cellular processes including cell death. These channels mediate these effects mainly by changing the cellular concentration of Ca2+, a prominent cellular second messenger. Measuring the current-voltage relationship and state of activation of TRP channels is of utmost importance for evaluating their contribution to a cellular process within a spatial and temporal context. The study of TRP channels and characterization of their mode of activation will benefit and progress our understanding of each channel’s role in specific cellular mechanisms. Many TRP channels exhibit constitutive activity, which is mostly observed in cell based expression systems. This constitutive activity can lead, in many cases, to cellular degeneration, which can be readily observed morphologically and by biochemical assays. This chapter describes in brief, different modes of TRP channel activity and their current voltage relationships. The chapter outlines methods for visualizing this activity and methods to correlate between TRP channel activity and cell death, and it illustrates mechanisms that prevent cell death in spite of constitutive activity. Finally, it describes methods for qualitatively and quantitatively measuring the accompanied cellular degeneration.
1. Introduction
TRP channels constitute a large superfamily of channel proteins with diverse roles in many transduction and sensory mechanisms. The superfamily, which is conserved through evolution, consists of seven subfamilies and its members are expressed in many cell types, including excitable as well as non excitable cells (Damann et al., 2008). These channels participate in many sensory modalities, and they either open directly in response to ligands or physical stimuli (e.g. temperature, osmotic pressure or noxious substance) or, indirectly, downstream of a signal transduction cascade (e.g. phototransduction, (Katz and Minke, 2009; Ramsey et al., 2006). In many reports on various TRP channels, the channels reveal constitutive activity, mainly when expressed in tissue culture cells. Moreover, since usually there is no easy access to the channels where they natively reside (inaccessible membrane, tissue or organelle), it is difficult to determine their actual activity under physiologically relevant conditions. A good example for a system in which the native signal can be accessed easily, is the phototransduction cascade in the Drosophila eye, in which TRP and TRP-like (TRPL) channels are activated in response to light (Hardie and Minke, 1992; Niemeyer et al., 1996). In the photoreceptor cells, the channels are closed in the dark and open upon illumination. As will be discussed in brief below, we use electroretinogram (ERG) and whole cell recordings from isolated grouped photoreceptor cells, in order to characterize the electrical activity of the cells. This activity changes in response to light, due to light induced channel openings, (Cosens and Manning, 1969; Hardie and Minke, 1992; Minke et al., 1975). However, it must be pointed out that complete characterization, which entails single channel analysis, is still difficult to obtain, due to inaccessible photoreceptor membrane which expresses the channels (Delgado and Bacigalupo, 2009). On the other hand, the TRPML1 channel protein is an example of a channel, which is in the most part non accessible for electrophysiological research in its native surroundings (Dong et al., 2008), due to exclusive native expression in intracellular vesicles, such as lysosomes (Vergarajauregui and Puertollano, 2006). Another difficulty arising from measurements of TRP channel activity in native surroundings is their spatial and temporal channel activity. In native systems, the complexity of the channels regulation (activation, trafficking and post translational modifications) is for the most part unknown. There is evidence showing that some TRP channels are dynamic in their location and in the activity they exhibit within a time frame. Our ability to perform experiments on these channels might be affected by their physiological state, because they reveal signal dependent translocation between the surface membrane and intracellular compartments (Bahner et al., 2002; Cronin et al., 2006; Meyer et al., 2006; Stein et al., 2006). This could hinder our ability to interpret correctly the data obtained from experiments on native systems. For these reasons, we and others perform much of the research on TRP channels in cell culture expression systems with the aim of gaining insight into channel function. The use of expression systems for TRP channel research has many advantages: (1) The channels are for the most part easily expressed in a functional manner.(2) The dissection of biophysical properties such as conductance, mean open time and permeability are readily obtained (Parnas et al., 2007). (3) In many cases, insight into the gating mechanisms can be achieved. (4) In many cases, TRP channels exhibit basal constitutive activity on the plasma membrane, which allows investigating TRP channels whose mode of activation is unknown. In spite of these advantages, care should be taken upon interpretation of results obtained from channels expressed in any expression system, which may show behavior differing from a native system. It is therefore advisable to compare between a specific TRP channel activity in a native system and that gained from an expression system. A good example for this methodology is the research on Drosophila TRPL channels conducted in our lab. Many of the properties were readily obtained in expression system and then verified with physiological relevance in the photoreceptor native system. In general, a major difficulty of TRP channel research, is the limited pharmacological tools (e.g. activators, inhibitors), which in many cases are non specific. The fact that many channels have basal activity means that they are expressed functionally. This activity is helpful in cases where TRP channels mode of activation and pharmocology are unknown. Not all TRP channels (e.g. Drosophila TRP channel) have been successfully expressed in heterologous expression systems ((Minke and Parnas, 2006) but see (Xu et al., 1997)). In such cases, the inaccessibility of these channels to single channel investigation in native cells puts a constraint on the full characterization of the channels. Without comparing the properties of a channel in both the native system and expression system, one cannot rule out that properties obtained in an expression system are different from those of the native system. Moreover, certain channels can display differing active states in different expression systems. For example, we have observed that the Drosophila TRPL channel reveals constitutive activity in the Schneider 2 (S2) and insect Spodoptera Frugiperda 9 (Sf9) expression systems (Chyb et al., 1999; Hu and Schilling, 1995), whereas when expressed in Human Embryonic Kidney (HEK) cells, these channels are closed, resembling the state of the channel in the photoreceptor cells, in the dark (Agam et al., 2000; Lev et al., 2010). This example demonstrates that different expression systems can affect channel properties differently. This point has to be taken into consideration when choosing an expression system. It would be advisable to compare activities of a TRP channels in several expression systems in order to gain insight into channel regulation modes. Furthermore, differences in the basal activity can also be attributed to the expression system at hand and not necessarily indicate for inherent properties. Constitutive activity of TRP channels can be attributed to the direct activation of the channel (Grimm et al., 2007), or the channel can be activated by a known or unknown upstream element, which is constitutively active. This is mostly true for those TRP channels which are activated downstream of a transduction cascade (e.g. TRPC channels which are receptor mediated). In this respect, the Drosophila TRPL channel expressed in S2 cells might be affected by constitutive endogenous phospholipase C (PLC) activity. Constitutive activity of TRP channels may lead to detrimental cellular degeneration because of their Ca2+ permeability, or because of a change in the resting membrane potential. This topic is extensively discussed below and constitutes a main subject in this review. As noted above, there are channels which show plasma membrane expression but no constitutive activity even in expression systems, such as wild type TRPML2 and TRPML3 channels expressed in (HEK) cells (Lev et al., 2010).
In conclusion, constitutive activity of a TRP channel can be beneficial in facilitating its characterization but can also lead to non physiological effects with phathological ramifications on both cellular and higher level functions. The scope of this chapter is to review TRP channels with and without constitutive activity, which lead to cellular degeneration in some but not all cases. We will also review several methods for determining the active state of TRP channels and describe methods for determining cellular degeneration and its quantification.
2. TRP channels and cellular degeneration
Constitutive and non-constitutive TRP channel activity can lead in many cases, to Ca2+ dependant cell death, via apoptotic and secondary necrotic processes (for review see (Dadon and Minke, 2010)). The degeneration is induced by Ca2+ overload or by change in Ca2+ homeostasis (Orrenius et al., 2003). Other cations entering the cells via TRP channels besides Ca2+ can lead to cell death (Carini et al., 1999) by changing the resting potential level of the cells (van Aken et al., 2008). The fact that most TRP channels exhibit Ca2+ permeability constitutes a potential role for these channels to participate in processes of cell death under physiological or pathophysiological conditions. TRPM2 was shown to affect the susceptibility of cells to death, both in native and heterologous TRPM2 expressing cells, in response to stimulation by oxidative stress (Hara et al., 2002; Zhang et al., 2003). TRPM7 has also been shown to be involved in cell death under conditions of ischemia (Sun et al., 2009). In addition, TRP channels from the TRPC subfamily have been implicated in apoptosis-mediated cell death when activated, possibly due to Ca2+ influx leading to Ca2+ overload (for review see (Dadon and Minke, 2010)). As stated above, in many cases, TRP channel activation has been linked to susceptibility to cellular degeneration. In addition, there are many examples of TRP channels which posses gain of function mutations, which also lead to cell death in a similar fashion. This is the case for the spontaneously occurring varitint-waddler mutation found in mouse TRPML3 expressing cells. In these cells, degeneration underlies cellular malfunction leading to behavioral pathology such as hearing loss and impaired vestibular function (Grimm et al., 2007; Nagata et al., 2008; Xu et al., 2007). We and others have shown that this mutation is conserved among other TRPML channels, leading to cellular degeneration as well (Lev et al., 2010).
To conclude, in some cases, TRP channels actively participate in programmed cell death. While in other cases, an increase in TRP channel activity (augmentation) is detrimental. Therefore, the activity level of TRP channels is crucial for maintaining cellular mechanisms at their physiological homeostatic point in native surroundings. As mentioned above, TRP channel activity in expression system can differ from the native system. Therefore caution must be taken when drawing conclusions from such systems.
3. Constitutive TRP channel activity which does not lead to cellular degeneration
In this section we briefly describe several TRP channels displaying activity which is not accompanied by cellular degeneration. We outline the current-voltage relationship (I–V curve) of different TRP channel types and point to those, which would probably not be associated with cellular degeneration. We also describe the methods used to measure the constitutive activity, mainly the whole-cell patch clamp recording technique. This technique is not a typical high throughput technique, in which a vast population of channel expressing cells can be evaluated, although there is advanced instrumentation in this direction by multiple electrodes performing simultaneous patch clamp recordings. The patch clamp technique gives insight into the I–V curve of a specific channel. Its power is in unfolding basic biophysical properties of a channel. This information is used to determine the characteristics and amplitude of the constitutive activity, which can be used to predict cell viability.
3.1 Examples of TRP channels displaying constitutive activity
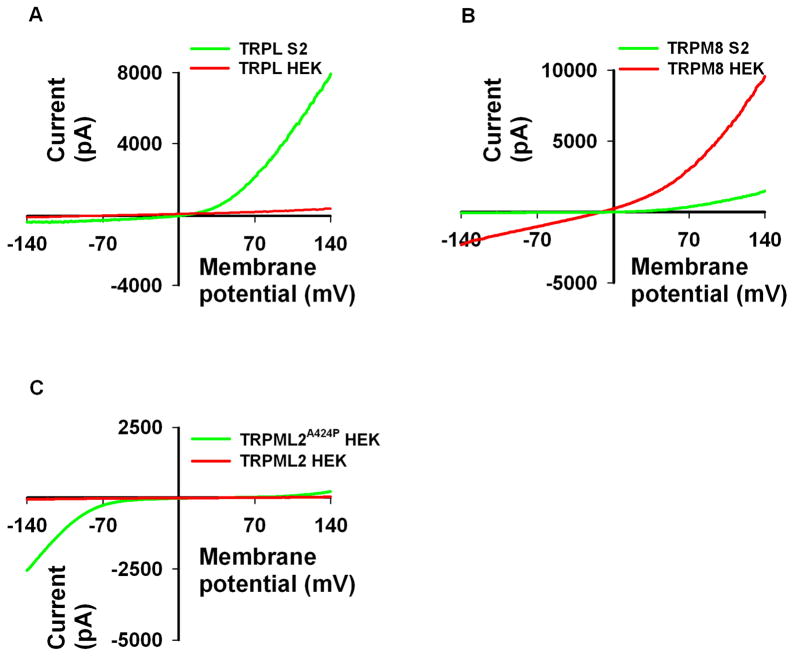
Since most TRP channels are permeable to Ca2+, it is plausible to assume that unregulated TRP channel activity, whether it is constitutive or not would not occur under normal physiological conditions. Nevertheless, many TRP channels reveal constitutive activity (e.g. TRPL, TRPM8, TRPV1, TRPV5/6) when expressed in tissue cultured cells. The open probability of these channels can be further enhanced by enzymatic or pharmacological means, which facilitates their whole cell currents. Some TRP channels reveal constitutive activity in one expression system and not in another ((Lev et al., 2010), Fig. 1A) and some reveal basal activity in all expression systems used (Fig. 1B). There are several examples of TRP channels which when mutated alter their activity and become constitutively active (Fig. 1C). This is observed for all TRPML channels possessing the alanine to proline (A to P) amino acid substitution (Va mutation) at the paralogous site in the transmembrane segment 5 (Dong et al., 2008; Grimm et al., 2007; Lev et al., 2010; Samie et al., 2009; Xu et al., 2007). This change in activity in a mutated channel was also observed for the Drosophila TRP channel with the phenyl alanine to isoleucine (F550I) mutational substitution, causing photoreceptor degeneration (Yoon et al., 2000). On the other hand, the wild type TRPML channels do not display any significant current ((Xu et al., 2007), Fig. 1C), albeit their surface membrane expression. The same is true for the Drosophila TRP channel in the dark.
Fig. 1. Constitutive activity of TRP channels in heterologous expression systems.
A, Representative I–V curves as measured from HEK and S2 cells expressing the Drosophila TRPL channel (red and green respectively). Note that TRPL in HEK cells does not reveal constitutive channel activity, which can be displayed by linoleic acid (LA) application, whereas when expressed in S2 cells, it displays a pronounce outwardly rectifying current. B, Representative I–V curves as measured from S2 and HEK cells expressing TRPM8 (green and red respectively). Note that TRPM8 reveals constitutive activity in both expression systems albeit differences in current magnitude. C, Representative I–V curves as measured from HEK cells expressing wild type human TRPML2 (red) and TRPML2A424P (i.e. the Va mutant, (green)). Note how the varritint waddler paralogous mutation conveys constitutive activity to TRPML2 in HEK cells. Wild type human TRPML2 has been shown to be surface membrane bound.
Constitutive activity of TRP channel can be investigated when upstream elements of its activating cascade, display constitutive activity. This was shown for the Drosophila TRP channels in the Drosophila retinal degeneration A (rdgA) phototransduction mutant (Raghu et al., 2000). In Drosophila photoreceptor cells, mutations in Diacylglycerol (DAG) kinase (RDGA), the enzyme which converts DAG into phosphatidic acid, induces constitutively active TRP and TRPL channels in the dark. This leads to photoreceptor degeneration. Similar constitutive activity of the TRP and TRPL channels was demonstrated upon anoxic conditions in the fly eye, which is thought to affect PLC activity (Agam et al., 2000).
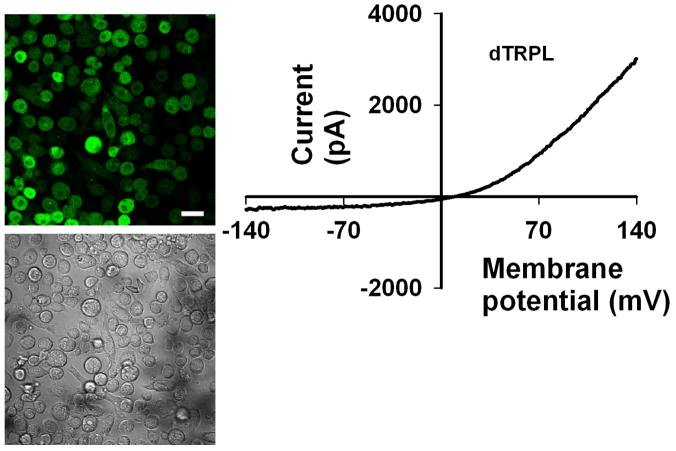
It must be emphasized that only a fraction of the TRP channels displaying constitutive activity cause cellular degeneration, (see below). A notable example for mammalian TRP channels showing constitutive activity of both native and heterologously expressed channels are the epithelial TRP channels TRPV5/6 (for review see (den et al., 2003). These are highly Ca2+ selective channels with distinctive physiological functions important for body Ca2+ homeostasis. Cells expressing these channels do not undergo degeneration in spite of their inwardly rectifying I–V curves (see below). A possible mechanism which prevents Ca2+ overload in cells expressing these channels is their strong Ca2+ dependent negative feedback regulation, leading to relatively fast Ca2+ dependent inactivation of the channels (den et al., 2003). Several of the channels described above are not associated with cellular degeneration, presumably because the channels display an outwardly rectifying I–V curve (see below) and a minute current flow into the cells at resting membrane potentials, due to Ca2+ dependent negative feedback mechanisms (i.e. TRPV1 (Lukacs et al., 2007; Rosenbaum et al., 2004), TRPM8 (Rohacs et al., 2005), TRPL, TRP ((Parnas et al., 2007), Fig. 2 left, images, right, I-V curve with outward rectification). However, when trying to predict a link between I-V curves and cell viability, one must consider the Ca2+ selectivity, resting potential and other endogenous channel activities present.
Fig. 2. TRP channels with constitutive activity and no degeneration.
Left, Wide-field confocal images showing representative cell morphology of S2 cells transfected with the indicated construct. Drosophila TRPL-GFP fluorescence (top) and DIC (bottom) images are presented, Scale bar 20 μm. Right, Representative I–V curve of whole-cell currents measured from S2 cells, expressing the indicated channel, displaying robust outward rectifying current.
3.2 Methods for determining constitutive activity
3.2.1 Whole cell patch clamp recordings
In this and the following sections, we do not describe standard protocols, which are readily available.
The most revealing technique for determination of channel activity is the whole cell patch clamp recording technique. This method is used for electrophysiological recordings of channel activity in native systems (such as isolated grouped photoreceptor cells) and in many expression systems (such as the Drosophila S2 cell line and the mammalian HEK cell line). In this method, a cell is approached with a borosilicate glass pipette. We suggest using capillaries containing glass filament inside them. The filament is important for easy back filling of the pipette with intracellular solution, due to capillary tension. Our experience leads us to recommend glass pipettes, which have a large inner radius and yet a large inner to outer segment, which allows the cell membrane attachment to the glass electrode with a tighter seal (higher resistance). We use glass pipettes with an outer 1.0 mm and inner 0.58 mm diameter for clamping photoreceptor and S2 cells. For clamping HEK cells, we use outer 1.5 mm and inner 1.1 mm diameter. In many cases we also briefly (less than one second) heat the tip in order to melt and smoothen out the tip (heat polishing). This also greatly helps in creating a tight seal between the electrode and the cell membrane.
Under stereo microscope visualization, using a micromanipulator, we approach the cell with the glass electrode applying positive pressure in the electrode in order to avoid particles from sticking to the tip upon entry into the extracellular solution. The pressure is applied via a “tygon” typed tubing connected to a 1cc syringe. We approach the cell gently within the first third of the cell until one sees a dent in the cell (this is indicated by an optical black contour lining). At this point in time we release the positive pressure and apply a minute negative pressure to form the gigaseal. Sometimes, the seal will form upon release of positive pressure. With some cells, applying negative pressure either with mouth or by way of the syringe will complete the seal. At this stage, it is possible to look at single channel activity in the cell attached mode. In this mode, it is possible to determine the voltage at the outer membrane side of the patch. The membrane potential is the voltage difference between the inner and outer parts of the membrane patch, while the physiological resting membrane potential in most cells is between −20 to −90 mV (depending on other channel activities present in cells, such as potassium channels and on specific cellular properties). In this part of the procedure, it is possible to pull and rupture the membrane patch from the cell (called inside out patch). Our experience leads us to suggest a quick and strong pull upwards with the micromanipulator (~ half a turn- 500μm). Sometimes it is advisable to lift the electrode outside the solution and back in, which causes a physical detachment of the membrane patch, due to air solution effect on membranes. If the inside-out technique is completed well, then a gigaseal is maintained. When doing so, any voltage clamping by the electrode is valid for the membrane potential of this patch. In this configuration it is also possible to look at single channel activity devoid of most of the cytosolic elements, which could affect channel activity. It has to be noted that in this configuration, the inner cytosolic part of the channel is facing towards the bath solution, which should be changed to intracellular solution, while the electrode solution should be changed to extracellular solution, in order to mimic physiological conditions. After performing a gigaseal, it is possible to rupture the membrane patch by applying pressure or by giving a short (0.2–1 msec duration) current pulse (zap) confined in duration. Rupturing the membrane connects chemically and electrically between the pipette content and the cell interior. In this mode (whole cell recording) it is important to make sure that the only conductance seen is that of the expressed channels. One has to make sure that the resistance of the cell is not impaired by a low resistance seal between cell and electrode (i.e. less than 1 gigaohm). In many cases (see below), the activity state of the channels can be large so that the input resistance is lower than 1 gigaohm. The input resistance can be displayed by applying a seal test (this function is a built in property of the amplifier). The amplitude of the test, together with the resting potential of the cell and the channel’s reversal potential (Erev) have to be considered when performing the seal test and receiving a value for the input resistance. Most TRP channels have an Erev around 0mV in normal conditions. If the channels display constitutive activity, the seal test might show a lower resistance just because these channels are open. It is therefore important to apply pharmacological agents or enzymatic intervention to close the channels in order to reveal endogenous currents, or currents arising from an improper seal (leak current). At this point, we advise to perform two kinds of voltage clamping protocols. One should perform I–V curves by stepping the holding voltages from negative to positive voltages. Each step should be at least several hundreds of milliseconds in duration to allow observation of fast current changes and steady state. The other protocol is a voltage ramp, in which we change the voltage from negative to positive potentials continuously within one second. If both protocols give the same I–V curve then it is easier to pursue all further experiments with the voltage ramp protocol. The advantage of this protocol is the ability to look at the full current voltage relationship within a short time scale. It allows application of voltage ramps repeatedly every few seconds, giving a kinetics profile of the activity at positive as well as negative membrane potentials.
3.2.1.1 I–V curves and channel voltage dependence
When measuring an I–V curve, there can be several possibilities related to the activity state of the channel: (1) the channel is integrated to the surface membrane but it is non-functional; (2) the channel is functional but it is not integrated to the plasma membrane; (3) the functional channel is integrated to the plasma membrane but does not have constitutive activity. As mentioned, many TRP channels display basal activity, which indicates functional integration to the surface membrane. Different TRP channels exhibit differing I–V curves. For example, in the presence of divalent cations, TRPC3/4/5/6, TRPM4/5/6/7/8, TRPV1/2/3 and Drosophila TRP and TRPL channels, all reveal outward rectifying I–V curves (Fig. 1, A, B, (Clapham, 2003; Hardie and Minke, 1994)). TRPML1/2/3 (Xu et al., 2007) and TRPV5/6 (Owsianik et al., 2006) have an inward rectifying I–V curve (Fig. 1C) and TRPM2/3/4/5 have a linear I–V curve (Clapham, 2003). The voltage dependence (i.e. rectification) observed in most TRP channels can be either an intrinsic property of the channels or due to divalent cation open channel block (Parnas et al., 2007). If a non linear I–V curve remains non linear even after removal of divalent cations, as in the case of TRPV1 or TRPM8 (Nilius et al., 2005), then the voltage dependence is an intrinsic property of the channel. When removal of divalent cations leads to a linear I–V curve, as in the case for TRPL or TRPM7 (Nadler et al., 2001; Parnas et al., 2007), then the non linearity arises from open channel block. A change in extra or intra cellular ion concentrations can underlie and facilitate the open probability of the channels in native systems, leading to inward cationic currents, which could affect cell viability (Wei et al., 2007). Furthermore, there are TRP channels which produce an inward current when lowering the intra or extra cellular divalent ion concentration (e.g. Mg2+ or Ca2+) as in the case for TRPV5 (Lee et al., 2005; Vennekens et al., 2000), TRPV6 (Bodding and Flockerzi, 2004) and Drosophila TRP (Hardie and Minke, 1994).
3.2.1.2 Endogenous currents in expression systems
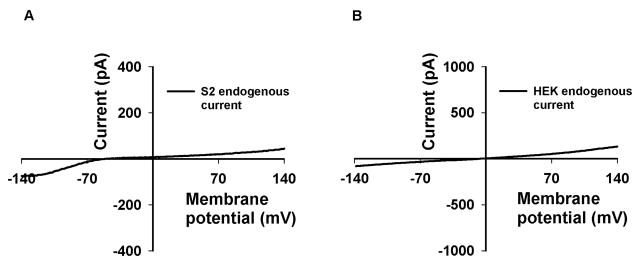
It is important to identify the endogenous channel activity of the cells used for TRP channel expression (Lev et al., 2010). Many tissues natively express multiple types of TRP channels and there too, it is essential to recognize the specific TRP channel activity. There are several ways to separate between the various channel activities: (1) A pharmacological approach, in which one blocks a specific activity, unveiling the other (e.g. application of μM La3+ to Drosophila photoreceptor cells blocks the TRP but not the TRPL channel activity (Hardie and Minke, 1992)). (2) A genetic approach, in which TRP channels are eliminated (silenced using RNAi). (3) Clamping the membrane potential to values in which one channel activity is prominent and the other is negligible. In our research project with the TRPML channels, we meticulously characterized the endogenous current in the S2 expression system used to express TRPML2/3. In S2 cells, the endogenous current is small and almost negligible. At positive membrane potentials, it is mostly nonexistent, whereas at negative membrane potentials, it appears to have a small saturating inward rectifying voltage dependence with an Erev at around −40 mV (Fig. 3A). In many cases, the endogenous current is nonexistent. In the HEK cell expression system that we also used for expression of TRPML1/2/3, the endogenous current is virtually absent when using intracellular solution containing Cs+ instead of K+ (Fig 3B). In all cases, we perform extensive control experiments to identify the TRP channel activity. Other control experiments are performed using the large, TRP channel impermeable, organic cation N-Methyl-D-glucamin (NMDG), in order to verify that the current is carried by cations, in a non specific manner (Lev et al., 2010). Several other experiments can be performed to identify the selectivity and ion permeability of a specific TRP channel (Kim et al., 2008; Kim et al., 2010; Xu et al., 2007).
Fig. 3. Endogenous currents in S2 and HEK expression systems.
A, Representative I–V curve of endogenous currents, as measured from S2 cells, by whole-cell patch-clamp recordings. Note the distinguishable inward rectifying, albeit small current, which is easily separated from currents in S2 cells expressing TRP channels (see Fig. 1A, B). B, Same as A except representative I–V curve from HEK cells in control conditions. Note the lack of detectable current arising from absence in channel activity. The non-significant linear leak current is displayed.
3.2.2 Ca2+ imaging
Calcium imaging is widely used to determine activity of TRP channels which have been activated (Siemens et al., 2006), or show prominent constitutive activity, which is characterized by inward rectification (Xu et al., 2007). Many TRP channels, which reveal outward rectification can be further activated (facilitated) resulting in opening of the channel at negative membrane potentials upon signaling, leading to increased intracellular Ca2+ (Varnai et al., 2006). The advantage of this technique is in its quick and high throughput readout. On the other hand, a channel can be in an open state and yet exhibit low or no Ca2+ influx (e.g. as is the case for TRPM4/5, which are Ca2+ impermeable). This can be explained by the channel’s I–V curve, open channel block or low permeability to Ca2+. Since most TRP channels are permeable to Ca2+, we recommend using Ca2+ imaging as a start point and later on applying electrophysiological tools to further characterize the observed activity. Many channels that exhibit constitutive activity display outward rectifying I–V curve. In this paradigm, the Ca2+ imaging technique might not show a change in Ca2+ levels, because of the small inward current, and therefore will not give an indication for constitutive activity. On the other hand, inwardly rectifying TRP channels with constitutive activity and a certain extent of Ca2+ permeability, will display this constitutive activity in terms of intracellular Ca2+ levels (Grimm et al., 2007; Xu et al., 2007). The Ca2+ indicator used should have a Kd which fits the calcium concentrations observed. In many cases it is advisable to apply ionomycin (a non specific Ca2+ ionophore) which serves as an inner positive control in cases where the amplitude of the calcium signal is not known.
The Ca2+ imaging method
Cells, which have been transfected with TRP channels, are then loaded with a Ca2+ indicator. Many indicators can be used, although the widely used reagents are the cell permeable Fura-2 AM and Fluo-4 AM. One should add Fluo-4 AM at a final concentration of ~1μM (higher concentrations may act as Ca2+ buffers) to clean medium (no antibiotics, no fetal calf serum) and incubate for half an hour. It is suggested to add the Fluo-4 AM, which is dissolved in a DMSO stock solution containing 10% Pluronic F127 to medium and then add the cells to that medium as to minimize DMSO toxicity. During the half hour incubation, the indicator enters the cell where the AM hydrophobic component is cleaved by an endogenous esterase enzyme. There is a partitioning of the indicator between the inside and extracellular solution. Some of the indicator is in the plasma membrane. After this incubation, the cells are washed twice with clean medium to rid the extracellular solution from any additional indicator. This is followed by incubation in clean medium with 2% Pluronic F127 for half an hour. This incubation is helpful in releasing the indicator from the plasma membrane into the cell, in order to avoid a continuance inflow of indicator into the cell during the experiment. After the incubation time, cells are rinsed thoroughly but gently with clean medium. The cells are now ready for imaging. We use the Fluo-4 AM indicator which is excited by Green Fluorescence Protein (GFP) excitation spectrum. For imaging, we use confocal microscopy and a monochrome laser at a wave length of 488 nm.
Since the cells are at resting membrane potential, constitutive activity will be unmasked only if there is robust inward current, which is also carried by Ca2+. Moreover, in order to measure the elevated Ca2+ levels, one should compare the indicator fluorescence to control (untransfected cells) or change the extracellular Ca2+ levels.
In a typical protocol, the cells are bathed in Ca2+-free solution and basal Ca2+ levels are monitored. Then, cells are exposed to normal (~2 mM) or higher Ca2+ levels, while observing a change in fluorescence inside the transfected cells. Then, the extracellular solution can be changed back again to a low Ca2+ level to exhibit reversibility. One should be aware of the change in intracellular Ca2+ levels, which occurs due to the Na+/Ca2+ exchanger (Fig. 4). This must be accounted for and separated from the signal arising from channel activity (Peretz et al., 1994).
Fig. 4. The intracellular basal Ca2+ concentration is affected by extracellular Ca2+.
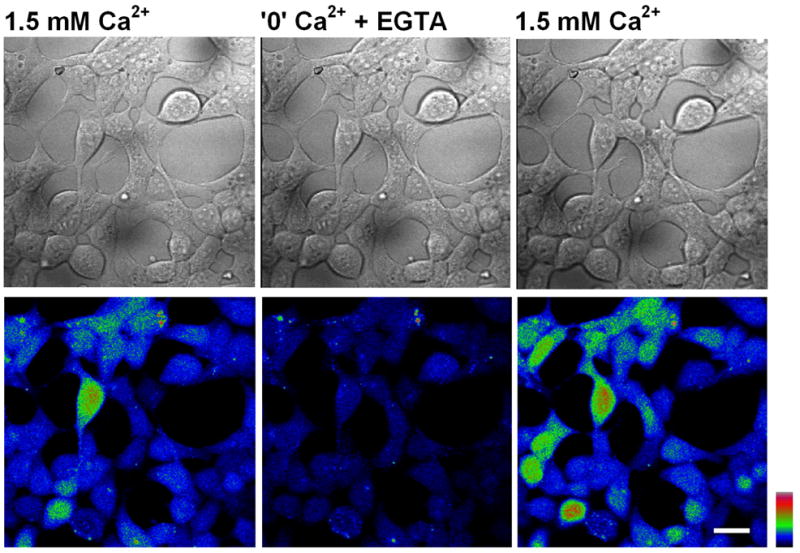
Wide-field confocal images of native HEK cells loaded with Fluo4-AM indicator, displaying resting [Ca2+]i in different levels of extracellular Ca2+, as indicated above (color coding indicates higher Ca2+ levels towards the warm colors). Note how the intracellular Ca2+ concentration decreases significantly, upon lowering of the extracellular Ca2+concentration, in a reversible manner (middle panel). This is most likely due to the activity of the Na- Ca2+ exchanger. Scale bar 20 μm.
3.2.3 Physiological mechanisms that are affected by constitutive channel activity
It is reasonable to assume that known physiological mechanisms can be altered when a constitutively active channel is present. In this case the activity does not have to be confined to the plasma membrane, and electrophysiological techniques would “miss” the activity. In order to show constitutive channel activity, the TRP channel has to be directly linked to a known cellular mechanism and this mechanism must have a clear readout. One example of such a mechanism is the phototransduction cascade in the fly, which allows the fly to see. The Drosophila TRP and TRPL channels constitute the last element in this transduction cascade, leading to cation influx and subsequent depolarization when light is turned on. Without these channels, the fly is blind. A phototaxis bio assay (i.e. the attraction of flies to light) can differentiate between loss of function, gain of function and “normal” activity (Choe and Clandinin, 2005; Cosens and Manning, 1969). One could anticipate a change in phototaxis behavior in flies expressing constitutively active TRP and TRPL channels.
4. Constitutive TRP channel activity which leads to cellular degeneration
In this section we describe TRP channels which have constitutive activity leading to cellular degeneration. The major causes of degeneration in cells expressing constitutively active TRP channels is Ca2+ influx leading to Ca2+ overload in the cells. The nature of the degeneration can be either necrotic or apoptotic, which can be differentiated easily by specific assays and also by the morphological changes, which accompany the degeneration process.
4.1 Examples of TRP channels which exert a degenerating effect
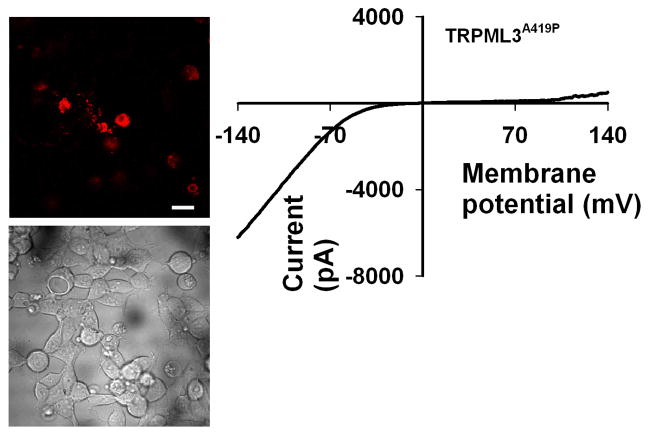
All three TRPML channels exhibit constitutive activity when possessing the Va mutation. The Va missense mutation (substitution of alanine or valine with helix breaking proline) is considered to introduce a kink in the transmembrane 5 segment (TM5 domain), thereby rendering the channel in the open state. This mutation was introduced into other TRP channels from other TRP channel subfamilies, reproducing the same effect as in TRPMLs (e.g. TRPV5 and TRPV6 (Grimm et al., 2007)). The channel activity is characterized by an inwardly rectifying I-V curve. The inward current is robust and is carried by cations including, but not limited to Ca2+. The Erev is around 0 mV, which is typical for non selective cation channels. In the case of all TRPMLs carrying the Va mutation, it is the Ca2+ overload which leads to cellular degeneration (Fig. 5 left, images of degeneration, right, I–V curve). Another TRP channel that leads to cellular degeneration is the founding member of the TRP superfamily, the Drosophila TRP channel. Photoreceptor degeneration is revealed in the constitutively active trpP365 mutant, because of unregulated channel activity leading to Ca2+ overload (Wang et al., 2005; Yoon et al., 2000). Although the mutated TRP channel displays mainly an outward current, a small but significant inward (mainly Ca2+) current is observed in the dark, leading to photoreceptor degeneration (Yoon et al., 2000). Later, it was shown that a single missense mutation (Phe-550 to Ile) in TM5 is sufficient to account for the degeneration observed in the original mutant, which harbors additional 3 mutations in TM4 and TM5 (Hong et al., 2002).
Fig. 5. TRP channels with constitutive activity show degeneration.
Left, Wide-field confocal images showing representative cell morphology of HEK cells transfected with the indicated construct. tdTomato-TRPML3A419P fluorescence (top) and DIC (bottom) images are displayed. Scale bar, 20 μm. Note that most fluorescent cells (top) also show degeneration, as displayed in transmitted image (bottom). Right, Representative I–V curve of whole-cell currents measured from HEK cells, expressing the indicated channel which displays robust inward rectifying currents.
Strikingly, metabolic stress induces constitutively active TRP and TRPL channels in the dark, in a reversible manner, in-vivo (Agam et al., 2000). A continuous uncontrolled and maximal activation of these channels, most likely leads to photoreceptor cell death due to Ca2+ overload (Agam et al., 2004). This conclusion is strongly supported by the trpP365 mutation which makes the channel constitutively active with a magnitude similar to anoxia.
4.2 Methods for determination of the degenerating effect
4.2.1 Morphological changes associated with cellular degeneration
As mentioned in section 2, it is easy to distinguish between normal viable cells and degenerating cells. It is always important to have a control which undergoes the same experimental manipulations with the tested cells expressing TRP channels to rule out possible degeneration effects originating from the experimental procedure.
We use S2 or HEK cell lines for transfection. The transfection procedure should be optimized to prevent a degenerating affect by virtue of transfection. A fluorescent marker or tag must be included in the transfection to allow separation between transfected and non transfected cells. In our experience, we have used, with best results, TRP channels conjugated to the color probe, tdTomato, because of its high fluorescent yield and the fact that cells usually do not have autofluorescence in the long wavelength range. It is advisable to have the channels conjugated rather than co expressed with a color probe to make sure that the TRP channel has been expressed. It is best to have several transfection plates to allow demonstration of the onset and kinetics of the degeneration process (start point and intensity). According to previous publications (Grimm et al., 2009) and our experience, degeneration starts around 10–15 hours post transfection with TRPML channels bearing the paralogous Va mutation in HEK cells, or wild type TRPML2 in S2 cells. The first step is to look at a cover slip with cells under low microscope magnification. This gives a good estimate whether the cells have undergone degeneration. Next, the cover slip is taken to a fluorescent microscope for complete evaluation of the channel expression and its effect on cell viability. One should count the number of degenerating cells out of the cell population, which have undergone TRP channel expression. The morphological markers used to separate between degenerating and non degenerating cells are: rounding up of the cells when observing HEK cells ((Xu et al., 2007), S2 cell are round under normal conditions), detachment of cells from the cover slip (this is to be considered when imaging those cells which are attached), swelling, total deterioration (loss of normal morphology), distorted contour of cells and membrane roughness of cell surface. Not all cells will have all morphological changes, because each cell can be at a different stage of the degenerating process. It is very important to recognize all features of the cell type used and how the cells look normally, before analyzing degeneration. The experiment should be carried out in a double blind fashion. Several areas of the plate are chosen randomly for scanning by confocal microscope, which gives a high spatial resolution (it is also possible to perform experiments with a regular fluorescent microscope, as long as morphology of the cells is readily seen). The cells should be imaged as Differential Interference Contrast (DIC) and fluorescence (Fig. 5A left bottom). Once the images have been acquired, the fluorescent cells are counted and the percent of degenerating cells is determined. This should formulate the degree of degeneration for a specific expressed channel. It is then possible to examine whether the degeneration results from Ca2+ overload by co expressing the calcium extrusion pump, PMCA2, which rescues the cells from degeneration (Grimm et al., 2009; Lev et al., 2010). Another option is to grow transfected cells in minimal Ca2+ medium before imaging and immediately after transfection, in order to rescue cells from cellular degeneration. This experimental procedure was used to show a causal relationship between TRPML’s constitutive activity and intracellular Ca2+ overload (Kim et al., 2008). According to unpublished observations made by us, when expressing TRPML2/3 harboring the paralogous Va mutation, we suggest reducing external Ca2+ from 1.5 mM Ca2+, (i.e. the physiological concentration in the extracellular solution), to ~ 50 μM. Lower Ca2+ concentrations, usually lead to lower cell viability.
4.2.2 Annexin based assay for determination of cellular degeneration
The Annexin based assay allows determination of the extent of degeneration, and whether it is necrotic or apoptotic. One can use other assays such as PI (Propidium Iodide, a general marker for cellular deterioration), Caspase 3 activity, nuclear staining and DNA laddering to describe cellular degeneration and its characteristics. The Annexin assay is based on the translocation of Phosphatidylserine (PS) to the outer leaflet from the inner leaflet of the plasma membrane at an early stage of the apoptotic process. This asymmetry, which is mediated by an inside-outside PS translocase, is necessary for the cells recognition by macrophages, for their removal (Allen et al., 1997). The fact that this process is an inherent part of programmed cell death in all cells is utilized as an early biochemical marker, which can indicate for apoptosis before secondary degeneration proceeds.
To apply the Annexin method, one needs to grow cells on coverslips and transfect them with fluorescent protein constructs. Eighteen hours post-transfection, one should dilute Alexa Fluor 647 Annexin V (BioLegend) in Binding Buffer (BioLegend) and incubate with transfected cells for 15 min at room temperature in the dark. Other Annexin conjugated probes are available depending on the fluorescent setup used and other fluorescent markers expressed in cells (conjugated fluorescent TRP channels). Following incubation with Annexin V, the cells are washed quickly with Binding Buffer and then fixed in the dark in 4% formaldehyde for 30 min at room temp. Following three washes with 1% NH4Cl (in PBS), coverslips are mounted onto glass slides with Antifade Solution (Vysis) and fluorescent images of Alexa Fluor 647 Annexin V staining, together with fluorescent protein emission are acquired in a confocal microscope (e.g. Olympus Fluoview 300 IX70). The readout of such an experiment is the number of Annexin positive cells from the total number of channel expressing cells (Fig. 6B). It is possible and also more accurate to use FACS analysis to quantify this degeneration. In combination with this assay, it is possible to look at various treatments such as co expression with the Ca2+ extrusion pump to assess the contribution of Ca2+ overload to the degenerating effect.
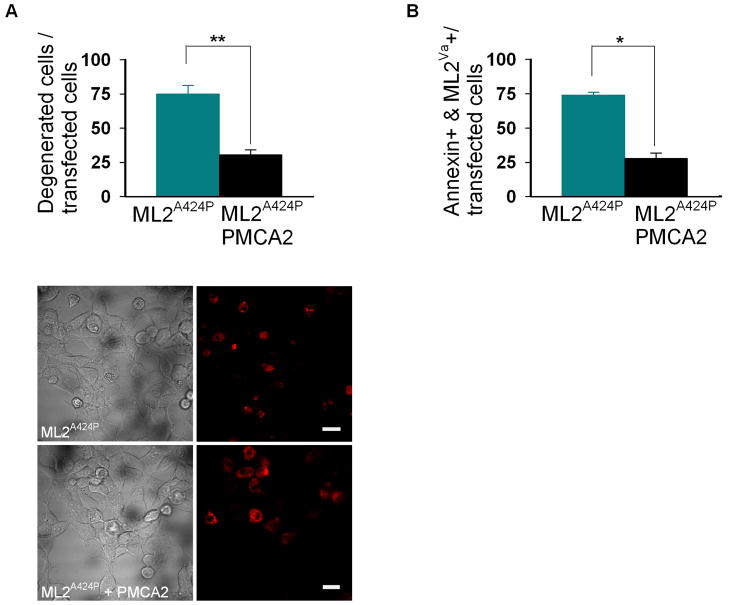
Fig. 6. Ca2+ induced degeneration caused by constitutive activity of h-TRPML2A424P is rescued by the calcium extrusion pump, PMCA2.
A, top, histogram showing the percent of degenerated HEK cells following co-transfection of h-TRPML2-A424P (ML2-A424P) together with PMCA2 or empty vector (**, P < 0.01). Note the rescue of cell degeneration in cells co-transfected with h-TRPML2A424P and PMCA2. A, bottom, wide field confocal images (left- DIC transmitted, right- fluorescent conjugated channel expressing cells) showing representative cell morphology in the two groups of co-transfected HEK cells described above (scale bar 20 μm). Note that most cells expressing the channel without PMCA2 display degeneration (distinct changes in morphology). B, histogram showing the percent of Annexin V-positive HeLa cells following co-transfection of h-TRPML2A424P with PMCA2 or with empty vector (*, P < 0.05). Note the rescue of cell degeneration in cells co-transfected with h-TRPML2A424P and PMCA2.
4.3 Summary of the methods for viewing cellular degeneration
As stated above, it is advisable to use morphological alterations to distinguish between viable and non viable cells when expressing a specific TRP channel in conjunction with other methods. Other methods use biochemical markers for determining degeneration, as we have done in our studies. Using several methods gives a more comprehensive description of the type of cell death and verification of each method is achieved. It is easy to see that both methods result in the same degree (percentage wise) of degeneration and that rescue of degeneration can also indicate the initial etiological effector which is Ca2+ overload in our case (Fig. 6, A, top histogram of morphological alterations, bottom, wide field images of cells exhibiting degeneration. B, histogram of Annexin positive TRP channel expressing cells).
Acknowledgments
We thank Ben Katz, Daniela Dadon and Maximilian Peters for careful and critical reading of the manuscript.
The experimental part of this review was supported by grants from the National Institute of Health (RO1-EY 03529), the German-Israel Foundation (GIF) the Israel Science Foundation (ISF) and the US-Israel Binational Science Foundation (BSF)
Reference List
- Agam K, Frechter S, Minke B. Activation of the Drosophila TRP and TRPL channels requires both Ca2+ and protein dephosphorylation. Cell Calcium. 2004;35:87–105. doi: 10.1016/j.ceca.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Agam K, von-Campenhausen M, Levy S, Ben-Ami HC, Cook B, Kirschfeld K, Minke B. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RT, Hunter WJ, III, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods. 1997;37:215–228. doi: 10.1016/s1056-8719(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Bodding M, Flockerzi V. Ca2+ dependence of the Ca2+-selective TRPV6 channel. J Biol Chem. 2004;279:36546–36552. doi: 10.1074/jbc.M404679200. [DOI] [PubMed] [Google Scholar]
- Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280–293. doi: 10.1006/excr.1999.4408. [DOI] [PubMed] [Google Scholar]
- Choe KM, Clandinin TR. Thinking about visual behavior; learning about photoreceptor function. Curr Top Dev Biol. 2005;69:187–213. doi: 10.1016/S0070-2153(05)69007-2. [DOI] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Lieu MH, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- Dadon D, Minke B. Cellular functions of Transient Receptor Potential channels. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–R889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J Neurophysiol. 2009;101:2372–2379. doi: 10.1152/jn.90578.2008. [DOI] [PubMed] [Google Scholar]
- den DE, Hoenderop JG, Nilius B, Bindels RJ. The epithelial calcium channels, TRPV5 & TRPV6: from identification towards regulation. Cell Calcium. 2003;33:497–507. doi: 10.1016/s0143-4160(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci U S A. 2007;104:19583–19588. doi: 10.1073/pnas.0709846104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Jors S, Heller S. Life and death of sensory hair cells expressing constitutively active TRPML3. J Biol Chem. 2009;284:13823–13831. doi: 10.1074/jbc.M809045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:409–427. doi: 10.1085/jgp.103.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YS, Park S, Geng C, Baek K, Bowman JD, Yoon J, Pak WL. Single amino acid change in the fifth transmembrane segment of the TRP Ca2+ channel causes massive degeneration of photoreceptors. J Biol Chem. 2002;277:33884–33889. doi: 10.1074/jbc.M204075200. [DOI] [PubMed] [Google Scholar]
- Hu Y, Schilling WP. Receptor-mediated activation of recombinant Trpl expressed in Sf9 insect cells. Bichem J. 1995;305:605–611. doi: 10.1042/bj3050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Li Q, Tjon-Kon-Sang S, So I, Kiselyov K, Soyombo AA, Muallem S. A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J. 2008;27:1197–1205. doi: 10.1038/emboj.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yamaguchi S, Li Q, So I, Muallem S. Properties of the TRPML3 pore and its stable expansion by the varitint-waddler causing mutation. J Biol Chem. 2010 doi: 10.1074/jbc.M109.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Cha SK, Sun TJ, Huang CL. PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+ J Gen Physiol. 2005;126:439–451. doi: 10.1085/jgp.200509314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Zeevi DA, Frumkin A, Offen-Glasner V, Bach G, Minke B. Constitutive activity of the human TRPML2 channel induces cell degeneration. J Biol Chem. 2010;285:2771–2782. doi: 10.1074/jbc.M109.046508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 2007;27:7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer NE, Joel-Almagor T, Frechter S, Minke B, Huber A. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J Cell Sci. 2006;119:2592–2603. doi: 10.1242/jcs.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Parnas M. Insights on TRP channels from in vivo studies in Drosophila. Annu Rev Physiol. 2006;68:649–684. doi: 10.1146/annurev.physiol.68.040204.100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- Nagata K, Zheng L, Madathany T, Castiglioni AJ, Bartles JR, Garcia-Anoveros J. The varitint-waddler (Va) deafness mutation in TRPML3 generates constitutive, inward rectifying currents and causes cell degeneration. Proc Natl Acad Sci U S A. 2008;105:353–358. doi: 10.1073/pnas.0707963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Talavera K, Owsianik G, Prenen J, Droogmans G, Voets T. Gating of TRP channels: a voltage connection? J Physiol. 2005;567:35–44. doi: 10.1113/jphysiol.2005.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Parnas M, Katz B, Minke B. Open channel block by Ca2+ underlies the voltage dependence of Drosophila TRPL channel. J Gen Physiol. 2007;129:17–28. doi: 10.1085/jgp.200609659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000;26:169–179. doi: 10.1016/s0896-6273(00)81147-2. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol. 2004;123:53–62. doi: 10.1085/jgp.200308906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samie MA, Grimm C, Evans JA, Curcio-Morelli C, Heller S, Slaugenhaupt SA, Cuajungco MP. The tissue-specific expression of TRPML2 (MCOLN-2) gene is influenced by the presence of TRPML1. Pflugers Arch. 2009;459:79–91. doi: 10.1007/s00424-009-0716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Zhou S, Piskorowski R, Nikai T, Lumpkin EA, Basbaum AI, King D, Julius D. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE. Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol. 2006;128:509–522. doi: 10.1085/jgp.200609576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- van Aken AF, Atiba-Davies M, Marcotti W, Goodyear RJ, Bryant JE, Richardson GP, Noben-Trauth K, Kros CJ. TRPML3 mutations cause impaired mechano-electrical transduction and depolarization by an inward-rectifier cation current in auditory hair cells of varitint-waddler mice. J Physiol. 2008;586:5403–5418. doi: 10.1113/jphysiol.2008.156992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. Journal of Cell Biology. 2006;175:377–382. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ. Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem. 2000;275:3963–3969. doi: 10.1074/jbc.275.6.3963. [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S, Puertollano R. Two di-leucine motifs regulate trafficking of mucolipin-1 to lysosomes. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Xu H, Oberwinkler J, Gu Y, Hardie RC, Montell C. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Wei WL, Sun HS, Olah ME, Sun X, Czerwinska E, Czerwinski W, Mori Y, Orser BA, Xiong ZG, Jackson MF, et al. TRPM7 channels in hippocampal neurons detect levels of extracellular divalent cations. Proc Natl Acad Sci U S A. 2007;104:16323–16328. doi: 10.1073/pnas.0701149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Li L, Dong X, Clapham DE. Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint-waddler mice. Proc Natl Acad Sci U S A. 2007;104:18321–18326. doi: 10.1073/pnas.0709096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZS, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Yoon J, Cohen Ben-Ami H, Hong YS, Park S, Strong LLR, Bowman J, Geng C, Baek K, Minke B, Pak WL. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci. 2000;20:649–659. doi: 10.1523/JNEUROSCI.20-02-00649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K, Miller BA. A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem. 2003;278:16222–16229. doi: 10.1074/jbc.M300298200. [DOI] [PubMed] [Google Scholar]