Abstract
We demonstrate that glycoprotein isolated from Dioscorea batatas (GDB) has immunostimulatory effects including macrophage activation. Analysis of infiltration of inflammatory cells into peritoneal cavity showed GDB treatment significantly increased the recruitment of macrophages, lymphocytes, neutrophils, and monocytes into the peritoneal cavity. Treatment of spleen cells isolated from C57BL/6 mice with GDB significantly increased the proliferation of B cells and T cells induced by LPS and ConA, respectively. Treatment with GDB significantly increased the cytolytic capacity of NK cells and macrophages against YAC-1 and B16 cells, respectively. In order to further confirm and investigate the mechanism of GDB on macrophage activation, we analyzed the effects of GDB on the cytokine expression including iNOS, IL-1β, and TNF-α in mouse macrophage cell line, RAW 264.7 cells. RT-PCR and ELISA showed that GDB increased the expression of IL-1β, and TNF-α, whereas iNOS was not induced by GDB. Collectively, this series of experiments indicates that GDB stimulates immune system including macrophage activation.
Keywords: Glycoprotein, Macrophages, TNF-α, IL-1β
INTRODUCTION
Dioscorea species are plants that have been used not only in traditional Chinese medicine, but also in modern medicine as a major source of steroid precursors. The previous studies have been revealed that plants of this genus possess immunomodulatory activity in vitro on the viability, cell-mediated cytotoxicity and interferon-γ (IFN-γ) secretion of splenic lymphocytes [1]. Phytochemical investigations have demonstrated that steroid saponins are active principles of Dioscorea species [2]. Among these, Dioscorea japonica, widely distributed in East Asia, has long been used in ethnomedicine for the treatment of poor appetite, chronic diarrhea, asthma, dry coughs, frequent or uncontrollable urination, diabetes, and emotional instability [2]. Yam is the common name for the plants of the genus Dioscorea of the Dioscoreaceae family.
Macrophages play major roles in both innate and acquired immunity. They can be stimulated by cytokines, such as IFN-γ, or microbial components, such as LPS [3,4]. For innate immunity, macrophages phagocytise and kill microbes, release inflammatory mediators such as NO by the action of iNOS. For acquired immunity, macrophages serve as antigen presenting cells and release cytokines such as tumor necrosis factor (TNF-α), interleukin (IL)-1, IL-6, and IL-12 to regulate the functions or development of helper T cells [5]. Activated macrophages inhibit the growth of a variety of tumor cells and foreign organisms, such as microorganisms [6]. Due to the critical role that macrophage activation plays in innate immune response, immunomodulating compounds targets macrophages [7-9].
In the present study we characterized glycoprotein (GDB) isolated form Dioscorea batatas and investigated the immunopotentiating effects in the animal models and in vitro effects on immune cell proliferation and NK cell- and macrophage-mediated cytotoxicity. We further analyzed the effects of GDB on the cytokine production in macrophage cell line RAW264.7.
METHODS
Animals
Male C57BL/6 mice weighing 21~23 g were housed under a 12-h light/dark cycle in a temperature-controlled room (22~24℃). Mice were given access to standard chow and water ad libitum. All animals were treated humanely under the Sungkyunkwan University Animal Care Committee guidelines.
Preparation of glycoprotein (GDB) from Dioscorea batatas
The tubers of Dioscorea batatas were harvested in May at the Okcheon County in North Chungcheong Province, South Korea, and were stored at 4℃ until used. For isolation of the proteins, whole tubers were used. Glycoproteins were isolated from Dioscorea hatatas according to the procedure of Gaidmashvili et al. [10] with minor modifications. Tubers were peeled and homogenized in an equal amount (w/w) of 50 mM sodium acetate buffer solution (pH 4.0). The homogenate was centrifuged at 15,000 g for 30 min. The supernatant was adjusted to pH 7.0 with 5 M NaOH. Ammonium sulfate was added to 25% saturation with stirring. The solution was centrifuged at 15,000 g for 30 min, and the supernatant was subjected to hydrophobic chromatography on a phenyl-Toyopearl 650M column (Tosoh, Japan) equilibrated with 50 mM Tris-HCl buffer (pH 7.0) containing ammonium sulfate to give 25% saturation. Elution was performed by a decrease of ammonium sulfate concentration from 25 to 0% in 50 mM Tris-HCl buffer (pH 7.0). The fractions yielding absorbance at 280 nm were pooled, dialyzed against distilled water, and lyophilized in a freeze dryer. The lyophilized proteins were stored at -20℃ until used. The lyophilized proteins were dissolved in 50 mM Tris-HCl buffer (pH 8.0) and subjected to an anion-exchange chromatography on a HiTrap Q HP column (5 ml) (GE Healthcare) equilibrated with 50 mM Tris-HCl buffer (pH 8.0). Fractionation of proteins was performed by a step-wise salt gradient (0.1, 0.2, 0.3, and 1 M) NaCl in equilibrium buffer.
Mitogen-induced cell proliferation
A modification of the method of Mosmann [11] was used. Spleens from male C57BL/6 mice were aseptically removed and dissociated into a single-cell suspension in culture medium. The concentration was adjusted to 2×106 cells/ml. The culture medium was RPMI 1640 (GIBCO, Grand Island, NY) containing 10% heat-inactivated FBS, penicillin (100 IU/ml), and streptomycin (100 µg/ml) (RPMI-FBS). Mitogenic stimulation was done as follows: 5×105 cells per well in a total volume of 50 µl were incubated in the absence (control) or presence of mitogens. The mitogens (50 µl) were 4 µg/ml concanavalin A (Con A) for T-cell activation and 10 µg/ml lipopolysaccharide (LPS) for B-cell activation. After an incubation period of 48 h, viable cells were measured by addition of 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) to each well. After additional 4 h incubation and the dissolution of the formazan crystals, the absorption was measured at 570 nm using a Molecular Devices microplate reader (Menlo Park, CA).
Assessment of natural killer (NK) cell cytotoxicity
Spleen cells of male C57BL/6 mice were tested as effector cells against YAC-1 mouse lymphoma cells (ATCC, Rockville, MD). For natural killer (NK) cell assays, 2.5×105 effector cells were co-incubated with 1×104 YAC-1 target cells in a final well volume of 200 µl in 96-well round-bottom plates (Costar Products, Cambridge, MA) for 24 h at 37℃ in a 5% CO2 humidified incubator. The effector-to-target cell ratio (E/T) of 25:1 was chosen after preliminary assays. The cell density was then assessed by incubating cells with 25 µg/ml of MTT for 4 h. The formazan produced was dissolved in dimethyl sulfoxide, and the optical density of each well was determined at 540 nm using a Molecular Devices microplate reader (Menlo Park, CA, USA). Cytolytic activity is expressed as the percentage of tumor cytotoxicity by the following formula:
% Cytotoxicity={1-O.D. of [(target cells+effector cells)-effector cells]/O.D. of target cells}×100
Isolation of inflammatory peritoneal macrophages
Thioglycollate-elicited peritoneal exudate cells were obtained from C57BL/6 mice following intraperitoneal injection of 1 ml Brewer Thioglycollate broth (4.05 g/100 ml) (Difco Laboratories, Detroit, MI, USA) and lavage of the peritoneal cavity with 5 ml of medium 3~4 days later. The cells were washed twice and resuspended in RPMI-1640 containing 10% heat-inactivated FBS, penicillin (100 IU/ml) and streptomycin (100 mg/ml) (RPMI-FBS). Macrophages were isolated from peritoneal exudate cells as described by Klimetzek and Remold [12]. Peritoneal exudate cells were seeded at densities of 5~6×105 cells/cm2 on Teflon-coated petri dishes (100×15 mm) and the macrophages were allowed to adhere for 2~3 h in a 5% CO2 humidified atmosphere. Teflon-coated petri dishes were prepared by spraying with aerosolized Teflon (Fisher Scientific, Pittsburgh, PA, USA) and sterilizing with UV light for 3 h. The non-adherent cells were removed by washing the dishes twice with 10 ml of pre-warmed medium and the dishes were incubated for 10 min at 4℃. The supernatants were then removed carefully and discarded and the plates were washed once with pre-warmed D-PBS (GIBCO). Cold PBS (15 ml) containing 1.5% FBS was then added, followed by 0.3 ml of 0.1 m EDTA (pH 7.0). The plates were incubated for 15 min at room temperature and the macrophages were removed by rinsing 10 times using a 10-ml syringe. The viability of the detached cells was assessed by trypan blue exclusion and proportion of macrophages was determined after cytoplasmic staining with acridine orange and examination under a fluorescence microscope. Cell preparations were greater than 95% viable and contained greater than 95% macrophages.
Macrophage-mediated cytotoxicity
The assay for macrophage cytotoxicity was performed by modification of the technique described previously [13]. Briefly, macrophages (1.0×105 cells/well) from B57BL/6 mice were first incubated in either medium alone or in medium supplemented with GDB (4~500 µg/ml) for 24 h in 96-well plates. Macrophages were co-incubated with B16 melanoma cells (1.0×104/well; an initial effector:target cell ratio of 10:1) in the presence of LPS (1 µg/ml) and IFN-γ (50 U/ml) at 37℃ in a 5% CO2 incubator. The cell density was then assessed by incubating cells with 25 µg/ml of MTT for 4 h. The formazan produced was dissolved in dimethyl sulfoxide, and the optical density of each well was determined at 540 nm using a Molecular Devices microplate reader (Menlo Park, CA, USA). Cytolytic activity is expressed as the percentage of tumor cytotoxicity by the following formula:
% Cytotoxicity={1-O.D. of [(target cells+macrophages)-macrophages]/O.D. of target cells}×100,
where the O.D. of target cells is the optical density of B16 melanoma cells and the O.D. of macrophages is the optical density of macrophages.
Nitrite determination
RAW 264.7 cells were stimulated with GDB for 24 h. Culture supernatants were collected and the accumulation of NO2- in culture supernatants was measured using the assay system described by Green et al. [14].
Cytokine determination
RAW 264.7 cells were stimulated with GDB for 24 h. Culture supernatants were collected and the concentrations of each cytokine (TNF-α and IL-1β) in the culture supernatants were determined using ELISA kits (BD Biosciences, San Diego, CA) according to the manufacturer's instructions.
RT-PCR
Total RNA was isolated with Tri Reagent (Molecular Researh Center, Cincinnati, OH, USA). The forward and reverse primer sequences are as follows: iNOS: 5'-CTG CAG CAC TTG GAT CAG GAA CCT G-3', 5'-GGG AGT AGC CTG TGT GCA CCT GGA A-3'; IL-1β: 5'-TGC AGA GTT CCC CAA CTG GTA CAT C-3', 5'-GTG CTG CCT AAT GTC CCC TTG AAT C-3'; TNF-α: 5'-CCT GTA GCC CAC GTC GTA GC-3', 5'-TTG ACC TCA GCG CTG AGT TG-3'; and β-actin: 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3', 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3'. Equal amounts of RNA were reverse transcribed into cDNA with oligo(dT)15 primers. PCR was performed with cDNA and each primer. Samples were heated to 94℃ for 5 min and cycled 30 times at 94℃ for 1 min, 55℃ for 1.5 min, and 94℃ for 1 min, after which an additional extension step at 72℃ for 5 min was included. PCR products were electrophoresed in 8% polyacrylamide gel followed by staining in ethidium bromide. The iNOS, IL-1β, TNF-α, and β-actin primers produce amplified products at 311, 387, 374, and 349 bp, respectively.
Statistical analysis
The mean±S.E.M. was determined for each treatment group in a given experiment. When significant differences occurred, treatment groups were compared to the vehicle control using a Dunnett's two-tailed t test [15].
RESULTS
Characterization of glycoprotein (GDB) isolated from Dioscorea batatas
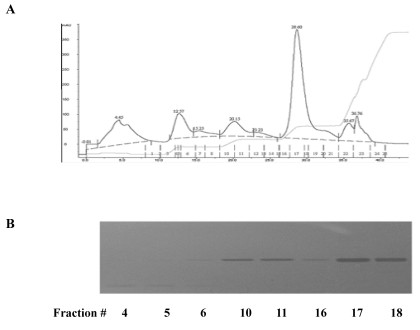
The lyophilized glycoproteins isolated from Dioscorea batatas were dissolved in 50 mM Tris-HCl buffer (pH 8.0) and subjected to an anion-exchange chromatography on a HiTrap Q HP column (5 ml) (GE Healthcare) equilibrated with 50 mM Tris-HCl buffer (pH 8.0). Fractionation of proteins with a step-wise salt gradient showed several peaks including major peak on fraction number 17 and 18 (Fig. 1A). SDS-PAGE analysis of the fractions showed that each fraction contains single band at 30 kD (Fig. 1B).
Fig. 1.
Characterization of glycoprotein isolated from Dioscorea batatas. (A) The lyophilized proteins were dissolved in 50 mM Tris-HCl buffer (pH 8.0) and subjected to an anion-exchange chromatography. Fractionation of proteins was performed by a step-wise salt gradient (0.1, 0.2, 0.3, and 1 M) NaCl in equilibrium buffer. (B) Peak fractions were further analyzed by SDS-PAGE.
Effects of GDB on inflammatory cell recruitment into the peritoneal cavity
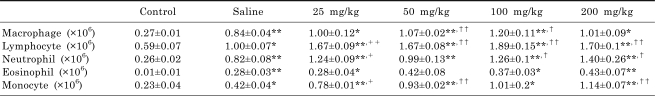
In order to examine the immunostimulatory effects of GDB, we analyzed the effects of GDB on the infiltration of inflammatory cells into the peritoneal cavity using an animal model. After treating mice with starch, differential counting of macrophages, lymphocytes, neutrophils, eosinophils, and monocytes was performed. Two weeks of GDB treatment significantly increased the recruitment of macrophages, lymphocytes, neutrophils, and monocytes into the peritoneal cavity (Table 1). Administration of 200 mg/kg GDB for 2 weeks showed no significant change including body weight from vehicle treated group (data not shown).
Table 1.
Effect of GDB on the recruitment of inflammatory cells
Animals orally received vehicle/GDB administrations (25, 50, 100 or 200 mg/kg) for 2 weeks. Differential counting for macrophages, lymphocytes, neutrophils, eosinophils, and monocytes was performed. The results are presented as the mean±S.E.M. of 10 mice per group *, **Denotes the significant differences (p<0.05, p<0.01) from the control group, †, ††denotes the significant differences (p<0.05, p<0.01) from the vehicle group.
Effects of GDB on lymphocyte proliferation
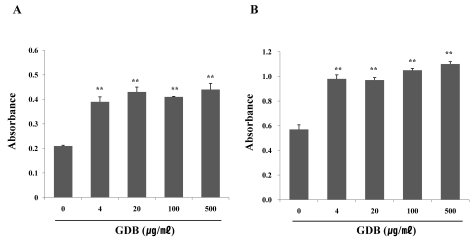
To further investigate the effects of GDB on immune cells, we isolated spleen cells from C57BL/6 mice and assessed the effects of GDB on LPS- or ConA-induced spleen cell proliferation. Treatment of spleen cells with GDB significantly increased the proliferation of B cells and T cells induced by LPS- and ConA, respectively (Fig. 2).
Fig. 2.
Effect of GDB on (A) LPS- and (B) ConA-stimulated splenic lymphocyte proliferation. Splenocytes were isolated from C57BL/6 mice. The results are presented as the mean±S.E.M. **Denote significant differences (p<0.05, p<0.01) vs. the control group. Experiments were repeated three times.
Effects of GDB on NK cell and macrophage function
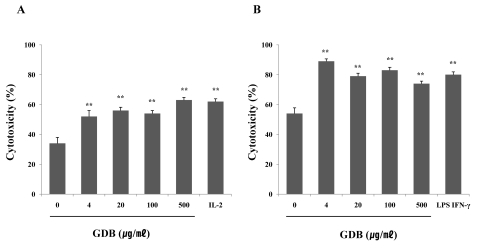
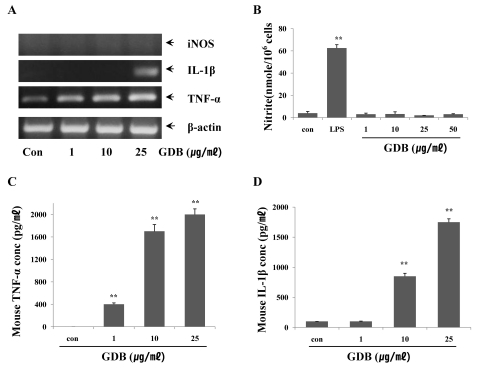
Treatment with GDB significantly increased the cytolytic capacity of NK cells by against YAC-1 in a dose-dependent manner, and this was comparable to the level reached by 1,000 U/ml of IL-2 (Fig. 3A). In the macrophage study, macrophage-induced cytotoxicity against B16 cells was also increased by GDB (Fig. 3B). In order to further confirm and investigate the mechanism of GDB on macrophage activation, we analyzed the effects of GDB on the cytokine expression including iNOS, IL-1β, and TNF-α in mouse macrophage cell line, RAW 264.7 cells. RT-PCR analysis showed that GDB increased the expression of IL-1β, and TNF-α, but not iNOS (Fig. 4A). To confirm the effect of GDB on iNOS expression, we tested the effect of GDB on the production of NO2-, an end product of NO. Fig. 4B showed that GDB has no effect on NO2- production, whereas LPS significantly increased the NO2- production. Since macrophages activated with LPS are known to secrete cytokines, we investigated the effect of GDB on the production of TNF-α and IL-1β. As shown in Fig. 4C and D, TNF-α and IL-1β production were significantly increased by GDB in a dose-related manner.
Fig. 3.
Effects of GDB on (A) NK cell- and (B) macrophage-mediated cytotoxicity. Cytotoxicity was measured as described in Methods and was expressed as the cytolytic percentage of target cells. IL-2 and LPS/IFN-γ were used as a positive control of NK cell- and macrophage-mediated cytotoxicity, respectively. The results are presented as the mean±S.E.M. **Denotes significant differences (p<0.01) vs. the control group. Experiments were repeated three times.
Fig. 4.
Effects of GDB on macrophage activation. (A) RAW264.7 cells were treated with GDB for 8 h. Total RNA was then isolated and analyzed for iNOS, IL-0, and TNF-α using RT-PCR. The production of (B) NO2-, (C) TNF-α, and (D) IL-1β were analyzed from the cell lysates treated with GDB for 24 h. The results are presented as the mean±S.E.M. **Denotes significant differences (p<0.01) vs. the control group. One representative of three experiments is shown.
DISCUSSION
In the present study, we demonstrate that GDB can exert significant immunomodulatory effects on specific immune responses mediated by macrophages, lymphocytes, and NK cells in vitro and in vivo. GDB treatment of C57BL/6 mice caused a significant increase in the numbers of lymphocytes and macrophages in the peritoneal cavity. Analysis of GDB activity in cell culture system further confirmed the immunostimulating activity. Mitogen-induced proliferation in splenocytes was markedly augmented by GDB, and these results indicate the potential stimulating effect of GDB on T cell and B cell proliferation, which may lead to increased immune responses. Macrophages and NK cells play an important role in the first line of immunological defense against tumor cells. We therefore accessed the effects of GDB on NK cell and macrophage function. The activity of NK cells and cytotoxicity of macrophages on tumor cells significantly increased in GDB-treated groups. Activated murine macrophages synthesize and release NO, which is considered cytotoxic agents toward certain tumor targets [16]. Our data showed that GDB could not enhance NO production even at the highest concentration tested. These results suggest that NO is not involved in the tumoricidal activity of macrophages induced by GDB.
Macrophages play a central role in the immune response by presenting antigen to lymphocytes during the development of specific immunity and by serving as supportive accessory cells to lymphocytes, in that they release soluble factors [17]. Macrophages also generate fundamental protective factors involved in host defense and inflammation. Once activated, macrophages produce a number of cytotoxic molecules [4]. Since there was a significant increase in IL-1β and TNF-α production by macrophage cell line RAW264.7 cells after GDB treatment, it is possible that the tumoricidal activity of macrophages could be mediated by cytotoxic molecules rather than by free radicals. The stimulant activity of GDB on macrophage functions suggests that phagocytic cells might be one of the targets for the immunopotentiating action of GDB. However, the mechanisms by which GDB exerts those effects on macrophages are not fully understood.
Even though the membrane receptor of GDB is not determined yet, some membrane proteins are assumed to act as receptors in macrophages. Possible examples are CD14, CR3, and Toll-like receptors (TLRs). CD14, a 55-kDa glycosylphosphatidylinositol-anchored protein expressed on the surface of monocytes and neutrophils [18], is known as LPS receptor and binds to LPS with high affinity. Complement receptor CR3 (also called Mac-1, CD11b/CD18, and αMβ2-integrin) is identified as the leukocyte membrane receptor for β-glucans [19]. They are expressed on the surface of neutrophils, monocytes, macrophages, and NK cells and are involved in numerous cell-cell and cell-substrate interactions [20]. TLRs constitute a mammalian transmembrane protein family and play crucial roles in innate immune recognition [21].
Immunostimulating activities of yam storage protein from Dioscorea alata and japonica have been described previously [22,23]. Yam is an important crop, which is basically made up of carbohydrates but also constitutes an important source of proteins accounting for 1~3% of the fresh tubers [24]. Two proteins have been identified in yam tubers, dioscorins and phytoglycoproteins [25,26]. Dioscorins isolated form D. alata activate MAP kinases (ERK, p38, and JNK) and NF-κB via the Toll-like receptor 4 (TLR-4) signal transduction pathway and stimulate pro-inflammatory cytokine expression, such as TNF-α, IL-1β, and IL-6, in RAW 264.7, murine bone marrow cells, and human monocytes ex vivo [23,27].
In summary, these experiments demonstrate that GDB is a potent enhancer of immune responses. Based on our findings, the most likely mechanism that can account for this biological effect involves the activation of macrophages. Due to the critical role that macrophage activation plays in innate immune response, the activation effects of GDB on macrophages suggest that this compound may represent useful immunopotentiating agents.
ABBREVIATIONS
- iNOS
inducibile nitric oxide synthase
- IFN-γ
interferon-γ
- LPS
lipopolysaccharide
- ConA
concanavalin A
References
- 1.Jin UH, Kim DI, Lee TK, Lee DN, Kim JK, Lee IS, Kim CH. Herbal formulation, Yukmi-jihang-tang-Jahage, regulates bone resorption by inhibition of phosphorylation mediated by tyrosine kinase Src and cyclooxygenase expression. J Ethnopharmacol. 2006;106:333–343. doi: 10.1016/j.jep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Wang C, Chang CT, Wang T. Effects of Taiwanese yam (Dioscorea japonica Thunb var. pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition. 2003;19:646–651. doi: 10.1016/s0899-9007(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi M, Higashi N, Taki H, Osawa T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and L-arginine-dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J Immunol. 1990;144:1425–1431. [PubMed] [Google Scholar]
- 4.Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am J Pharm Educ. 2006;70:102. doi: 10.5688/aj7005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon YJ, Kim YK, Lee M, Park SM, Han SB, Kim HM. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopolysaccharide-stimulated macrophages. J Pharmacol Exp Ther. 2000;294:548–554. [PubMed] [Google Scholar]
- 8.Lee KY, Jeon YJ. Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression in murine macrophages. Int Immunopharmacol. 2003;3:1353–1362. doi: 10.1016/S1567-5769(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 9.Li MH, Kothandan G, Cho SJ, Huong PT, Nan YH, Lee KY, Shin SY, Yea SS, Jeon YJ. Magnolol Inhibits LPS-induced NF-κB/Rel Activation by Blocking p38 Kinase in Murine Macrophages. Korean J Physiol Pharmacol. 2010;14:353–358. doi: 10.4196/kjpp.2010.14.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaidamashvili M, Ohizumi Y, Iijima S, Takayama T, Ogawa T, Muramoto K. Characterization of the yam tuber storage proteins from Dioscorea batatas exhibiting unique lectin activities. J Biol Chem. 2004;279:26028–26035. doi: 10.1074/jbc.M402139200. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Klimetzek V, Remold HG. The murine bone marrow macrophage, a sensitive indicator cell for murine migration inhibitory factor and a new method for their harvest. Cell Immunol. 1980;53:257–266. doi: 10.1016/0008-8749(80)90327-5. [DOI] [PubMed] [Google Scholar]
- 13.Gifford GE, Flick DA, AbdAllah NA, Fisch H. Production of a cytotoxin from phorbol myristate acetate-treated human promyelocytes. J Natl Cancer Inst. 1984;73:69–73. [PubMed] [Google Scholar]
- 14.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 16.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 17.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 18.Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le Beau MM. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988;239:497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- 19.Thornton BP, Větvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 20.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 21.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 22.Lin PL, Lin KW, Weng CF, Lin KC. Yam storage protein dioscorins from dioscorea alata and dioscorea japonica exhibit distinct immunomodulatory activities in mice. J Agric Food Chem. 2009;57:4606–4613. doi: 10.1021/jf8038499. [DOI] [PubMed] [Google Scholar]
- 23.Fu SL, Hsu YH, Lee PY, Hou WC, Hung LC, Lin CH, Chen CM, Huang YJ. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem Biophys Res Commun. 2006;339:137–144. doi: 10.1016/j.bbrc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Shewry PR. Tuber storage proteins. Ann Bot. 2003;91:755–769. doi: 10.1093/aob/mcg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh PS, Lim KT. HeLa cells treated with phytoglycoprotein (150 kDa) were killed by activation of caspase 3 via inhibitory activities of NF-kappaB and AP-1. J Biomed Sci. 2007;14:223–232. doi: 10.1007/s11373-006-9140-4. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Lim KT. Phytoglycoprotein inhibits interleukin-1beta and interleukin-6 via p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated RAW 264.7 cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:45–54. doi: 10.1007/s00210-007-0253-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu YW, Shang HF, Wang CK, Hsu FL, Hou WC. Immunomodulatory activity of dioscorin, the storage protein of yam (Dioscorea alata cv. Tainong No. 1) tuber. Food Chem Toxicol. 2007;45:2312–2318. doi: 10.1016/j.fct.2007.06.009. [DOI] [PubMed] [Google Scholar]