Abstract
Neurofibrillary tangle (NFT) is a characteristic hallmark of Alzheimer's disease. GSK3β has been reported to play a major role in the NFT formation of tau. Dysfunction of autophagy might facilitate the aggregate formation of tau. The present study examined the role of GSK3β-mediated phosphorylation of tau species on their autophagic degradation. We transfected wild type tau (T4), caspase-3-cleaved tau at Asp421 (T4C3), or pseudophosphorylated tau at Ser396/Ser404 (T4-2EC) in the presence of active or enzyme-inactive GSK3β. Trehalose and 3-methyladenine (3-MA) were used to enhance or inhibit autophagic activity, respectively. All tau species showed increased accumulation with 3-MA treatment whereas reduced with trehalose, indicating that tau undergoes autophagic degradation. However, T4C3 and T4-2EC showed abundant formation of oligomers than T4. Active GSK3β in the presence of 3-MA resulted in significantly increased formation of insoluble tau aggregates. These results indicate that GSK3β-mediated phosphorylation and compromised autophagic activity significantly contribute to tau aggregation.
Keywords: Tau, Autopahgy, Glycogen synthase kinase 3β, Trehalose, Neurofibrillary tangles
INTRODUCTION
Intracellular accumulation of the microtubule-associated protein tau as the NFTs is one of the characteristic features of several diseases known as tauopathies, which include AD and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17). NFTs area mainly composed of paired helical filaments (PHFs), which are formed from abnormally hyperphosphorylated tau [1]. There is increasing evidence that inappropriate phosphorylation of tau, which leads to tau dysfunction, results in decreased cell viability. Indeed, in all neurodegenerative diseases in which tau pathology has evidence that glycogen synthase kinase 3β (GSK3β) may play a major role in regulating tau phosphorylation in pathological conditions [2]. Increased expression of GSK3β results in the increased phosphorylation of tau at numerous sites including PHF-1 epitope [3]. Furthermore, there is evidence for abnormally increased activation of GSK3β in AD brain [4].
Along with aberrant phosphorylation, caspase cleavage of tau has been reported to play an important role in the aggregation of tau [5]. Although tau is present predominantly as an intact and full-length form in AD brain, tau truncated at Asp421 has been found in AD brain. Caspase-cleaved tau, albeit not a large amount, may play a significant role in the formation of NFTs, given that truncated tau is more fibrillogenic than wild type tau [5] and contributes to the nucleation-dependent filament formation of tau [6]. Although the role of NFTs as a toxic mediator in neuronal dysfunction and death in AD is still not clear, the tau aggregation is closely related with the pathology of AD [7].
Autophagy has been reported to play important roles in many diseases such as cancer [8] and neurodegenerative diseases [9]. Soluble, misfolded proteins in the cytosol are normally degraded by the ubiquitin-proteasome system. However, when the levels of such proteins overload the ubiquitin-proteasome system, they may form toxic oligomers or large aggregates that cause neurodegeneration [10]. Therefore, dysfunction of autophagic pathway might contribute to the pathology of various neurodegenerative disorders including AD and Parkinson's disease (PD) [9]. Mice with neuronally confined autophagy-gene knockouts develop intraneuronal aggregates and neurodegeneration [11]. It has been reported that alterations of autophagic degradation pathway are present in AD. For instance, the number of lysosomal-endosomal compartments is increased in vulnerable neuronal populations of AD brains [12]. The expression of cathepsins is increased and concentrated in senile plaques [13].
The present study was undertaken to determine the role of autophagic degradation pathway in the accumulation and its consequent aggregation of post-translationally modified tau. To achieve this goal wild type or abnormal tau species were expressed in the presence of constitutively active GSK3β or kinase-dead GSK3β in the milieu of modified autophagic activity, and the propensity of intracellular accumulation and aggregation of tau was examined. The present study demonstrates that constitutively active GSK3β and 3-MA-induced inhibition of autophagy resulted in increased intracellular accumulation and aggregation of tau and that wild type tau phosphorylated by GSK3β is resistant to autophagic degradation. These results strongly indicate that compromised autophagic activity significantly facilitates the accumulation and aggregate formation of post-translationally modified tau.
METHODS
Plasmid constructs
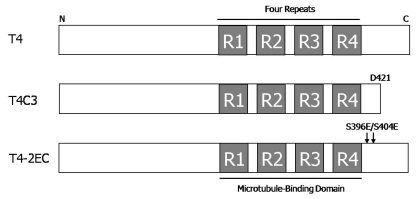
All plasmid constructs (Fig. 1) used in this study have been previously described [14,15]. T4 contains human tau with four microtubule-binding repeats but without exons 2 and 3. T4C3, the last 20 amino acids deleted, mimics caspase-cleaved tau and T4-2EC, pseudophosphorylated at serine 396 and 404E residues, mimics phosphorylation at PHF-1 epitope. All the mutations were confirmed by DNA sequencing analysis. GSK3β-S9A-HA, which is a constitutively active form of GSK3β, was constructed in pcDNA3.1(-) and kinase dead-GSK3β-HA construct, in which lysine residues at 85 and 86 were mutated to alanines, was a generous gift of G. Meares at the University of Alabama at Birmingham, AL, USA.
Fig. 1.
Plasmid constructs of human tau. All types of human tau constructs are contained four microtubule-binding repeats but without exons 2 and 3. T4 is wild type of tau in pcDNA3.1(-). T4C3 is mimicking caspase-cleaved tau form which was deleted the last 20 amino acids at D421 in pcDNA3.1(+). Mutant constructs T4-2EC (S396E/S404E) is generated by mutating the serine 396 and 404 sites to glutamic acid to mimic phosphorylation.
Cell culture
Chinese hamster ovary (CHO) cells were grown in D-MEM/F-12 (Gibco, Dulbecco's Modified Eagle's Medium: Mixture F-12 (1:1)) supplemented with 5% fetal bovine serum (Gibco) and 2 mM L-glutamine (Gibco), 10 units/ml penicillin (Gibco), 10 µg/ml streptomycin at 37℃ in a humidified incubator with 5% CO2.
Transient transfections and drug treatments
Tau constructs alone or with either GK3β-S9A or GSK3 β-kinase-dead were transiently transfected into CHO cells using LT-1 (Mirus) transfection reagent according to the manufacturer's protocol. After transfection, the cells were treated 5 mM 3-MA (Sigma) or 200 mM trehalose (Sigma). Twenty-four hrs after drug treatments, cells were washed with ice-cold PBS and then harvested and processed for below assays.
Immunoblotting
Cell lysates were sonicated briefly and centrifuged. Protein concentrations in the supernatants were determined using the bicinchoninic acid assay (Sigma). Equal amounts of proteins were diluted with 2X protein loading buffer (0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 25 mM dithiothreitol, 2% SDS, and 10% glycerol with bromophenol blue as the tracking dye), incubated in a boiling water bath for 5 min, separated on 8~15% SDS-polyacrylamide gels, transferred to PVDF membrane (GE Healthcare), and blots were blocked in 5% nonfat dry milk in TBST (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.05% Tween 20) for 30 min at room temperature, and probed with the indicated antibodies in same buffer overnight at 4℃. The membranes were then washed with TBST and incubated with HRP-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch) or HRP-conjugated goat anti-mouse IgG (Jackson Immuno-Research) for 2 hrs at room temperature. The membranes were rinsed with TBST. The blots were visualized with enhanced chemiluminescence (GE Healthcare) and exposed on film (Kodak). Gel images were scanned and quantified using Image Quant software (Molecular Dynamics, Sunnyvale, California, USA). For fair comparison, total protein levels in each β-actin levels were used as loading control. The following antibodies were used in this study; anti-5A6 for total tau (phosphorylation-independent); anti-LC 3 antibody (MBL) for autophagic activity; Anti-GSK3β antibody (Cell Signaling); anti-β-actin (Sigma).
Sarkosyl fractionation assay
The assay was carried out as described previously [15]. CHO cells were co-transfected with one of tau constructs and with or without either active GSK3β or kd-GSK3β. Twenty four hours after transfection, the cells were treated 5 mM 3-MA or 200 mM trehalose for 24 hrs, then rinsed with ice-cold PBS, collected by spinning at 13,000 rpm for 10 min at 4℃, resuspended in RAB buffer (100 mmol/l Mes, 1 mmol/l EGTA, 0.5 mmol/l MgSO4, 750 mmol/l NaCl, and 20 mmol/l NaF) containing protease inhibitors; and then homogenized with 30 strokes using a tissue grinder. The homogenates were centrifuged at 13,000 rpm for 20 min and the supernatants were collected as RAB fraction. The pellets were resuspended with RIPA buffer (50 mmol/l Tris-HCl at pH 8.0, 150 mmol/l NaCl, 1% Nonidet P-40, 5 mmol/l EDTA, 0.5% sodium deoxycholate, and 0.1% SDS) containing protease inhibitors, sonicated briefly and then incubated on ice for 30 min. The homogenates were centrifuged at 13,000 rpm for 20 min and the supernatants were collected as RIPA fraction. The pellets were resuspended with RAB buffer containing 1% N-lauroylsarcosine (sarkosyl) in Mes buffer (20 mmol/l Mes at pH 6.8, 80 mmol/l NaCl, 1 mmol/l MgCl2, 2 mmol/l EGTA, 10 mmol/l NaH2PO4, and 20 mmol/l NaF) with protease inhibitors, vortexed for 30 min at 15~25℃, incubated overnight at 4℃, and then centrifuged at 13,000 rpm for 30 min at 15~25℃. The supernatants (sarkosyl-soluble fraction) were collected and diluted with protein loading buffer, and the pellets (sarkosyl-insoluble fraction) were resuspended in 2X protein loading buffer and incubated in a boiling water bath for 5 min. Samples were separated by SDS-polyacrylamide gel electrophoresis, transferred, and blotted with 5A6 antibody.
Immunocytochemistry
These procedures were modified from previously described protocols [16]. The cells were transiently transfected individually with each tau construct in the absence or presence of active GSK3β. Twenty four hours after transfection cells were treated 5 mM 3-MA. Two days after transfection the cells were rinsed with PBS and fixed at room temperature for 1 hr in fixation buffer (3% paraformaldehyde, 0.2% glutaraldehyde, 1 mM MgCl2, 1 mM EGTA, 30% (v/v) glycerol in 70 mM PIPES, pH 6.8). Cells were washed 3 times with PBS then permeabilized with 0.2% Triton X-100 in PBS for 2 min. After rinsing with PBS, cells were blocked with 4% bovine serum albumin in PBS to reduce background before staining. The cells were incubated for 1.5 hrs with a 5A6 antibody diluted in 0.4% bovine serum albumin. After rinsing with PBS, the cells were incubated with Texas Red-conjugated goat anti-mouse IgG (Jackson Laboratories). Cells were then washed extensively in PBS before being incubated in a thioflavin-S solution (Sigma) (0.005%) for 10 min. The cells were then washed in 70% ethanol and water before mounting [17]. Cells images were captured with OLYMPUS Fluoview FV1000 Confocal Microscope and processed with Olympus FluoView software.
Statistical analysis
All data were analyzed by ANOVA using the statistical software SPSS (version 7.5). Values were expressed as mean±SE and considered significantly different when p<0.05.
RESULTS
Tau is degraded via autophagic degradation pathway
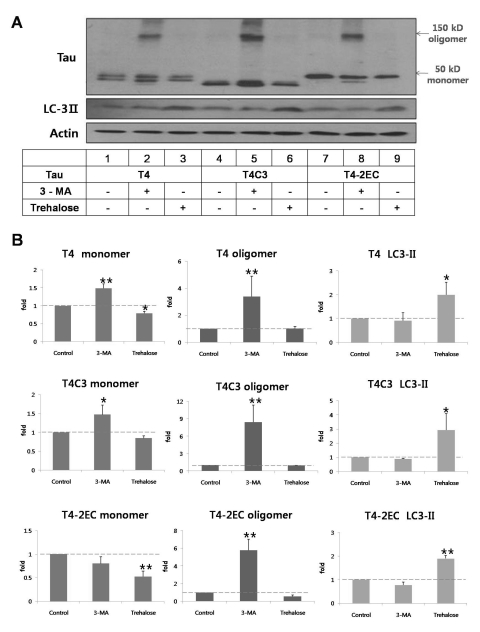
To determine the role of autophagy in tau degradation, tau levels were measured in the presence of 3-MA, autophagic inhibitor [18] or trehalose, autophagic inducer [19]. In all types of tau species, intracellular levels of tau were significantly increased with 3-MA treatment (Fig. 2). Especially, oligomeric form of tau, which is presumed to precede the aggregate formation, was observed with 3-MA treatment in all tau species (Fig. 2). T4C3 showed highest amount of oligomers compared to other tau species, albeit not significant. To the contrary, trehalose treatment exhibited significantly decreased level of tau in all tau species. In addition, no oligomers were observed with trehalose treatment (Fig. 2). Facilitation of autophagic degradation with trehalose was confirmed with increased level of LC3-II, an autophagic degradation marker. The fact that modulation of autophagic activity directly affects the intracellular level of tau clearly demonstrates that tau is degraded via autophagy pathway.
Fig. 2.
Tau is degraded via the autophagic pathway. Representative immunoblot (A) and quantitative analysis (B) of intracellular tau levels. Tau constructs were transiently transfected into CHO cells. One day after transfection, 3-MA (5 mM) or trehalose (200 mM) was added to the medium immunobloted and incubated for 24 hrs. Lysates were immunobloted with tau (5A6), LC3, or β-actin antibodies. (B) The intensity of monomers and oligomers was quantitated by a densitometer and calculated as a fold to untreated control of each tau. Results are plotted as mean±SD from three independent experiments (n=3). 3-MA treatment caused the significant accumulation of oligomers in all tau species. Trehalose treatment exhibited decreased intracellular tau levels. Increased LC3-II levels indicate facilitated autophagic activity with trehalose treatment. Arrows indicate monomeric (~50 kDa) and oligomeric (~150 kDa) tau, respectively. *p<0.05, **p<0.01 to each control.
GSK3β-induced phosphorylation of T4 is resistant to trehalose-facilitated autophagic degradation
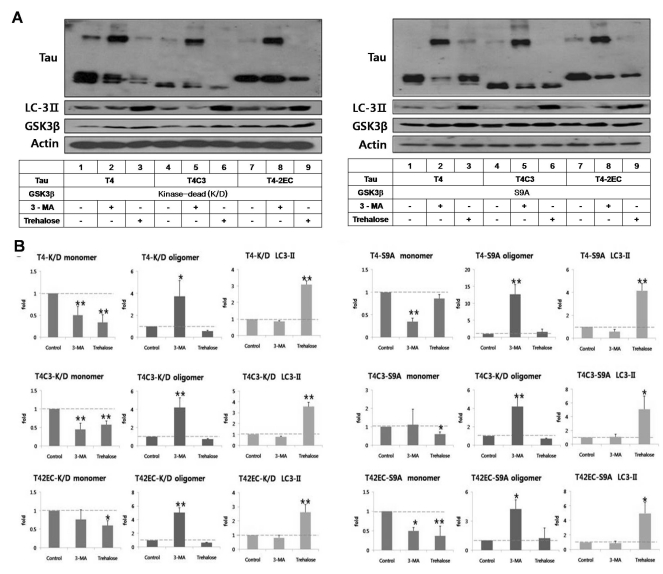
To determine the role of GSK3β-mediated phosphorylation in autophagic degradation of tau, intracellular level of tau was examined in the presence of enzyme-inactive (kinase-dead) or constitutively active GSK3β (S9A) (Fig. 3). As shown in Fig. 2A & C, intracellular level of each tau species in the presence of kinase-dead GSK3β was similar to tau-only expression in Fig. 1. However, level of wild type tau (T4) was not attenuated with trehalose treatment in the presence of active GSK3β, suggesting that GSK3β-mediated phosphorylation might interfere autophagic degradation of tau (Fig. 3). Interestingly, attenuated clearance of tau was not observed in abnormal tau species, T4C3 and T4-2EC, even in the presence of active GSK3β. This phenomenon might be due to the fact that wild type tau is more efficiently phosphorylated than abnormal tau species by GSK3β as previously [20]. 3-MA treatment significantly increased the formation of oligomers in all tau species.
Fig. 3.
GSK3β-phosphorylated T4 is less efficient in trehalose-facilitated autophagic degradation. (A) Representative immunoblots of tau levels with enzyme-inactive GSK3β (kinase-dead (k/D), left) or constitutively active GSK3β (S9A, right), respectively. Tau and each GSK3β constructs were transiently co-transfected into CHO cells. One day after transfection, 3-MA (5 mM) or trehalose (200 mM) was added to the medium and incubated for 24 hrs. Lysates were immunobloted with tau (5A6), LC3, GSK3β, or β-actin antibodies. (B) Quantitative analysis of tau levels. The intensity of monomers and oligomers was quantitated and calculated as a fold to untreated control of each tau. Results are plotted as mean±SD from three independent experiments (n=3). In the presence of kinase-dead GSK3β, 3-MA treatment resulted in the shift of monomeric tau to oligomeric tau in all tau species and trehalose treatment showed significantly decreased intracellular tau level of all tau species. In the presence of active GSK3β, 3-MA treatment resulted in the increased accumulation of oligomeric tau in all tau species. Trehalose treatment resulted in facilitated clearance of T4C3 and T4-2EC. However, phosphorylated T4 by GSK3β was not efficiently degraded, which indicates phosphorylation hinders autophagic degradation. LC3-II levels indicate facilitated autophagic activity with trehalose treatment. Arrows indicate monomeric (~50 kDa) and oligomeric (~150 kDa) tau, respectively. *p<0.05, **p<0.01 to each control.
Active GSK3β and 3-MA treatment result in the formation of thioflavin-S-positive inclusions
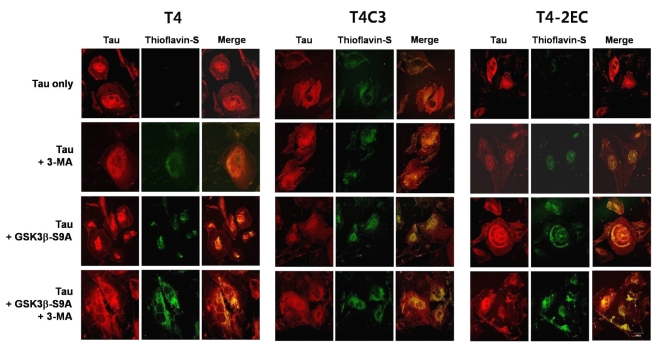
To determine whether intracellular accumulation of tau proceeds to the aggregate formation, thioflavin-S staining was carried out. Thioflavin-S binding is a known indicator of insoluble protein aggregates with β-pleated sheet structure and thioflavin-S readily stains the intracellular neurofibrillary tangles in AD brain [21]. Thioflavin-S-positive inclusions were not observed in T4- or T4-2EC- expressing cells (Fig. 4). However, T4C3 expressing cells showed thioflavin-S-positive inclusions even in basal condition (Fig. 4). These data suggest that T4C3 is more fibililogenic than T4 and T4-2EC. Expression of kinase-dead GSK3β did not affect the formation of thioflavin-S-positive inclusions in all tau species (data not shown).
Fig. 4.
Activ me GSK3β and 3-MA treatment result in the formation of thioflavin-S-positive inclusions of tau in a co-operative manner. Each tau and active GSK3β or enzyme-inactive GSK3β constructs were transiently co-transfected into CHO cells on coverslips. One day after transfection, 3-MA (5 mM) or trehalose (200 mM) was added to the medium and incubated for 24 hrs. Cells fixed with 3% PFA were immunostained with an antibody that recognizes total tau (red) and counterstained with thioflavin-S (green). When tau and thioflavin-S staining co-localize, the merged image is yellow/orange. 3-MA treatment or active GSK3β exhibited thioflavin-S-positive tau inclusion in all tau species. Inhibition of autophagic activity and GSK3β-induced phosphorylation facilitated aggregate formation of tau in a co-operative manner. The scale bar shows 50 µm.
Expression of constitutively active GSK3β resulted in the formation of thioflavin-S-positive inclusions in all tau species. Autophagic inhibition with 3-MA also exhibited the formation of thioflavin-S positive inclusions in all tau species. Immunostaining with tau antibody showed that thioflavin-S-positive inclusions were composed of tau. 3-MA treatment further increased GSK3β-induced thioflavin-S-positive inclusions in all tau species (Fig. 4), suggesting that GSK3β-mediated phosphorylation and autophagic inhibition facilitate the formation of tau aggregates in a cooperative manner. In addition, expression of kinase-dead GSK3β exhibited negligible effect on the 3-MA-induced facilitation of thioflavin-S-positive inclusions in all tau species (data not shown), suggesting that the enzyme activity of GSK3β may be necessary to affect the propensity of inclusion formation of tau.
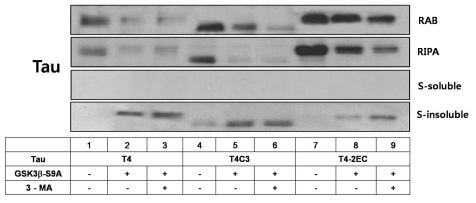
3-MA potentiates GSK3β-mediated formation of Sarkosyl-insoluble aggregates
To determine whether thioflavin-S-positive tau inclusions eventually form insoluble aggregates, the presence of tau-positive Sarkosyl-insoluble materials was examined. Tau levels were decreased with active GSK3β and 3-MA treatment at RAB and RIPA fractions. However, tau-positive Sarkosyl-insoluble materials were observed with active GSK3β and further increased with concurrent 3-MA treatment (Fig. 5). These results suggest that GSK3β-mediated phosphorylation and 3-MA-induced inhibition of autophagy shift the intracellular pool of tau toward more fibrillogenic forms, favoring aggregate formation of tau.
Fig. 5.
Co-operative facilitation of sarcosyl-insoluble aggregation with active GSK3β and 3-MA treatment. Cells were transiently transfected with each tau construct in the presence of constitutively active GSK3β or kinase-dead GSK3β. One day after transfection, the cells were treated with 3-MA for 24 hrs. Cell lysates were homogenized and then fractionated into RAB, RIRA, Sarkosyl-soluble, and Sarkosyl-insoluble fractions. After sarkosyl fractionation assay, each fraction was separated by SDS-PAGE and immunobloted with total tau antibody (5A6). Even in the absence of active GSK3β, partitioning into the sarkosyl-insoluble fraction was observed in T4C3, an aggregation-prone tau species, whereas not observed in T4 and T4-2EC. In the presence of active GSK3β, sarkosyl-insoluble aggregates were observed in all tau species and 3-MA treatment resulted in the increased amount of sarkosyl-insoluble aggregates.
DISCUSSION
The present study demonstrates that inhibition of autophagic degradation pathway results in the accumulation and subsequent aggregation of tau and that GSK3β-mediated phosphorylation of wild type tau significantly interfered autophagic degradation of tau. Intracellular buildup of oligomeric tau species may be the initial step in the aggregation process of tau given that the aggregation of tau may be concentration-dependent [22].
The underlying mechanism of tau degradation is still not clear. It has been reported that tau is not normally degraded by the proteasome given the fact that tau degradation by proteasome requires polyubiqutination of tau but it is difficult to observe polyubiqutinated tau in cells [23]. It has been suggested that autophagy might play a key role in the degradation of protein aggregates in age-related neurodegenerative diseases [24]. Activation of autophagic activity such as proliferation of lysosomes and increased expression of lysosomal hydrolases, was observed during the progression of neurodegenerative disorders [25], suggesting that compensatory activation of autophagy was made to counteract the accumulation of insoluble proteins. In the present study, data showed that autophagic activity regulates turnover of tau. Inhibition of autophagic activity resulted in the accumulation of tau in all tau species whereas activation of autophagy facilitated the clearance of tau species. In accordance with a previous report that autophagic inhibition with chloroquine resulted in the formation of oligomers of wild type tau [25], inhibition of autophagic degradation with 3-MA exhibited the formation of tau oligomers, which are presumed to precede the formation of insoluble aggregates. However, no differential accumulation pattern of oligomers was observed among tau species.
It has been suggested that post-translational modifications of tau such as aberrant phosphorylation contribute to the formation of NFTs. Several studies have provided evidence that phosphorylation of key sites on tau has a strong impact on the normal function of tau and likely contributes to its pathological role including the tendency of aggregate formation [26,27]. It has been also reported that phosphorylation of tau impedes turnover of tau, resulting in the intracellular accumulation of phosphorylated tau [28]. In this study, the phosphorylation of T4 by GSK3β resulted in significantly increased intracellular level of tau, indicating that phosphorylation of T4 by GSK3β causes the intracellular accumulation, which is supported by a previous report that phosphorylated tau is less efficiently degraded [28]. Present data demonstrate that extensive phosphorylation of wild type tau by active GSK3β makes tau less efficient in the autophagic degradation. Given that presence of active GSK3β does not affect the autophagic degradation of other tau species, which are less efficiently phosphorylated tau forms by GSK3β, phosphorylation is considered to be the key mediator that interferes autophagic degradation of tau. However, further studies are necessary to understand the mechanism by which phosphorylation of tau interferes autophagic activity of wild type tau. Although GSK3β-mediated phosphorylation of wild type tau caused an accumulation of phosphorylated tau including phosphorylation at the PHF-1 epitope, which can form aggregates in vitro [29], aggregation of tau was observed in situ. These data suggest that phosphorylation may not be sufficient to induce the formation of insoluble aggregates and other factors be necessary to facilitate aggregate formation of full length tau in situ. Interestingly, aggregation of T4-2EC was observed in the presence of active GSK3β, although T4-2EC is not an efficient substrate of GSK3β. In addition, T4-2EC showed increased sarkosyl-insoluble inclusions with the presence of active GSK3β. The aggregation of T4-2EC by active GSK3β might be due to further phosphorylation at other sites by active GSK3β. However, it is still possible that GSK3β may phosphorylate other intracellular mediators, which in turn facilitate the aggregate formation of tau, given the numerous roles of GSK3β in cells [2]. Inhibition of autophagic activity facilitated aggregate formation of T4-2EC.
Caspase cleavage of tau has been reported to facilitate aggregate formation of tau [6,30], given the fact that tau is a substrate for caspase-3 and its cleaved product truncated at Asp421 aggregates more efficiently [30]. The present data demonstrated that T4C3 formed increased sarkosyl insoluble aggregates in the presence of active GSK3β. Although T4C3 was not efficiently phosphorylated by GSK3 β compared to wild type tau [16], minimally phosphorylated T4C3 by GSK3β may act as a nucleation seed for the remaining unphosphorylated T4C3. Autophagic activation facilitated the degradation of T4C3 whereas 3-MA-induced inhibition of autophagy resulted in the accumulation of oligomers. However, presence of active GSK3β does not affect autophagic degradation of T4C3, which is presumably due to less efficient phosphorylation status of T4C3.
Taken together the present study demonstrates that inhibition of autophagic degradation pathway causes the intracellular accumulation and subsequent inclusions of tau. Furthermore, GSK3β-mediated phosphorylation of wild type tau significantly interferes autophagic degradation of tau. Therefore, the results strongly suggest that compromised autophagic activity might be a key factor in the aggregation of tau, which is posttranslationally modified.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-E00028).
ABBREVIATIONS
- AD
Alzheimer's disease
- NFT
neurofibrillary tangle
- FTDP-17
parkinsonism linked to chromosome 17
- PHFs
paired helical filaments
- GSK3β
glycogen synthase kinase 3β
References
- 1.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubuleassociated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Lovestone S, Hartley CL, Pearce J, Anderton BH. Phosphorylation of tau by glycogen synthase kinase-3 beta in intact mammalian cells: the effects on the organization and stability of microtubules. Neuroscience. 1996;73:1145–1157. doi: 10.1016/0306-4522(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 4.Leroy K, Boutajangout A, Authelet M, Woodgett JR, Anderton BH, Brion JP. The active form of glycogen synthase kinase-3beta is associated with granulovacuolar degeneration in neurons in Alzheimer's disease. Acta Neuropathol. 2002;103:91–99. doi: 10.1007/s004010100435. [DOI] [PubMed] [Google Scholar]
- 5.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol Med. 2005;11:164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Kim NY, Suh YA, Lee C. Involvement of ROS in curcumin-induced autophagic cell death. Korean J Physiol Pharmacol. 2011;15:1–7. doi: 10.4196/kjpp.2011.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 10.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 12.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Takeda M, Suzuki H, Hattori H, Tada K, Hariguchi S, Hashimoto S, Nishimura T. Abnormal distribution of cathepsins in the brain of patients with Alzheimer's disease. Neurosci Lett. 1991;130:195–198. doi: 10.1016/0304-3940(91)90395-a. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Matthews TA, Johnson GV. Site-specific phosphorylation and caspase cleavage differentially impact tau-microtubule interactions and tau aggregation. J Biol Chem. 2006;281:19107–19114. doi: 10.1074/jbc.M511697200. [DOI] [PubMed] [Google Scholar]
- 15.Chun W, Johnson GV. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J Biol Chem. 2007;282:23410–23417. doi: 10.1074/jbc.M703706200. [DOI] [PubMed] [Google Scholar]
- 16.Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem. 2004;279:54716–54723. doi: 10.1074/jbc.M403364200. [DOI] [PubMed] [Google Scholar]
- 17.Sun A, Nguyen XV, Bing G. Comparative analysis of an improved thioflavin-s stain, Gallyas silver stain, and immunohistochemistry for neurofibrillary tangle demonstration on the same sections. J Histochem Cytochem. 2002;50:463–472. doi: 10.1177/002215540205000403. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alphasynuclein. J Biol Chem. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 20.Chun W, Waldo GS, Johnson GV. Split GFP complementation assay: a novel approach to quantitatively measure aggregation of tau in situ: effects of GSK3beta activation and caspase 3 cleavage. J Neurochem. 2007;103:2529–2539. doi: 10.1111/j.1471-4159.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Tatebayashi Y, Akagi T, Chui DH, Murayama M, Miyasaka T, Planel E, Tanemura K, Sun X, Hashikawa T, Yoshioka K, Ishiguro K, Takashima A. Aberrant tau phosphorylation by glycogen synthase kinase-3beta and JNK3 induces oligomeric tau fibrils in COS-7 cells. J Biol Chem. 2002;277:42060–42065. doi: 10.1074/jbc.M202241200. [DOI] [PubMed] [Google Scholar]
- 22.Kuret J, Chirita CN, Congdon EE, Kannanayakal T, Li G, Necula M, Yin H, Zhong Q. Pathways of tau fibrillization. Biochim Biophys Acta. 2005;1739:167–178. doi: 10.1016/j.bbadis.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Feuillette S, Blard O, Lecourtois M, Frebourg T, Campion D, Dumanchin C. Tau is not normally degraded by the proteasome. J Neurosci Res. 2005;80:400–405. doi: 10.1002/jnr.20414. [DOI] [PubMed] [Google Scholar]
- 24.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16:75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- 25.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 26.Rankin CA, Sun Q, Gamblin TC. Pseudo-phosphorylation of tau at Ser202 and Thr205 affects tau filament formation. Brain Res Mol Brain Res. 2005;138:84–93. doi: 10.1016/j.molbrainres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM. Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 28.Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006;400:511–520. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci USA. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun W, Johnson GV. The role of tau phosphorylation and cleavage in neuronal cell death. Front Biosci. 2007;12:733–756. doi: 10.2741/2097. [DOI] [PubMed] [Google Scholar]