Abstract
Obesity is an exaggeration of normal adiposity and is a central player in the pathophysiology of diabetes mellitus, insulin resistance, dyslipidemia, hypertension, and atherosclerosis, largely due to its secretion of excessive adipokines. Obesity is a major contributor to the metabolic dysfunction involving lipid and glucose, but on a broader scale, it influences organ dysfunction involving cardiac, liver, intestinal, pulmonary, endocrine, and reproductive functions. Inflammatory, insulin-resistant, hypertensive, and thrombotic-promoting adipokines, which are atherogenic, are counterbalanced by anti-inflammatory and anti-atherogenic adipocyte hormones such as adiponectin, visfatin, and acylation-stimulating protein, whereas certain actions of leptin and resistin are pro-atherogenic. Adiponectin is protective against liver fibrosis due to its anti-inflammatory effect, whereas inflammatory cytokines such as tumor necrosis factor-α are detrimental for both fatty liver and pancreatic insulin release. Obesity contributes to immune dysfunction from the effects of its inflammatory adipokine secretion and is a major risk factor for many cancers, including hepatocellular, esophageal, and colon. Because of the accelerating effects that obesity has on the worsening of metabolic syndrome and cancer, it has the potential to be profoundly detrimental to our species if major methods of prevention and/or effective treatment are not realized. It is essential then to institute major educational efforts aimed at promoting better eating habits and physical exercise.
Keywords: Pathophysiology, obesity, lipotoxicity, inflammatory, cytokines, clinical correlations
General Considerations
Much has been learned in the past decade regarding the regulation of obesity as it relates to the molecular regulation of appetite that affects energy homeostasis, particularly as positive energy balance upsets lipid and glucose metabolism.1,2 Furthermore, obesity appears to play a central role in the dysregulation of cellular metabolism that accounts for insulin resistance in diabetes mellitus type 2. Excess adipocytes secrete numerous cytokines that contribute to vascular dysfunction in hypertension and dyslipidemia, as manifested by hypercholesterolemia and triglyceridemia. These conditions eventually contribute to significant atherosclerosis, and when associated with obesity and/or diabetes and insulin resistance, they constitute the metabolic syndrome.3,4 New knowledge related to fatty liver and its association with inflammation, as well as visceral adiposity's effect on gastroesophageal reflux, gallstone disease, and cancer of the bowel, also make the liver and gut vulnerable to comorbidities of obesity.4–7 A detailed explanation of the pathophysiology of obesity, or excess adiposity, and its comorbidities follows.
Dysregulation of Lipid and Glucose Metabolism: Lipotoxicity and Insulin Resistance in Obesity
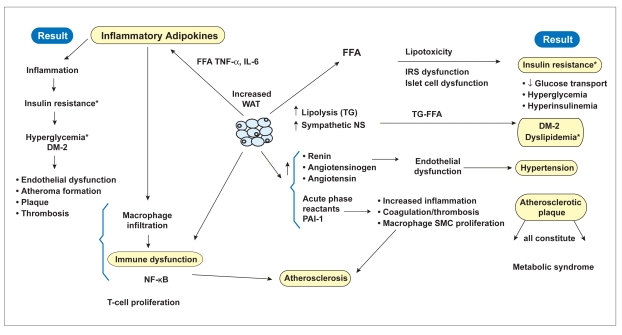
The abundance of stored fat is required for survival during nutritionally deprived states such as starvation. In times of prolonged abundance of food, however, very efficient fat storage results in the excessive storage of fat, eventually resulting in obesity.8–10 It has been hypothesized that the storage of fatty acid as triacylglycerol within adipocytes protects against fatty acid toxicity; otherwise, free fatty acids would circulate freely in the vasculature and produce oxidative stress by disseminating throughout the body. However, the excessive storage that creates obesity eventually leads to the release of excessive fatty acids from enhanced lipolysis, which is stimulated by the enhanced sympathetic state existing in obesity. The release of these excessive free fatty acids then incites lipotoxicity, as lipids and their metabolites create oxidant stress to the endoplasmic reticulum and mitochondria. This affects adipose as well as nonadipose tissue, accounting for its pathophysiology in many organs, such as the liver and pancreas, and in the metabolic syndrome.11,12 The free fatty acids released from excessively stored triacylglycerol deposits also inhibit lipogenesis, preventing adequate clearance of serum triacylglycerol levels that contribute to hypertriglyceridemia. Release of free fatty acids by endothelial lipoprotein lipase from increased serum triglycerides within elevated β lipoproteins causes lipotoxicity that results in insulin-receptor dysfunction. The consequent insulin-resistant state creates hyperglycemia with compensated hepatic gluconeogenesis. The latter increases hepatic glucose production, further accentuating the hyperglycemia caused by insulin resistance. Free fatty acids also decrease utilization of insulin-stimulated muscle glucose, contributing further to hyperglycemia.13,14 Lipotoxicity from excessive free fatty acids also decreases secretion of pancreatic β-cell insulin, which eventually results in β-cell exhaustion (Figure 1).15
Figure 1.
Role of lipotoxicity and inflammation on obesity. White adipose tissue (WAT) releases pre–fatty acids and adipokines, which are lipotoxic and inflammatory and result in diverse effects, outlined in the left-hand columns. Their correlation to the metabolic syndrome is shown on the right-hand column, whereas all the effects culminate in atherosclerosis on the bottom of the figure.
*Perturbed glucose and lipid metabolism.
- DM-2
- diabetes mellitus-2
- FFA
- free fatty acids
- IL
- interleukin
- IRS
- insulin receptor substrate
- NF-KB
- nuclear factor kappa beta
- NS
- nervous system
- PAI-1
- plasminogen activator inhibitor-1
- SMC
- smooth muscle cell
- TG
- triglyceride
- TNF
- tumor necrosis factor.
The Specific Role of Adipocyte Inflammatory Secretagogues (Adipocytokines), Including Effects of Hypertension, Macrophage, and Immune Functions
Sites and Function of Adipokines
Adipocytes, consisting of over one billion cells, not only store triacylglycerol in fat depots in various body sites to provide energy reserves, but in aggregate constitute the largest endocrine tissue that constantly communicates with other tissues by adipocyte-released secretagogues, such as the proteohormones lectin, adiponectin, and visfatin. Along with insulin, these proteohormones help regulate body-fat mass.16,17 Other gene groups that contribute to adipocyte adipokines include cytokines, growth factors, and complement proteins.17 These include the inflammatory adipokines tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 that cause local steatonecrosis, but are also distributed by the vascular system and cause inflammation elsewhere.18 The enhanced fat content in muscle becomes so significant in severe obesity that whole-body magnetic resonance imaging reveals cumulative fat depots in muscle sites similar in size to that of total visceral adipose tissue.19 Buttock fat appears to be largely inert with respect to endocrine function, as this fat is used largely for long-term energy reserves.20 Visceral fat depots release inflammatory adipokines, which, along with free fatty acids, provide the pathophysiologic basis for comorbid conditions associated with obesity such as insulin resistance and diabetes mellitus type 2.21 Visceral adipokines are transported by the portal vascular system to the liver, enhancing nonalcoholic steatohepatitis (NASH), and also by the systemic circulation to other diverse sites. Along with fatty-acid lipotoxicity, visceral adipokines also contribute to the adipokine inflammatory injury that leads to pancreatic β-cell dysfunction, which, in turn, decreases insulin synthesis and secretion.
Role of Specific Adipokines
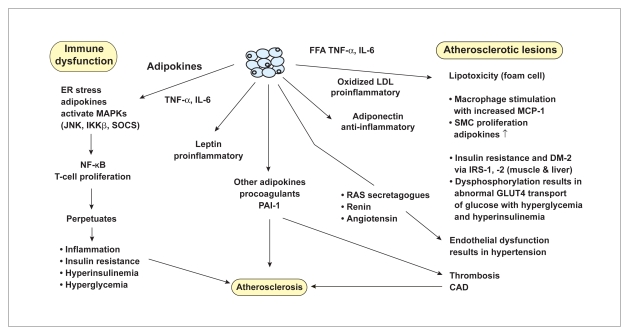
Dyslipidemia, hypertension, and atherogenesis are comorbid conditions, in addition to insulin resistance, that are associated with obesity and adversely influenced by the secretion of diverse inflammatory adipokines, particularly from white adipose tissues (WAT) in visceral fat depots.22 Specific adipokines enhance endothelial vasomotor tone by secreting renin, angiotensinogen, and angiotensin II, which are similar to those within the renal renin-angiotensin system (RAS), but when secreted from adipocytes, enhance hypertension in obese patients.23 TNF-α secretion increases in proportion to increased total body-fat mass and enhances inflammation in fatty livers and fat depots elsewhere, particularly in pancreas, mesentery, and gut visceral sites.24 Inflammatory markers that are increased in obesity commonly contribute to inflammatory conditions such as NASH25 and in the bronchial tree of patients with obstructive sleep apnea.26 These markers include not only TNF-α and IL-6, but also acute-phase reactants such as C-reactive protein, α1 acid glycoprotein, and the specific amyloid antigen, particularly in the fatty liver.23 The acute-phase reactants are important inflammatory markers that are also upregulated in the insulin-resistant state associated with diabetes mellitus type 2 and NASH.25,27 Adipocytes also stimulate fat-associated macrophages that also secrete monocyte chemoattractant protein 1 (MCP-1), macrophage migration inhibiting factor (MMIF), and resistin, all of which decrease insulin sensitivity (ie, enhance insulin resistance).23,24,28–30 These macrophages contribute to the enhanced inflammatory state and, as immune stimulators, enhance the mitogenactivated protein kinase family (C-Jun N-terminal Kinase, inhibitor of nuclear factor kappa beta [NF-KB] Kinase b, and phosphatidylinositol 3-Kinase), inducing the transcription factor NF-KB that allows dephosphorylation of the IRS-1 and -2 docking proteins. The latter inhibits the GLUT4 transporter of glucose, resulting in insulin resistance (Figure 2).31,32
Figure 2.
Role of inflammation and immune dysfunction in obesity. The immune dysfunction (left column) and inflammation (center column with arrows) are correlated with atherosclerotic lesions (right column).
- CAD
- coronary artery disease
- DM
- diabetes mellitus
- ER
- endoplasmic reticulum
- FFA
- free fatty acids
- IKKβ
- inhibitor of NF-KB kinase b
- IL
- interleukin
- IRS
- insulin receptor substrate
- JNK
- Jun N-terminal kinase
- LDL
- low-density lipoproteins
- MAPK
- mitogen-activated protein kinase
- MCP-1
- monocyte chemotactic protein
- NF-KB
- nuclear factor kappa beta
- PAI-1
- plasminogen activator inhibitor-1
- RAS
- renin angiotensin system
- SMC
- smooth muscle cell
- SOCS
- suppressor of cytokine signaling
- TNF
- tumor necrosis factor.
The progressive proinflammatory state resulting from increased obesity that promotes insulin resistance also perpetuates atherogenesis throughout its development, from early endothelial fatty streaks to late-plaque formation, rupture, and thrombosis. Endothelial modulators—such as vasoactive endothelial growth factor,33 plasminogen activator inhibitor-1,34 angiotensinogen, renin, and angiotensin II31—are secreted by white fat cells, in particular by perivascular fat tissues that contribute to vasomotor dysfunction and cause hypertension and endothelial injury.35 This process is followed by the formation of foam cells following the enhanced endothelial uptake of oxidized low density lipoproteins, free fatty acids, and other lipid metabolites that accumulate as a result of fatty acid peroxidation—all of which originate from dyslipidemic β-lipoproteins. Both endothelial and adipose cell lipoprotein lipase activity are also decreased by inflammatory cytokines such as IL-6, so that by inhibiting lipolysis they increase serum triacylglycerol levels accentuating hyper-triglyceridemia.32,36–38 Later, as atherosclerosis progresses with macrophage and smooth–muscle cell infiltration, there is additional secretion of other cytokines, such as MCP-1, MMIF, and endothelin-1, that enhance the evolving inflammatory lesions of atherosclerotic plaques within the vascular wall.4,28 Other adipokine procoagulants include plasminogen activator inhibitor-1, IL-6, tumor growth factor-β, and TNF-α, which cause thrombosis, particularly from ruptured atherosclerotic plaques.6,34 Progression of atherosclerosis with plaque formation and remodeling of collagen results from the action of matrix metalloproteinases also secreted by adipocytes.39 This activity causes atheroma cap thinning and plaque rupture that precipitates release of the tissue factor, also promoting intravascular thrombosis.39 Adipokines also enhance angiogenesis and promote adipogenesis by neovascularization enhancement of WAT.34 Figure 2 shows immune and inflammatory mediator effects on the comorbidities of obesity, including atherosclerosis.
Anti-inflammatory Secretagogues
To counter these injurious inflammatory secretagogues, adipose cells also secrete anti-inflammatory hormones, such as adiponectin, visfatin, and the complement-related acylation-stimulating protein, which exert beneficial effects inhibiting inflammatory adipokines. In this fashion, protective hormones and complement proteins become both anti-inflammatory and anti-atherogenetic in action, as they concomitantly enhance insulin sensitivity and improve vascular endothelium dysfunction. This effect is most obvious when these anti-inflammatory adipokines become deficient, as when adiponectin levels decrease with increasing obesity.17 It is probable that adiponectin receptor deficiency, inflammatory adipokines, as well as excessive fatty acids, all contribute to insulin resistance and other comorbidities of obesity—including hypertension, dyslipidemia, and atherosclerosis—as obesity is the common cause of these disorders. Interestingly, leptin may act as both an anti-inflammatory and pro-inflammatory secretagogue, in that it enhances insulin sensitivity for glucose uptake in muscle but promotes inflammation and angiogenesis at other sites (Table 1).
Table 1.
Adipocyte Secretagogues—Mechanism of Action
| Adipokine Promoters | Inhibitors or Atheroprotective |
|---|---|
| Inflammatory | Anti-inflammatory |
| IL-1, IL-6, TNF-α, IFN-α, IFN-β IL-8, IP-10, MCP-1, TGF-β, leptin Resistin, RANTES, markers of inflammation,* immune regulators† | IL-10, IL-4, TGF-β |
| Hypertensive | Antihypertensive |
| Renin, angiotensin system, angiotensinogen, angiotensin II | Angiotensin II receptor blocker |
| Insulin resistance | Insulin sensitivity |
| TNF-α, IL-6, resistin | Adiponectin, leptin, AgRP, MMIF, acylation-stimulating protein (stimulates glucose transport) |
| Procoagulant | Anticoagulant |
| Plasminogen activator inhibitor-1, tissue factor TNF-α, IL-6, TGF-β | Adiponectin |
| Angiogenetic | Atheroprotective |
| Leptin, IL-8, VEGF, FGF-2, MCP-1, IP-10, VCAM, ICAM-1, monobutyrin | Adiponectin |
| Lipogenetic (adipogenesis) | Lipolysis |
| Agouti protein insulin-like growth factor 1, angiotensinogen, angiotensin II, acylation-stimulating protein, visfatin | TNF-α, IL-6 |
Markers of inflammation: CRP, SAA, fibrinogen (acute phase reactants).
Immune regulators: IL-6, IL-8, MMIF, leptin.
- AgRP
agouti-related protein
- CRP
C-reactive protein
- FGF
fibroblastic growth factor
- ICAM
intercellular adhesion molecule
- IFN
interferon
- IL
interleukin
- IP-10
interferon &gamma
- inducible protein-10
- MCP
monocyte chemoattractant protein
- MMIF
macrophage migration inhibiting factor
- RANTES
regulate upon activation of novel T-cell expression sequences
- SAA
serum amyloid A
- TGF
tumor growth factor
- TNF
tumor necrosis factor
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor.
In summary, inflammatory, insulin-resistant, hypertensive, and thrombotic-promoting adipokines that are atherogenic are counterbalanced by anti-inflammatory and anti-atherogenic adipocyte hormones, such as adiponectin, visfatin, and acylation-stimulating protein, whereas certain actions of leptin and resistin are pro-atherogenic.
The Clinical Manifestations of Obesity
The Associated Inflammatory State in Obesity as a Major Contributor to the Metabolic Syndrome X
The understanding of the pathophysiology of obesity and its comorbidities reveals the central role that obesity plays as a result of the action of inflammatory adipokines in metabolic syndrome X. These comorbidities include diabetes mellitus type 2, whereby insulin resistance is worsened by TNF-α and other inflammatory adipocyte secretagogues21; endothelial dysfunction and hypertension, which results from the activity of RAS-secreting adipokines33,40; and dyslipidemia, which is caused by hypercholesterolemia and hypertriglyceridemia. These comorbidities and the effects of fatty acid lipotoxicity41 culminate to promote atherogenesis, including coronary artery disease. All these disorders are adversely affected by enhanced upregulation of NF-KB from visceral WAT inflammatory adipokines.3,42,43 Other conditions that appear to contribute to metabolic syndrome include chronic renal disease,44 obstructive sleep apnea,45 and nonalcoholic fatty-liver disease.46 In each disorder, immune responses engendered by inflammation (Figure 2) accentuate the dysfunction of the involved tissue, whether in adipose tissue, muscle, or vascular endothelium, or in multiple organs such as the liver, heart, and kidney. In summary, the profound effect that these inflammatory adipokines have on obesity and its comorbidities, including atherosclerosis,3,4,5,6 makes obesity the number one preventable public health problem in the United States and an increasing risk factor in the rest of the world.47–50
Obesity and Cancer
Besides the profound effect that obesity has on the manifestations of inflammation in many tissues and organs, it is a major risk factor for many forms of cancer, including breast, colon, endometrial, esophageal, hepatocellular, renal, and prostate cancer. A mechanism for this association was first realized when hyperinsulinemia was found to be a risk factor for colon cancer in obese patients.51 The combined effects of diabetes, insulin resistance, and increased body-mass index (BMI) were all later determined to contribute to the pathogenesis of colorectal cancer.51 Obesity accounts for 20–33% of the risk for breast, esophageal, endothelial, and kidney cancer.52,53 Mechanisms of carcinogenesis or tumor growth include perturbed cellular proliferation, dedifferentiation and/or apoptosis, angiogenesis, and chronic adipokine-associated inflammation, along with the effects of cancer genes and/or environmental toxins that enhance inflammation. Examples of adipose tissue adipokines that promote cancer include stimulating insulin-like growth factor-1 and other growth hormone secretagogues, such as leptin that enhance cellular proliferation and/or dedifferentiation.54,55
In a landmark paper, Calle and colleagues56 determined that among men who did not initially have cancer and who were then followed for 16 years, those with BMIs of 30–34.9 had a 20% higher death rate from prostate cancer, whereas those with BMIs of 35–39.9 had a 34% higher death rate compared with men with normal BMIs. Although testosterone itself is a key prostate growth factor that may enhance cellular proliferation, it was speculated that enzymatic conversion of testosterone to estradiol within cells of benign prostatic hypertrophy caused them to dedifferentiate into prostate cancer cells.57–59 A meta-analysis of multiple studies showed a relationship between obesity and advanced cancer but not early prostate cancer.60,61
Hepatocellular cancer is also linked to the associated comorbidity of fatty liver in obesity, which, after progressing from steatonecrosis to cirrhosis, becomes a risk factor for hepatocellular cancer. High leptin levels are also found in these obese patients and may be a growth-promoting factor for this cancer.62 Pancreatic cancer may be linked to obesity as a result of associated inflammatory adipokines, which not only upset glucose transport, causing insulin resistance, but combined with hyperinsulinemia, hyperglycemia, and lipotoxicity, all may lead to pancreatic β-cell inflammation and their exhaustion. It is speculated that the pancreatic dysplasia resulting from chronic inflammation associated with chronic pancreatitis promotes progression to pancreatic adenocarcinoma.7,63 Although depression and hypercoagulable states are features of pancreatic cancer, both conditions are enhanced in obese patients with pancreatic cancer.
Similarly, multiple etiologies may contribute to both obesity and chronic inflammation that are risk factors for esophageal carcinoma. The chronic inflammatory state related to the chronic esophageal acid reflux common in obesity results in Barrett esophagus, whose pathologic hallmark is intestinal metaplasia. This may also be accentuated by chronic adipokine injury from visceral periesophageal adiposity appearing to enhance the progression of metaplasia to high-grade dysplasia, the premalignant precursor to esophageal carcinoma. Further complications of visceral adiposity include hiatal hernial formation and its associated decreased esophageal sphincter function, which, with increased abdominal pressure from visceral adiposity, further enhances gastric reflux.64 In addition, increases in the leptin levels seen in obesity may also contribute to cellular proliferation, dedifferentiation, and inhibition of apoptosis in this cancer.65
Adipokine secretagogues such as unbound insulin-like growth factor also enhance angiogenesis, which promotes cancer growth in general.66 Adiponectin, the adipocyte-secretory proteohormone, protects against angiogenesis, but decreased adiponectin levels in obesity allows the progression from enhanced angiogenesis to cancer.67,68 Cancer-promoting factors enhanced by estrogenization occur in breast, endometrial, ovarian, and prostate cancers, whereas increased leptin levels have been found in renal, esophageal, and hepatocellular carcinomas. Much more information is needed to explain the molecular biology of obesity that would be responsible for the development of individual cancers, particularly those due to cancer genes and environmental toxins that could compound inflammation engendered by inflammatory adipokines in obesity.
Other Comorbidities Related to Obesity
Comorbidities also result from the burden of weight and space-occupying effects of obesity. These include enhanced degenerative joint disease that results from increased weight-bearing on joints due to increased adiposity and the injurious effects that inflammatory adipokines such as resistin have on joint synovia and muscle function.69,70 Comorbidities involving the respiratory system include obstructive sleep apnea, which results from accumulation of extra adipose tissue within the confines of the upper respiratory tract, and hypopharynx, which adversely affects ventilation, with secondary hypoxia and even hypercapnia. Excessive bronchial and peribronchial adipose cells secrete inflammatory adipokines that enhance bronchial mucosal and submucosal inflammation, causing reactive airway disease including asthma in women.26 Pulmonary embolism also occurs at higher rates in patients with obesity, particularly in those with decreased mobility.
Cholesterol gallstone disease is also associated with obesity, particularly in overweight women of child-bearing age. During fasting, there is enhanced mobilization of cholesterol from fat depots, which pass through the liver into the biliary ducts. This allows increased biliary cholesterol secretion and supersaturation of bile in the gallbladder, promoting gallstone formation.71 Such gallstones invoke a local inflammatory state, which, when chronic, becomes a risk factor for gallbladder cancer.
Obesity is also a feature of polycystic ovarian syndrome, in which adipocyte secretagogues enhance the metabolic abnormalities of hyperandrogenemia, insulin resistance, and, along with inflammatory adipokines, increase the incidence of diabetes mellitus type 2 in this disorder.72 Obesity is a risk factor for preeclampsia and eclampsia of pregnancy, in which increased adipokines include RAS, prostaglandins, and other fatty-acid derivatives. Adipocytes also secrete these substances, which exacerbates hypertension and fluid retention in this syndrome. Endarteritis within the placenta may also be related to the increased inflammatory adipokines that contribute to preeclampsia.73 Many of these disorders improve or even disappear with the elimination of obesity. Obesity in women causes a predisposition to depression, amenorrhea, menorrhagia, infertility, and urinary stress incontinence. Obesity is associated with poor wound-healing, which in morbid obesity is magnified by chronic renal failure and calcific necrosis or calciphylaxis.74
Summary
Obesity is an exaggeration of normal adiposity and is a central player in the pathophysiology of diabetes mellitus, insulin resistance, dyslipidemia, hypertension, and atherosclerosis, largely because of its secretion of excessive adipokines. Obesity is a major contributor to the metabolic dysfunction involving lipid and glucose, but on a broader scale, it influences organ dysfunction involving cardiac, liver, pulmonary, endocrine, and reproductive functions. Finally, obesity contributes to immune dysfunction from the effects of its secretion of inflammatory adipokines and is a major risk factor for many cancers. Because of the accelerating effects it has on the worsening of metabolic syndrome and cancer, obesity has the potential to be profoundly detrimental to our species if major methods of prevention and/or effective treatment are not realized.47,48 It is essential then to institute major educational efforts aimed at promoting better habits of eating and physical exercise. These lifestyle changes may be complemented by pharmacologic therapy.
References
- 1.Schoonjans K, Staels B, Auwerx J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res. 1996;37:907–925. [PubMed] [Google Scholar]
- 2.Verges B. Clinical interest of PPARs ligands. Diabetes Metab. 2004;30:7–12. doi: 10.1016/s1262-3636(07)70083-6. [DOI] [PubMed] [Google Scholar]
- 3.Shirai K. Obesity as the core of the metabolic syndrome and the management of coronary heart disease. Curr Med Res Opin. 2004;20:295–304. doi: 10.1185/030079903125003008. [DOI] [PubMed] [Google Scholar]
- 4.Rajala MW, Scherer PE. Minireview: The adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 5.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 6.Lau DCW, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: Molecular links between obesity and atherosclerosis. Am J Physiol Heart Circ Physiol. 2005;288:H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E. Review article: Steatosis, the metabolic syndrome and cancer. Aliment Pharmacol Ther. 2005;22(suppl 2):40–43. doi: 10.1111/j.1365-2036.2005.02594.x. [DOI] [PubMed] [Google Scholar]
- 8.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 9.Ravussin E, Bogardus C. Relationship of genetics, age, and physical fitness to daily energy expenditure and fuel utilization. Am J Clin Nutr. 1989;49(5 suppl):968–975. doi: 10.1093/ajcn/49.5.968. [DOI] [PubMed] [Google Scholar]
- 10.Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: Implications for obesity. Nat Rev Neurosci. 2003;4:901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- 11.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 12.Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 14.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 16.Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. 2004;15:362–369. doi: 10.1016/j.tem.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 18.Lafontan M. Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol. 2005;45:119–146. doi: 10.1146/annurev.pharmtox.45.120403.095843. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan GD, Goossens GH, Humphreys SM, Vidal H, Karpe F. Upper and lower body adipose tissue function: a direct comparison of fat mobilization in humans. Obes Res. 2004;12:114–118. doi: 10.1038/oby.2004.15. [DOI] [PubMed] [Google Scholar]
- 21.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 22.Miner JL. The adipocyte as an endocrine cell. J Anim Sci. 2004;82:935–941. doi: 10.2527/2004.823935x. [DOI] [PubMed] [Google Scholar]
- 23.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and inflammation: from basic science to clinical applications. Int J Obes Relat Metab Disord. 2003;27(suppl 3):S41–S45. doi: 10.1038/sj.ijo.0802499. [DOI] [PubMed] [Google Scholar]
- 24.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 25.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/s1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron C, Boulet LP, Hamid Q. Obesity, allergy and immunology. J Allergy Clin Immunol. 2005;115:1102–1104. doi: 10.1016/j.jaci.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- 28.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Li SH, Wang CH, Fedak PWM, Li RK, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 31.Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, et al. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg AS, Nordan RP, McIntosh J, Calvo JC, Scow RO, Jablons D. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 1992;52:4113–4116. [PubMed] [Google Scholar]
- 33.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 34.Abo-Auda W, Benza RL. Therapeutic angiogenesis: review of current concepts and future directions. J Heart Lung Transplant. 2003;22:370–382. doi: 10.1016/s1053-2498(02)00665-4. [DOI] [PubMed] [Google Scholar]
- 35.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, et al. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 36.Nonogaki K, Fuller GM, Fuentes NL, Moser AH, Staprans I, et al. Interleu-kin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology. 1995;136:2143–2149. doi: 10.1210/endo.136.5.7720663. [DOI] [PubMed] [Google Scholar]
- 37.Stouthard JM, Romijn JA, Van der Poll T, Endert E, Klein S, et al. Endocrinologic and metabolic effects of interleukin-6 in humans. Am J Physiol. 1995;268(5 pt 1):E813–E819. doi: 10.1152/ajpendo.1995.268.5.E813. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Real JM, Broch M, Vendrell J, Richart C, Ricart W. Interluekin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab. 2000;85:1334–1339. doi: 10.1210/jcem.85.3.6555. [DOI] [PubMed] [Google Scholar]
- 39.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 40.Francis GA, Annicotte JS, Auwerx J. PPAR agonists in the treatment of atherosclerosis. Curr Opin Pharmacol. 2003;3:186–191. doi: 10.1016/s1471-4892(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 41.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith CS, Jr, Lenfant C. American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 42.Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord. 2002;26:1205–1210. doi: 10.1038/sj.ijo.0802026. [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, et al. The metabolic syndrome and chronic kidney disease in US. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 45.Simard B, Turcotte H, Marceau P, Biron S, Hould FS, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 2004;14:1381–1388. doi: 10.1381/0960892042584021. [DOI] [PubMed] [Google Scholar]
- 46.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 47.Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet. 2005;366:1578–1582. doi: 10.1016/S0140-6736(05)67341-2. [DOI] [PubMed] [Google Scholar]
- 48.James WPT, Rigby N, Leach R. International Obesity Task Force. The obesity epidemic, metabolic syndrome and future prevention strategies. Eur J Cardiovasc Rehabil. 2004;11:3–8. doi: 10.1097/01.hjr.0000114707.27531.48. [DOI] [PubMed] [Google Scholar]
- 49.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 50.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:1–149. backcover. [PubMed] [Google Scholar]
- 51.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 52.IARC Working Group.; IARC. IARC Working Group on the evaluation of cancer-preventive strategies. In: Vanio H, Bianchini F, editors. Handbooks of Cancer Prevention (Volume 6) Weight Control and Physical Activity. Lyon, France: IARC Press; 2002. [Google Scholar]
- 53.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33:1055–1059. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 54.Kim S, Popkin BM. Commentary: Understanding the epidemiology of overweight and obesity—a real global public health concern. Int J Epidemiol. 2006;35:60–67. doi: 10.1093/ije/dyi255. [DOI] [PubMed] [Google Scholar]
- 55.Campos JD, Saguy A, Ernsberger P, Oliver E, Gaesser G. The epidemiology of overweight and obesity: public health crisis or moral panic? Int J Epidemiol. 2006;35:55–60. doi: 10.1093/ije/dyi254. [DOI] [PubMed] [Google Scholar]
- 56.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 57.Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, et al. Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol. 2003;169:1670–1675. doi: 10.1097/01.ju.0000062674.43964.d0. [DOI] [PubMed] [Google Scholar]
- 58.Schatzl G, Madersbacher S, Thurridl T, Waldmüller J, Kramer G, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 59.Freedland SJ. Obesity and prostate cancer: a growing problem. Clin Cancer Res. 2005;11:6763–6766. doi: 10.1158/1078-0432.CCR-05-1305. [DOI] [PubMed] [Google Scholar]
- 60.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 61.MacInnis RJ, English DR. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 62.Wang SN, Yeh YT, Yang SF, Chai CY, Lee KT. Potential role of leptin expression in hepatocellular carcinoma. J Clin Pathol. 2006;59:930–934. doi: 10.1136/jcp.2005.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calle EE, Kaaks S, Calle EE. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 64.Chow WH, Blot WJ, Vaughan TL, Risch HA, Gammon MD, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 65.Ogunwobi O, Mutungi G, Beales ILP. Leptin stimulates proliferation and inhibits apoptosis in Barrett's esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology. 2006;147:4505–4516. doi: 10.1210/en.2006-0224. [DOI] [PubMed] [Google Scholar]
- 66.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 69.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin: an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 70.Schaffler A, Ehling A, Neumann E, Nerfarth H, Tarner I, et al. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 71.Redinger RN, Small DM. Bile composition, bile salt metabolism and gallstones. Arch Intern Med. 1972;130:618–630. [PubMed] [Google Scholar]
- 72.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:1435. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 73.Barden A. Pre-eclampsia: contribution of material constitutional factors and the consequences for cardiovascular health. Clin Exp Pharmacol Physiol. 2006;33:826–830. doi: 10.1111/j.1440-1681.2006.04448.x. [DOI] [PubMed] [Google Scholar]
- 74.Bazari H, Jaff MR, Mannstadt M, Yan S. Case records of the Massachusetts General Hospital. Case 7-2007. A 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med. 2007;356:1049–1057. doi: 10.1056/NEJMcpc069038. [DOI] [PubMed] [Google Scholar]