Abstract
The purpose of our study was to investigate changes in immunological parameters induced by weaning stress (including milk restriction) in calves. Fifteen Holstein calves were subjected to weaning at 6 weeks of age. Blood samples were collected at -14, -7, -2, 1, 3, and 5 days post-weaning (DPW; 0 DPW = 42 days). Weaning caused significant (p < 0.01) increases in the neutrophil (NE):lymphocyte (LY) ratio at 5 DPW with a significant (p < 0.05) reduction of LYs. The concentration of acute-phase proteins (haptoglobin and serum amyloid A) also increased significantly (p < 0.05) at 3 and 5 DPW compared to -2 DPW. Levels of the iron-binding protein lactoferrin decreased significantly (p < 0.05) after weaning. Serum tumor necrosis factor-α and cortisol levels were elevated (p < 0.05) at 3 DPW, while those of serum interferon-γ decreased (p < 0.05) at 1 and 3 DPW compared to levels observed before weaning. Weaning significantly (p < 0.05) decreased the percentage of CD25+ T cells in the peripheral blood. In conclusion, weaning stress affected the NE:LY ratio along with the levels of acute phase proteins, lactoferrin, cortisol, and inflammatory cytokines in the peripheral blood of calves. Weaning stress may induce an acute phase response possibly through the elevation of cortisol production and modulation of inflammatory cytokines.
Keywords: acute phase protein, immune response, inflammatory cytokines, stress, weaning
Introduction
Stress can be defined as the biological response elicited when a threat to homeostasis is perceived. The effects of several types of stressors, such as transportation, heat, and weaning, have been studied in various animals [2,10, 11,30]. Stress is an important factor in the animal industry because it has been directly linked to growth, reproduction, meat quality, animal welfare, and disease susceptibility [27,28,37], thereby giving it the potential for making a substantial economic impact [8]. Weaning and transportation are regarded as the top stressors that calves may experience [14,27]. Although the effects of all stages of transportation on cattle are well-documented in the literature, there has been limited research on the effects of weaning alone or weaning and grouping on calves. The majority of reports focus on weaning and transportation together since both of these stressors often occur at the same time in agriculture [11]. Previous studies have found that weaning stress alters the concentration of plasma acute phase protein (APP) [24] and the neutrophil (NE): lymphocyte (LY) ratio, and increases norepinephrine and cortisol levels [6,8,12]. Lactoferrin (Lf) and transferrin (Tf), two iron-binding proteins, play many physiological roles including regulation of iron metabolism, protection against microbial infection, and regulation of immune functions [33]. The acute phase response is considered to be an exclusive biomarker of inflammation and/or infection [20]. Accordingly, the concentration of APPs is a useful indicator of stress response in calves. The elevation of APPs by transportation and weaning stress has already been reported [2,21,25]. Triggered by inflammatory signals (predominantly cytokines), the circulating concentrations of APPs are elevated in a nonspecific manner in animals [32]. In particular, inflammatory cytokines including interleukin (IL)-1, IL-6, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) are considered to be the mediators of immunological and pathological responses to stress and infection [15,35].
The goal of this study was to elucidate the interaction between immune response and stress response by profiling the changes of various immune-related biomarkers in Holstein calves after a weaning challenge (including milk restriction and grouping). We investigated the changes of physiological (cortisol and iron-binding proteins) and immunological mediators (leukocytes, immunoglobulins (Igs), inflammatory cytokines, and APPs). We hypothesized that weaning is a significant stressor in calves that induces the production of cortisol and alters immune-physiological parameters that result in an acute phase response.
Materials and Methods
Animal management and blood sample collection
All experiments were carried out at the Dairy Science Division at the National Institute of Animal Science (Korea). All experimental procedures were reviewed and approved by the Ethics Committee on the Use of Animals in Research of the National Livestock Research Institute (Korea). Holstein calves (n = 15; 8 males and 7 females, BW 37.06 ± 2.12 kg) were separated from their dams within 2 h of birth, weighed, and moved into individual pens (1.5 × 2.5 m; with wood shavings as bedding) where they were fed colostrum in an amount equivalent to 10% of their body weight for the first 3 day. Pens had sides constructed with solid iron rods; openings in the front and rear of the pens allowed the calves free access to calf starter, a mixture of chopped grass and hay (43% orchard grass, 43% tall fescue, and 14% white clover based on dry weight), and water from a bowl in each pen. Calf starter and mixed grass hay were given starting at the first and fourth wk of age, respectively. All calves were fed whole milk using mobile plastic bottles (2 L capacity) fitted with soft rubber nipples using the step-down milking method as previously described [16]. The calves were fed quantities of milk equivalent to 20% of their body weight until 28 days of age. When the calves were between 29 to 30 days old, the amount of milk was gradually reduced to 10% and was maintained at this level until weaning. All calves were weaned (milk restriction) at 42 days of age [0 day post weaning (DPW)]. On the morning of the day of weaning, all calves were moved from their individual pens to the assigned group pens and fed a concentrated mixture (crude protein = 17.50%, fat = 2.57%, ash = 6.44%, total digestible nutrient = 71.43%) and forage, but were not given milk. Blood samples (5 mL) were collected from jugular veins at 40 (-2 DPW) and 47 (5 DPW) days of age. To determine the concentration of APPs, iron binding proteins, pro-inflammatory cytokines, and cortisol, additional blood samples (10 mL) were collected into evacuated tubes coated with the anti-coagulant libitum-heparin (BD Vacutainer; BD, USA) at 43 (1 DPW) and 45 (3 DPW) days of age. To determine the concentration of total serum immunoglobulins, additional blood samples (5 mL) were also collected at 28 (-14 DPW) and 35 (-7 DPW) days of age.
Hematology
Plasma was harvested from uncoagulated blood after centrifugation at 1,600 × g at 4℃ for 15 min and stored at -80℃ until analyzed. NE, LY, platelet, monocyte, and leukocyte populations in whole blood were measured using an automatic analyzer (Hemavet 850; Drew Scientific, USA).
Enzyme immunoassay (EIA) and ELISA
All serum samples were analyzed in triplicate. The amount of total serum immunoglobulins (IgG and IgA) and iron-binding proteins (Lf and Tf) were determined using commercial ELISA kits (Bethyl Laboratory, USA). In brief, 96-well immunoplates (Nalgene Nunc International, USA) were coated with bovine capture antibody (1 µg/mL) and incubated for 1 h. The plates were then washed with washing buffer (0.05% Tween 20 in phosphate buffered saline) three times and blocked with blocking buffer (1% bovine serum albumin in phosphate buffered saline) for 30 min, After washing, the plates were incubated with the bovine serum samples (100 µL) and the respective proteins standards for 1 h at room temperature. The plates were then washed and antibody binding was detected with a secondary antibody (1:4000 dilution) conjugated with biotin that was added to the plates incubated for 1 h. Specific binding was detected using streptavidin-horse radish peroxidase and tetramethylbenzidine substrate (Sigma-Aldrich, USA). The reaction was stopped with 2 N H2SO4. Absorbance was measured at 450 nm using a microplate reader (Molecular Devices, USA). Serum haptoglobin (Hp; Life Diagnostics, USA) and serum amyloid A (SAA; Tridelta Development, Ireland) were measured using ELISA kits according to the manufacturer's instructions. Serum concentration of cortisol was measured using an EIA kit (Oxford Biomedical Research, USA). Serum concentrations of TNF-α and IFN-γ were assayed using the Duoset ELISA kit according to the manufacturer's protocol (R&D Systems, USA) with detection ranges of 6.25 to 800 pg/mL and 78 to 10,000 pg/mL for TNF-α and IFN-γ, respectively.
Flow cytometry analysis
CD4+, CD8+, and CD25+ T cells were quantified by single-color flow cytometry analysis. Cells (5 × 105) were harvested and stained with CD4-APC, or CD8-APC and CD25-PE (1 µg/mL) (VMRD, USA). After incubating for 30 min at 37℃, the cells were washed three times with phosphate buffered saline, and differences in the expression of cell surface molecules were detected using a FACScanto flow cytometer (BD Bioscience, USA). All data were further analyzed with FACSDiva software (BD Bioscience, USA).
Statistical analysis
Data were analyzed with Proc Mixed (version 8.2; SAS, USA) using calves as the experimental unit and the fixed effects of time relative to the pre-weaning time points. Data are presented as the mean ± SE, and all effects of time relative to weaning were deemed significant when p < 0.05.
Results
Leukocytes
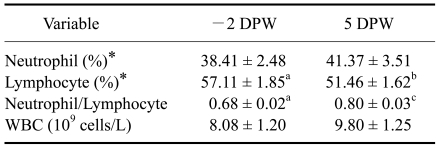
Weaning exerted significant effects on leukocytes as demonstrated by changes in the NE:LY ratio relative to pre-weaning (-2 DPW) values (Table 1). The NE:LY ratio at 5 DPW (0.80 ± 0.03) was greater than that at -2 DPW (0.68 ± 0.02; p < 0.01), mainly due to a significant (p < 0.05) decrease in LY (57.11 ± 1.85% vs. 51.46 ± 1.62%, respectively). Statistically, there was no change in the concentration of white blood cells after weaning (Table 1).
Table 1.
Changes in the leukocyte populations of the calves pre- and post-weaning
*Percentage in blood. a,bValues for the mean with different letters are significantly different between -2 DPW and 5 DPW, abp < 0.05, acp < 0.01. -2 DPW: 40 days of age. 5 DPW: 47 days of age. The values are expressed as the mean ± SE.
Concentration of APPs
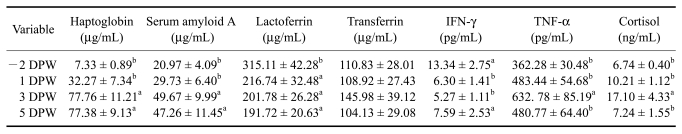
Weaning clearly induced an increase in the acute phase response in calves by elevating serum concentrations of Hp and SAA. Average concentrations of serum Hp (Table 2) were higher at 3 and 5 DPW (77.76 ± 11.21 µg/mL and 77.38 ± 9.13 µg/mL, respectively) than at -2 DPW (7.33 ± 0.89 µg/mL; p < 0.05 for both). An elevated Hp level (> 50 ng/mL) was observed in eight out of 15 calves within 5 days after weaning (data not shown). A minor elevation of Hp levels (30~50 ng/mL) was observed in five out of 15 calves within 5 days after weaning (data not shown). The average concentration of SAA (Table 2) was higher at 3 and 5 DPW (49.67 ± 9.99 µg/mL and 47.26 ± 11.45 µg/mL, respectively) than at -2 DPW (20.97 ± 4.09 µg/mL; p < 0.05 for both). An elevated SAA level (> 50 ng/mL) was observed in 10 out of 15 calves after weaning (data not shown).
Table 2.
Changes in the concentration of immunophysiological and stress-related parameters in the blood of calves after the weaning challenge
a,bValues of the mean with different letters were significantly different cytokines levels between pre-weaning and post-weaning time points (p < 0.05). 1 DPW: 43 days of age. 3DPW: 45 days of age. IFN-γ: interferon-γ, TNF-α: tumor necrosis factor-α. The values are expressed as the mean ± SE.
Concentration of iron binding proteins
To identify the effect of weaning (milk restriction) on iron binding proteins level, changes of serum concentrations of Lf and Tf were observed (Table 2). Serum Lf concentrations decreased drastically at 1 DPW (216.74 ± 32.48 µg/mL) compared to pre-weaning levels (315.11 ± 42.28 µg/mL; p < 0.05) and remained lower at 3 and 5 DPW (201.78 ± 26.28 µg/mL and 191.72 ± 20.63 µg/mL, respectively). Among 15 calves, 13 responded to weaning stress with reduced Lf levels (data not shown). The concentration of Tf increased at 3 DPW, but not significantly (Table 2).
Concentrations of serum inflammatory cytokines and cortisol
The concentrations of serum inflammatory cytokines were investigated in order to assess changes the production of inflammatory cytokines upon weaning stress. As shown in Table 2, serum IFN-γ levels declined within a day after weaning. The concentration of IFN-γ decreased significantly from 13.34 ± 2.75 pg/mL at -2 DPW to 6.30 ± 1.41 at 1 DPW and 5.27 ± 1.11 pg/mL at 3 DPW (p < 0.05 for both). Among 15 calves, 11 responded to weaning with reduced IFN-γ levels within 5 days (data not shown). Conversely, the concentration of TNF-α (Table 2) increased from 362.28 ± 30.48 pg/mL at -2 DPW to 632.78 ± 85.19 pg/mL at 3 DPW (p < 0.05). Nine calves showed elevated serum TNF-α levels within 5 days after weaning (data not shown). The level of serum cortisol was examined (Table 2), and it was found that stress led to increased cortisol levels in the circulation. The concentration of serum cortisol rose from 6.74 ± 0.40 ng/mL at -2 DPW to 17.10 ± 4.33 ng/mL at 3 DPW (p < 0.05), and then returned to the initial level at 5 DPW (7.24 ± 1.55 ng/mL). Among 15 calves, 10 displayed a rise in cortisol levels after weaning (data not shown).
Concentrations of total serum IgG and IgA
Total serum IgG and IgA levels were determined after weaning to investigate the effects of weaning stress on antibody production associated with the B-cell immune response. Weaning stress did not affect the concentrations of total serum IgG or IgA (Table 3), but different trends in the IgG and IgA profiles were observed. The concentration of total serum IgG did not change over the entire experimental period, but total serum IgA increased in a time-dependent manner. The total serum IgA concentration was higher at 5 DPW (132.69 ± 16.12 ng/mL) than at -14 and -7 DPW (63.27 ± 5.62 µg/mL and 79.50 ± 7.21 µg/mL, respectively; p < 0.05 for both).
Table 3.
Changes in total serum IgG and IgA concentrations in calves during the experimental period
a,bValues of the mean with different letters were significantly different Ig levels between pre-weaning and post-weaning time points (p < 0.05). -14 DPW: 28 days of age. -7 DPW: 35 days of age. Ig: immunoglobulin. The values expressed as the mean ± SE.
CD4+, CD8+, and CD25+ T cells
The percent change in composition (%) of CD4+, CD8+, and CD25+ cells in peripheral blood was investigated to determine the effects of weaning stress on the LYs. Weaning stress caused a significant decreased in the percentage of CD25+ T cells in the peripheral blood (Table 4) from 4.02 ± 0.09% at -2 DPW to 3.19 ± 0.08% at 5 DPW (p < 0.05). This result indicated that the proportion of expressed IL-2 receptor was decreased in the peripheral blood. Although the percentage of CD4+ T cells was greater at 5 DPW, no significant changes in CD4+, CD8+, or CD4+/CD8+ cells were observed (Table 4).
Table 4.
Changes in peripheral lymphocyte subsets in calves pre- and post-weaning
a,bValues of the mean with different letters were significantly different peripheral lymphocytes composition between -2 DPW and 5 DPW (p < 0.05). The values are expressed as the mean ± SE.
Discussion
All animals maintain homeostasis and have delicate mechanisms that enable them to cope with environmental stimuli. A variety of environmental factors can cause strain on animals and evoke stress responses. Stressors can be separated into three main types: (1) psychological stress, (2) physical stress, and (3) a combination of both. A psychological stressor does not physically affect the body directly, but can be perceived as stressful or dangerous. For example, when animals are transported, handled, and mixed and/or put in isolation, they view these situations as psychological stress [2,10,14,31]. Physical stress, including extreme temperatures or food shortage, directly induces stress responses in the body. Some stressors such as noise, pain, restraint, and weaning combine both psychological and physical stress [14]. In particular, weaning stress is composed of the psychological stress of breaking maternal and social bonding and the physical stress of nutritional change. Accordingly, weaning stress in animals is recognized as one cause of many health problems in cattle with important economic implications [4,18,19].
The immune response is one of the mechanisms through which animals defend themselves against environmental challenges. A common theory is that stress responses suppress immune activity, thus increasing the susceptibility of an animal to disease [26,29,36]. The underlying mechanisms of immune responses to stress remain unclear because of the complexity of both the immune system and stress responses, and other physiological factors such as age, nutrition, genetics, and gender that influence such interactions. Our examination of the immune-physiological characteristics in calves with weaning stress might help define the interaction between stress and immune responses in animals.
In this study, we found that weaning stress influenced leukocyte populations. Weaned calves showed a significantly elevated NE:LY ratios, mainly due to a significant reduction in the percentage of LYs with a minimal elevation of NE percentages. Previous studies have investigated changes in leukocytes as potential biomarkers of physiological stress. Dexamethasone induces an elevation of NE:LY ratios in animals [1], and isolation of calves from their dams also increases the NE:LY ratio [5]. Hickey et al. [12] suggested that glucocorticoids are a contributing factor to the alteration of the NE:LY ratio. Together with the results of previous studies, our findings suggest that a weaning challenge affects leukocyte levels, and that the NE:LY ratio may be an effective biomarker of stress responses.
APPs refer to a group of hepatic glycoproteins that are stimulated by inflammatory mediators and serve as initial reactors to infection, inflammation, or trauma in animals [20]. Circulating concentrations of APPs can be a useful tool for monitoring the status of animal health because blood concentrations of acute-phase proteins increase in cattle in response to stress stimuli [6,12]. Hp and SAA are considered to be representative APPs in cattle. Hp concentrations in healthy cattle are often undetectable, but can increase as much as 50~100 times during an acute phase response. In contrast, the change of SAA concentrations is remarkably moderate in cattle, increasing by only 2~5 times during an acute phase response [6,23]. The APP response is considered to be almost an exclusive marker of inflammation and/or infection, but there is also an essential linkage between non-inflammatory stress and the acute phase response [22]. The hypothesis that explains the induction of the APP response in stressed animals is based on the idea that non-inflammatory and psychological stresses activate the sympatho-adrenal and hypothalamic-pituitary-adrenal (HPA) axis via the afferent sensory nervous system [13,19]. The sympatho-adrenal axis releases catecholamines which stimulate immune-related cells and cause them to produce pro-inflammatory cytokines in response to stress [13]. The HPA pathway controls the release of glucocorticoids from the adrenal cortex [19]. Both glucocorticoids and pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α are potentially direct stimulators of the acute phase response [17]. Therefore, we postulate that weaning as a stressor induced two major stress pathways and stimulated the production of hepatic APPs. However, there is some contradiction related to the production of pro-inflammatory cytokines in the stress response. Both physical and psychological stressors have been shown to suppress blastogenesis of T- and B-lymphocytes, natural killer cell activity, and cytokine (IL-2 and IFN-γ) production [7]. Glucocorticoids also suppress the synthesis and release of cytokines [25,36]. Such complexities make it difficult to estimate changes in serum cytokine levels associated with the stress response.
For this study, we examined the levels of cortisol, serum inflammatory cytokines (TNF-α, IFN-γ) and APPs (Hp and SAA). At 3 DPW, serum cortisol levels were higher than at -2 DPW and acute phase responses were observed in this study as demonstrated by drastic elevation of Hp and SAA levels at both 3 and 5 DPW. Furthermore, elevation of serum TNF-α levels and reduction of IFN-γ levels were observed at the same time. Although in this study, we did not evaluate changes in catecholamine levels in this study, suggesting that weaning stress might have induced the HPA axis pathway and resulted in the release of glucocorticoids in the stressed calves. Further study on changes in catecholamines as well as the involvement of other inflammatory cytokines and interleukins together with APPs could help elucidate the interaction between stress and acute phase responses. Further examination of the interaction among various serum pro-inflammatory cytokines produced by immune cells treated with stress hormones are necessary for better understanding the complicated actions of cytokines involved in the stress response.
Stress hormones released in response to activation of the HPA axis have all been shown to have an effect on aspects of the immune system [30]. In vivo activation of glucocorticoid release via adrenocorticotropic hormone injection reduces antibody production in cattle and pigs [3,34]. Intramuscular injection of short-acting dexamethasone followed by long-acting dexamethasone induces leukocytosis, increases in CD4+ cells, and decreases in CD8+ cells [1]. Although total serum IgA levels increased in a time-dependent manner in the current study, no significant (p > 0.05) change in total serum IgG or IgA levels was observed with weaning stress. These results suggest that total serum IgA levels increased when the calves were 4 to 7 weeks of age, and that the weaning challenge did not induce dramatic changes in serum IgG and IgA levels. Weaning stress significantly (p < 0.05) reduced the number of CD25+ (IL-2 receptor activated cells) cells, but not CD4+ (helper T-cells) or CD8+ (cytotoxic and suppressor T-cells) cells. This result suggested that weaning stress might affect the expression of the IL-2 receptor on LYs in peripheral blood. The α-chain of the IL-2 receptor, CD25, is expressed on activated T-cells, B-cells, and monocytes. Formation of the high-affinity IL-2 receptor allows T-cell proliferation and differentiation to be driven by IL-2 [9]. Although decreased IL-2 receptor expression is not direct evidence of immune attenuation, this result indicated that stress-associated metabolites such as cortisol might influence the proliferation or differentiation of immune cells.
We also investigated changes in two iron-binding proteins, Lf and Tf, due to weaning stress in calves. Lf plays many roles in different physiological functions including regulation of iron metabolism, protection against microbial infection, and regulation of immune responses [33]. In the current study, serum Lf levels were dramatically reduced at 1 DPW compared to the -2 DPW levels, and this level was maintained at 3 and 5 DPW. These results suggest that Lf contained in milk might affect serum Lf levels during the calving period. One of the fundamental issues to be resolved by future studies is whether orally administered Lf or its functional fragments can be absorbed in the intestinal tract and exert protective effects during stress. There is the possibility that orally ingested Lf can enter the systemic circulation during the calving period. In the current study, we investigated changes of various immune-related parameters such as APPs, inflammatory cytokines, total serum immunoglobulins, iron-binding proteins, and leukocyte levels before and after a weaning challenge in order to elucidate the interaction between immune responses and stress responses. Although we did not include a negative control in this study, we believe that there is a non-significant effect of natural ageing on the drastic changes of biomarkers such as cortisol, APPs, NE:LY ratio, and cytokines because we observed the profile of parameters within 1 week after weaning. In agreement with these findings, previous studies have shown that these biomarkers do not show significant changes within a short time [2,11,12,32].
In this study, leukocyte subsets and levels of circulating cortisol, inflammatory cytokines, and APPs were altered after the weaning challenge. These results indicated that a weaning challenge as a stressor not only alters stress-related factors, but also affects immune-physiological parameters. It is worth noting that weaning appears to induce an acute phase response accompanied by increased cortisol production and the modulation of inflammatory cytokines. The results obtained in the present study suggest that APPs play an important role in stress response which might be helpful for future studies on weaning stress and immune system attenuation. Further study on the production of cytokines and activity of bovine immune cells treated with stress-related hormones will helpful for better understanding the effects immune attenuation following stress challenges.
Acknowledgments
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant from the Korean Ministry of Education, Science and Technology (R01-2008-000-10854-0).
References
- 1.Anderson BH, Watson DL, Colditz IG. The effect of dexamethasone on some immunological parameters in cattle. Vet Res Commun. 1999;23:399–413. doi: 10.1023/a:1006365324335. [DOI] [PubMed] [Google Scholar]
- 2.Arthington JD, Eicher SD, Kunkle WE, Martin FG. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci. 2003;81:1120–1125. doi: 10.2527/2003.8151120x. [DOI] [PubMed] [Google Scholar]
- 3.Blecha F, Baker PE. Effect of cortisol in vitro and in vivo on production of bovine interleukin 2. Am J Vet Res. 1986;47:841–845. [PubMed] [Google Scholar]
- 4.Burton JL, Kehrli ME., Jr Regulation of neutrophil adhesion molecules and shedding of Staphylococcus aureus in milk of cortisol- and dexamethasone-treated cows. Am J Vet Res. 1995;56:997–1006. [PubMed] [Google Scholar]
- 5.Church JS, Hudson RJ. Comparison of the stress of abrupt and interval weaning of farmed wapiti calves (Cervus elaphus) Small Rumin Res. 1999;32:119–124. [Google Scholar]
- 6.Conner JG, Eckersall PD, Ferguson J, Douglas TA. Acute phase response in the dog following surgical trauma. Res Vet Sci. 1988;45:107–110. [PubMed] [Google Scholar]
- 7.Cunnick JE, Lysle DT, Kucinski BJ, Rabin BS. Evidence that shock-induced immune suppression is mediated by adrenal hormones and peripheral β-adrenergic receptors. Pharmacol Biochem Behav. 1990;36:645–651. doi: 10.1016/0091-3057(90)90270-r. [DOI] [PubMed] [Google Scholar]
- 8.Fike K, Spire MF. Transportation of cattle. Vet Clin North Am Food Anim Pract. 2006;22:305–320. doi: 10.1016/j.cvfa.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Foote MR, Nonnecke BJ, Fowler MA, Miller BL, Beitz DC, Waters WR. Effects of age and nutrition on expression of CD25, CD44, and L-selectin (CD62L) on T-cells from neonatal calves. J Dairy Sci. 2005;88:2718–2729. doi: 10.3168/jds.S0022-0302(05)72951-9. [DOI] [PubMed] [Google Scholar]
- 10.Grandin T. Assessment of stress during handling and transport. J Anim Sci. 1997;75:249–257. doi: 10.2527/1997.751249x. [DOI] [PubMed] [Google Scholar]
- 11.Herzog KR. The effects of weaning stress on the serum protein profile of calves: a proteomic analysis. Saskatoon: University of Saskatchewan; 2007. Master thesis. [Google Scholar]
- 12.Hickey MC, Drennan M, Earley B. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 2003;81:2847–2855. doi: 10.2527/2003.81112847x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 14.Kelley KW. Stress and immune function: a bibliographic review. Ann Rech Vet. 1980;11:445–478. [PubMed] [Google Scholar]
- 15.Kelley KW, Johnson RW, Dantzer R. Immunology discovers physiology. Vet Immunol Immunopathol. 1994;43:157–165. doi: 10.1016/0165-2427(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 16.Khan MA, Lee HJ, Lee WS, Kim HS, Kim SB, Ki KS, Ha JK, Lee HG, Choi YJ. Pre- and postweaning performance of Holstein female calves fed milk through step-down and conventional methods. J Dairy Sci. 2007;90:876–885. doi: 10.3168/jds.S0022-0302(07)71571-0. [DOI] [PubMed] [Google Scholar]
- 17.Kurash JK, Shen CN, Tosh D. Induction and regulation of acute phase proteins in transdifferentiated hepatocytes. Exp Cell Res. 2004;292:342–358. doi: 10.1016/j.yexcr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Khan MA, Lee WS, Kim HS, Ki KS, Kang SJ, Hur TY, Khan MS, Choi YJ. Growth, blood metabolites, and health of Holstein calves fed milk replacer containing different amounts of energy and protein. Asian-Australas J Anim Sci. 2008;21:198–203. [Google Scholar]
- 19.Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 20.Marinkovic S, Jahreis GP, Wong GG, Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989;142:808–812. [PubMed] [Google Scholar]
- 21.Murata H, Miyamoto T. Bovine haptoglobin as a possible immunomodulator in the sera of transported calves. Br Vet J. 1993;149:277–283. doi: 10.1016/S0007-1935(05)80173-3. [DOI] [PubMed] [Google Scholar]
- 22.Murata H. Stress and acute phase protein response: an inconspicuous but essential linkage. Vet J. 2007;173:473–474. doi: 10.1016/j.tvjl.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Heegaard PMH, Godson DL, Toussaint MJM, Tjørnehøj K, Larsen LE, Viuff B, Rønsholt L. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet Immunol Immunopathol. 2000;77:151–159. doi: 10.1016/S0165-2427(00)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips WA, Juniewicz PE, Zavy MT, Von Tungeln DL. The effect of the stress of weaning and transport on white blood cell patterns and fibrinogen concentration of beef calves of different genotypes. Can J Anim Sci. 1989;69:333–340. [Google Scholar]
- 25.Richards DF, Fernandez M, Caulfield J, Hawrylowicz CM. Glucocorticoids drive human CD8+ T cell differentiation towards a phenotype with high IL-10 and reduced IL-4, IL-5 and IL-13 production. Eur J Immunol. 2000;30:2344–2354. doi: 10.1002/1521-4141(2000)30:8<2344::AID-IMMU2344>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Salak-Johnson JL, McGlone JJ, Norman RL. In vivo glucocorticoid effects on porcine natural killer cell activity and circulating leukocytes. J Anim Sci. 1996;74:584–592. doi: 10.2527/1996.743584x. [DOI] [PubMed] [Google Scholar]
- 27.Schaefer AL, Jones SD, Stanley RW. The use of electrolyte solutions for reducing transport stress. J Anim Sci. 1997;75:258–265. doi: 10.2527/1997.751258x. [DOI] [PubMed] [Google Scholar]
- 28.Shawhat Ali MD, Kang GH, Joo ST. A review: influence of pre-slaughter stress on poultry meat quality. Asian-Australas J Anim Sci. 2008;21:912–916. [Google Scholar]
- 29.Shinde PL, Dass RS, Garg AK, Chaturvedi VK. Immune response and plasma alpha tocopherol and selenium status of male buffalo (Bubalus bubalis) calves supplemented with vitamin E and selenium. Asian-Australas J Anim Sci. 2007;20:1539–1545. [Google Scholar]
- 30.Sivakumar AVN, Singh G, Varshney VP. Antioxidants supplementation on acid base balance during heat stress in goats. Asian-Australas J Anim Sci. 2010;23:1462–1468. [Google Scholar]
- 31.Sunil Kumar BV, Singh G, Meur SK. Effects of addition of electrolyte and ascorbic acid in feeding during heat stress in bufalloes. Asian-Australas J Anim Sci. 2010;23:880–888. [Google Scholar]
- 32.Tizard IR. Veterinary Immunology: an Introduction. 7th ed. Philadelphia: Saunders; 2004. [Google Scholar]
- 33.Wakabayashi H, Yamauchi K, Takase M. Lactoferrin research, technology and applications. Int Dairy J. 2006;16:1241–1251. [Google Scholar]
- 34.Wallgren P, Wilén IL, Fossum C. Influence of experimentally induced endogenous production of cortisol on the immune capacity in swine. Vet Immunol Immunopathol. 1994;42:301–316. doi: 10.1016/0165-2427(94)90075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren EJ, Finck BN, Arkins S, Kelley KW, Scamurra RW, Murtaugh MP, Johnson RW. Coincidental changes in behavior and plasma cortisol in unrestrained pigs after intracerebroventricular injection of tumor necrosis factor-α. Endocrinology. 1997;138:2365–2371. doi: 10.1210/endo.138.6.5180. [DOI] [PubMed] [Google Scholar]
- 36.Westly HJ, Kelley KW. Physiologic concentrations of cortisol suppress cell-mediated immune events in the domestic pig. Proc Soc Exp Biol Med. 1984;177:156–164. doi: 10.3181/00379727-177-41926. [DOI] [PubMed] [Google Scholar]
- 37.Yamane H, Kurauchi I, Denbow DM, Furuse M. Central functions of amino acids for the stress response in chicks. Asian-Australas J Anim Sci. 2009;22:296–304. [Google Scholar]