Abstract
A large body of evidence has indicated that induction of endogenous antioxidative proteins seems to be a reasonable strategy for delaying the progression of cell injury. In our previous study, cilostazol was found to increase the expression of the antioxidant enzyme heme oxygenase-1 (HO-1) in synovial cells. Thus, the present study was undertaken to examine whether cilostazol is able to counteract tumor necrosis factor-α (TNF-α)-induced cell death in endothelial cells via the induction of HO-1 expression. We exposed human umbilical vein endothelial cells (HUVECs) to TNF-α (50 ng/ml), with or without cilostazol (10 µM). Pretreatment with cilostazol markedly reduced TNF-α-induced viability loss in the HUVECs, which was reversed by zinc protoporphyrine IX (ZnPP), an inhibitor of HO-1. Moreover, cilostazol increased HO-1 protein and mRNA expression. Cilostazol-induced HO-1 induction was markedly attenuated not only by ZnPP but also by copper-protoporphyrin IX (CuPP). In an assay measuring peroxisome proliferator-activated receptor-γ (PPAR-γ) transcription activity, cilostazol directly increased PPAR-γ transcriptional activity which was completely abolished by HO-1 inhibitor. Furthermore, increased PPAR-γ activity by cilostazol and rosiglitazone was completely abolished in cells transfected with HO-1 siRNA. Taken together, these results indicate that cilostazol up-regulates HO-1 and protects cells against TNF-α-induced endothelial cytotoxicity via a PPAR-γ-dependent pathway.
Keywords: Cilosatzol, PPAR-γ, HO-1, Endothelial cells
INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs), members of the nuclear receptor superfamily of ligand-activated transcriptional regulators, have been implicated in a number of diverse physiological functions including the control of cellular differentiation, embryonic development, lipid metabolism, and regulation of energy balance [1]. Among PPAR subclasses, PPAR-γ is expressed in monocytes, macrophages, smooth muscle cells, and endothelium [2,3]. PPAR-γ is activated by several natural ligands including nitro lipids [4]. It is also activated by synthetic ligands such as thiazolidinediones [3,4]. Activation of PPAR-γ inhibits the proliferation of vascular smooth muscle cells [5,6] and also has anti-inflammatory effects [7,8]. In addition, PPAR-γ plays a central role in regulating the expression of genes related to lipid trafficking, cell proliferation, and inflammatory signaling [9]. In vitro experiments using human endothelial cells indicated that PPAR-γ inhibits interferon-r (IFN-r)-induced chemokine expression and decreases lymphocyte chemotaxis [10]. Although PPAR-γ has been recognized as an important regulator in endothelial biology [3], the precise role of PPAR-γ in the regulation of endothelial survival and proliferation is still largely unknown.
Cilostazol, an inhibitor of phosphodiesterase type III, has been reported to produce potent anti-inflammatory effects. Several lines of evidence have suggested that cilostazol provides beneficial effects that help to protect cells from injuries from diverse causes. Kim et al. [11] reported a protective effect of cilostazol against lipopolysaccharide (LPS)-induced apoptosis in human umbilical vein endothelial cells (HUVECs). Hong et al. [12] demonstrated the ability of cilostazol to prevent tumor necrosis factor-α (TNF-α)-induced cell death in human neuroblastoma cells. An in vivo study in an animal model of middle cerebral artery occlusion and reperfusion also provided evidence of the protective effect of cilostazol against cerebral infarct through anti-apoptotic actions. These reports have provided consistent evidence that cilostazol can reduce apoptotic cell death by inhibiting the mitochondria-dependent apoptotic signaling pathway [13]. In our recent study, cilostazol was found to suppress cytokine production in synovial cells via mediation of cAMP-dependent protein kinase activation-coupled NF-E2 related factor (Nrf2)-linked heme oxygenase-1 (HO-1) expression [14]. HO-1 is an inducible isoform of HO, and its induction has been shown to be cytoprotective [15]. HO catalyzes the breakdown of heme into iron, biliverdin, and carbon monoxide (CO) [16]. Both biliverdin and CO cause vasculature dilation and inhibit the proliferation of vascular smooth muscle cells [17]. Induction of HO-1 by either genetic approaches or pharmacological intervention has been shown to be effective in preventing or treating pulmonary hypertension in animal models [18,19]. Indeed, several studies support the notion [19] that HO-1 exerts an essential protective role in the vessel wall during atherogenesis [20,21]. However, the mechanism underlying the protection of endothelial cells by HO-1 from TNF-α-induced cytotoxicity remains to be clarified. The demonstration that PPAR-γ activation promotes the reversal of multiple metabolic abnormalities in moderately obese diabetic men [22], combined with its emerging role in the endothelium, suggests PPAR-γ represents a viable therapeutic target. A recent study has suggested that activation of PPAR-γ induces expression of HO-1 in human umbilical vein endothelial cells and human umbilical artery or vein smooth muscle cells [23].
METHODS
Cell cultures
HUVECs (CRL-1730; American Type Culture Collection, Manassas, VA) were cultured in the endothelial cell basal media-2 (EGM-2) Bullet kit media (Clonetics, BioWhittaker, San Diego, CA). Cells were grown to confluence at 37℃ in 5% CO2 and used for experiments before passage 6.
Cell viability assay
Cell viability was assessed by the mitochondrial tetrazolium (MTT) assay. Briefly, cells were seeded with 1×105 cells/well in a 96-well tissue culture dish. The confluent cells were incubated in cell culture medium plus drugs and then exposed to TNF-α for 24 h. MTT solution (20 µl/well) was added and the samples were incubated for 4 h. The absorbance of the sample was measured using an enzyme-linked immunosorbent assay (ELISA) reader (BioTek Instruments, Winooski, VT).
Reverse transcription polymerase chain reaction (PCR)
Total RNA was extracted from HUVECs using TRIzol reagent (Invitrogen, San Diego, CA). Sequences of the PCR primers for amplification of the HO-1 gene were as follows: 5'CAGGCAGAGAATGCTGAGTTC-3' (sense) and 5'-GATGTTGAGCAGGAACGCAGT-3' (antisense). The PCR cycles consisted of 95℃ for 1 min, followed by a hybridization step at 55℃ for 1 min and an elongation step at 72℃ for 2 min. Relative abundance of mRNA was calculated after normalization to GAPDH.
Western blot analysis
Total protein (30 µg) was separated on 10% SDS-polyacrylamide electrophoresis gel and transferred to nitrocellulose membranes (Amersham Bioscience, Inc.). The blocked membrane was then incubated with antibodies against HO-1 (Stressgen, Victoria, British Columbia, Canada). The immunoreactive bands were visualized with a SuperSignal West Dura Extended Duration Substrate kit (Pierce Chemical). The signals of the bands were quantified using a GS-710 calibrated imaging densitometer (Bio-Rad, Hercules, CA).
Transfection and luciferase reporter assay
HUVECs were transfected using Lipofectamine2000 (Invitrogen, Carlsbad, CA) for promoter activity analysis [24]. Cells were grown to 70~80% confluence in 12-well dishes and Opti-MEM medium. Cells were transfected with pGL3-basic, peroxisome proliferator response element (PPRE)-pGL3, and a renilla luciferase control reporter vector. After 6 h of incubation, the medium was replaced by cell culture medium. After 24 h, cells were lysed in reporter lysis buffer then luciferase activity was measured using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) and the transfection efficiencies were normalized by renilla luciferase activity.
Small interfering RNA preparation and transfection
The HO-1 siRNA oligonucleotide (GenBank accession no.NM_002133) was synthesized by Invitrogen. A siRNA negative control duplex was used as a control oligonucleotide. The siRNA molecules were transfected into HUVECs Lipofectamine 2000 (Invitrogen).
Drugs
Cilostazol (OPC-13013), (6-[4-(1-cyclohexyl-1H-tetrazol-5-yl) butoxy]-3,4-dihydro-2-(1H)-quinolinone), was generously donated by Otsuka Pharmaceutical Co. Ltd. (Tokushima, Japan) and dissolved in dimethyl sulfoxide to make a 10 mM stock solution. TNF-α (Upstate Biotechnology, Lake Placid, NY) was dissolved in phosphate-buffered saline to make a 10 µg/ml stock solution. MTT and GW9662 (2-chloro-5-nitrobenzanilide) were from Sigma-Aldrich (St. Louis, MO). ZnPP, CuPP and rosiglitazone were purchased from Alexis (San Diego, CA).
Statistical analysis
The results are expressed as the mean±SEM. The significance of the results was analyzed by a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. p values less than 0.05 were considered significant.
RESULTS
Effects of cilostazol on TNF-α-mediated cytotoxicity
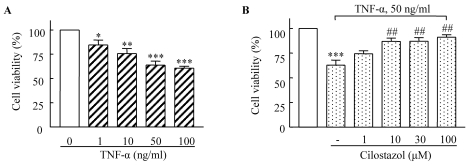
HUVECs were stimulated with different concentrations of TNF-α for 18 h, and then cell viability was determined using MTT assay. As shown in Fig. 1A, the number of viable cells gradually decreased in a TNF-α concentration-dependent manner. Cell viability after 18 h of incubation was significantly reduced to 62.87±4.95% (p<0.001) in response to 50 µg/ml TNF-α. Pretreatment of the cells with cilostazol prevented the loss of viable cells in a dose-dependent manner (Fig. 1B).
Fig. 1.
Dose-dependent cytotoxic effects of TNF-α (1~100 ng/ml) on HUVECs (A) and suppression of these effects by cilostazol (B). Cells were pretreated with cilostazol (1~100 µM) for 4 h, and then stimulated with TNF-α (50 ng/mL) for 18 h. Results are expressed as the mean±S.E.M. of four experiments. *p<0.05, **p<0.01, ***p<0.001 vs. none, ##p<0.01 vs. TNF-α alone.
Potential role of HO-1 in the cytoprotective effect of cilostazol
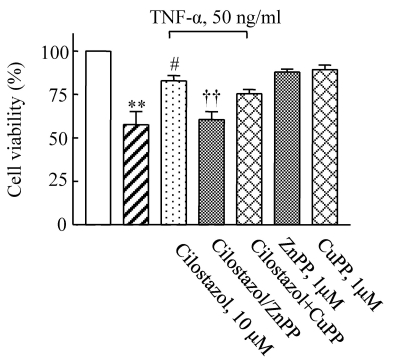
To determine the potential protective effect of cilostazol against TNF-α-induced cytotoxicity, HUVECs were pretreated with cilostazol in the presence or absence of various HO-1-related compounds including ZnPP and CuPP. While ZnPP and CuPP had no effects on endothelial cell viability, the cytoprotective effect of cilostazol in TNF-α-stimulated cells was attenuated by the HO-1 inhibitor, ZnPP, but not by CuPP (Fig. 2). This suggests potential involvement of the HO-1 pathway in the action of cilostazol.
Fig. 2.
Effect of an HO-1 inhibitor on the protective effects of cilostazol against TNF-α-mediated cytotoxicity in HUVECs. Cells were pretreated with cilostazol in the presence or absence of an HO-1 inhibitor (ZnPP, 1 µM) and then stimulated with TNF-α (50 ng/ml) for 18 h. Results are expressed as the mean±S.E.M. of four experiments. **p<0.01 vs. none, #p<0.05 vs. TNF-α alone, ††p<0.01 vs. TNF-α+cilostazol.
Cilostazol induces HO-1 expression
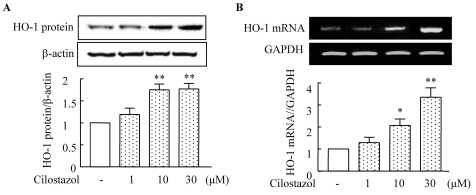
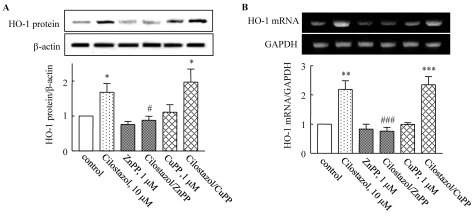
Both HO-1 protein and mRNA expression were significantly increased by treatment with cilostazol (1~30 µM) in a concentration-dependent manner (Fig. 3). Protein expression induced by cilostazol (10 µM) increased to 1.75±0.21-fold compared to that of the control (p<0.05), and was significantly suppressed by ZnPP. Increased expression of HO-1 mRNA stimulated by cilostazol (10 µM) was also suppressed by ZnPP (Fig. 4). These results suggest that cilostazol increases HO-1 expression via the regulation of HO-1 transcription.
Fig. 3.
Cilostazol induces HO-1 expression. HUVECs were stimulated with different concentrations of cilostazol for 12 h. The expression of HO-1 mRNA and protein was determined using reverse transcription PCR and Western blotting, respectively. GAPDH and β-actin were used as loading controls. Results are expressed as the mean±S.E.M. of four experiments. *p<0.05, **p<0.01 vs. vehicle.
Fig. 4.
Effect of an HO-1 inhibitor on cilostazol-induced HO-1 expression. HUVECs were pretreated with an HO-1 inhibitor (ZnPP), and then stimulated with cilostazol (10 µM) for 12 h. The expression of HO-1 mRNA and protein was determined using reverse transcription PCR and Western blotting, respectively. GAPDH and β-actin were used as loading controls. Results are expressed as the mean±S.E.M. of three or four experiments. *p<0.05; **p<0.01, ***p<0.001 vs. vehicle, #p<0.05; ###p<0.001 vs. cilostazol alone.
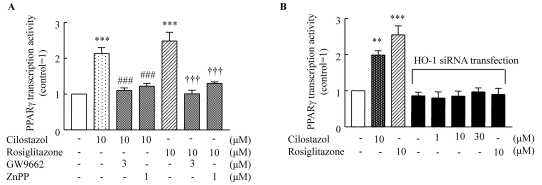
Role of HO-1 in PPAR-γ transcriptional activity
To examine the potential involvement of PPAR-γ in the action of cilostazol, PPAR-γ transcriptional activity in cells treated either with cilostazol or rosiglitazone were determined using a luciferase activity assay. As shown in Fig. 5, PPAR-γ transcriptional activity stimulated by cilostazol (10 µM) increased to 2.13±0.67-fold of the control (p<0.001), and that stimulated by rosiglitazone (10 µM) increased to 2.48±0.25-fold of the control (p<0.001). These effects of cilostazol and rosiglitazone were completely abolished by an HO-1 inhibitor. Furthermore, increased PPAR-γ transcriptional activity by cilostazol and rosiglitazone was not observed in cells transfected with HO-1 siRNA.
Fig. 5.
HO-1 mediates the effect of cliostazol and rosiglitazone on PPAR-γ transcriptional activity. (A) HUVECs were transiently transfected with PPRE-pGL3, a renilla luciferase control reporter vector, and then treated either with cilostazol (10 µM) or rosiglitazone (10 µM) for 24 h. PPAR-γ transcriptional activity was determined using a luciferase activity assay. (B.) HUVECs were transfected with siRNA specific for HO-1 then treated with cilostazol (10 µM) or rosiglitazone (10 µM) for 24 h. **p<0.01, ***p<0.001 vs. no treatment, ###p<0.001 vs. cilostazol, †††p<0.001 vs. rosiglitazone.
DISCUSSION
The present study demonstrated that cilostazol up-regulated HO-1 expression in vascular endothelial cells and provided protection against TNF-α-induced cytotoxicity. In addition, these cilostazol-mediated cellular events were effectively blocked by ZnPP (1 µM), an HO-1 competitive inhibitor. The present study adds new insight into the signal transduction pathway that regulates cilostazol-mediated protection against endothelial cell injury and emphasizes the involvement of the HO-1 pathway. HO-1 is the inducible form of HO that catalyzes the rate-limiting step of the conversion of heme into biliverdin, CO, and free iron. HO-1 and its products exert anti-apoptotic, anti-inflammatory, anti-oxidant, and pro-angiogenic effects in the vasculature [3,25]. HO-1 is up-regulated in endothelial cells and vascular smooth muscle cell (VSMC) by PPAR-α and PPAR-γ agonists in vitro [23].
In the present study, we demonstrated that cilostazol increased the expression of HO-1 mRNA and protein in HUVECs. Previously, HO-1 gene expression was demonstrated to be induced by activation of the cAMP signal pathway via a transcriptional mechanism in primary rat hepatocyte cultures. Dibutylyl cAMP-dependent induction of HO-1 mRNA expression was prevented by pretreatment with PKA inhibitor, KT5720, but not by pretreatment with a PKG inhibitor, KT5823 [26]. Additionally, PI3K/Akt pathways have been reported to be involved in HO-1 expression and Nrf2-dependent transcription [27].
The diversity of stimuli that may induce HO-1 expression suggests that the molecular mechanisms regulating HO-1 expression are complicated. Our previous studies showed that cilostazol inhibits cellular apoptosis under several circumstances [11,13,28,29]. These results raise the interesting possibility that cilostazol can be used as a therapeutic tool for treating vascular diseases associated with excess apoptosis. Although the mechanism by which cilostazol protects cells against apoptosis remains far from clear, previous studies [11,13] have shown that cilostazol reverses decreases in Bcl-2 protein and increases in Bax protein production as well as cytochrome c release. Moreover, this compound has also been found to suppress of NAD(P)H oxidase-dependent superoxide production [29]. Thus, it was suggested that cilostazol might have a protective role against vascular diseases through multiple mechanisms. Interestingly, in the present study using HUVECs, pretreatment of cells with cilostazol markedly reduced TNF-α-induced cell viability loss, which was reversed by ZnPP. Moreover, cilostazol increased the expression of HO-1 mRNA and protein in HUVECs in a concentration-dependent manner. Based on our present experimental results, it was suggested that increased expression of HO-1 by cilostazol may be correlated with the protective effect against TNF-α-induced endothelial cell death.
Although the mechanism by which HO-1 inhibits endothelial injury via cilostazol is not fully understood, some studies suggested that PPAR activity might have anti-inflammatory and vasculoprotective benefits [23,30-32]. Thus, it was hypothesized that the cytoprotective effect of HO-1 against inflammatory cytokine-induced endothelial injury is dependent on PPAR activity. PPARs are members of the nuclear receptor family, a group of transcription factors which play a critical role in mammalian physiology [1]. Following ligand activation, PPARs may activate or repress gene transcription, a process that is also controlled by ligand-induced recruitment or release of co-activators and co-repressors [33]. Li et al. demonstrated that activation of PPAR-γ by rosiglitazone induces significant HO-1 expression in primary cultured VSMCs. However, considering the diversity of stimuli that may induce HO-1 expression, it was suggested that the molecular mechanisms that regulate PPAR-γ activity are also complicated [34]. In the present study, cilostazol directly increased PPAR-γ transcriptional activity which was completely abolished by an HO-1 inhibitor. Furthermore, increased PPAR activity by cilostazol and rosiglitazone was completely abolished in cells transfected with HO-1 siRNA. These results indicate that cilostazol up-regulates PPAR activity via induction of HO-1 in endothelial cells.
TNF-α is commonly found in atherosclerotic lesions of blood vessels [35,36] and mediates vascular inflammation [37]. The development of new therapeutic agents that preserve the beneficial effects of acute vascular inflammation would have major clinical value. Recently, Wu et al. reported that PPAR-γ overexpression protects mitochondrial membrane potential and prevent apoptosis by upregulating the expression of anti-apoptotic Bcl-2 family proteins [38]. In TNF-α-activated endothelial cells, upregulation of PPAR-γ in cells inhibits NF-κB activity [39]. Therefore, considering these previous reports along with the importance of PPAR-γ in the regulation of NF-κB which is a major regulator of susceptibility to vascular injuries [3], the experimental results of our study in which cilostazol activated PPAR-γ in endothelial cells suggests that cilostazol has beneficial effects on TNF-α-induced endothelial injury through PPAR-γ activation and subsequent suppression of NF-κB activity. However, further experiments are necessary to elucidate the precise molecular mechanisms involved in cilostazol-mediated protection against endothelial injury by various cytokines.
In conclusion, the data reported here support a potential role for cilostazol in preventing TNF-α-induced endothelial injury through the HO-1/PPAR pathway. Considering that vascular endothelial injury and associated endothelial dysfunction represent critical early steps in atherogenesis and are targets for novel preventative therapies, our findings may have important implications in the prevention of vascular complications associated with vascular inflammation by modulating the activities of HO-1 and PPAR. However, further studies will be needed to further examine whether HO-1 and PPAR-γ function in a codependent and cooperative fashion to confer protection from inflammatory cytokine-mediated endothelial injury.
ACKNOWLEDGMENTS
This study was supported for two years by a Pusan National University Research Grant.
ABBREVIATIONS
- HO-1
heme oxygenase-1
- Nrf2
NF-E2 related factor
- HUVECs
human umbilical vein endothelial cells
- TNF-α
tumor necrosis factor-α
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- ZnPP
zinc protoporphyrin IX
- CuPP
copper-protoporphyrin IX
References
- 1.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan SZ, Ivashchenko CY, Usher MG, Mortensen RM. PPAR-gamma in the Cardiovascular System. PPAR Res. 2008;2008:745804. doi: 10.1155/2008/745804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vascul Pharmacol. 2006;45:19–28. doi: 10.1016/j.vph.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Nisbet RE, Sutliff RL, Hart CM. The role of peroxisome proliferator-activated receptors in pulmonary vascular disease. PPAR Res. 2007;2007:18797. doi: 10.1155/2007/18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPAR-gamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The Role of PPARs in Lung Fibrosis. PPAR Res. 2007;2007:71323. doi: 10.1155/2007/71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol. 2006;6:421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Chen YE, Fu M, Zhang J, Zhu X, Lin Y, Akinbami MA, Song Q. Peroxisome proliferator-activated receptors and the cardiovascular system. Vitam Horm. 2003;66:157–188. doi: 10.1016/s0083-6729(03)01005-7. [DOI] [PubMed] [Google Scholar]
- 10.Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002;300:709–715. doi: 10.1124/jpet.300.2.709. [DOI] [PubMed] [Google Scholar]
- 12.Hong KW, Kim KY, Shin HK, Lee JH, Choi JM, Kwak YG, Kim CD, Lee WS, Rhim BY. Cilostazol prevents tumor necrosis factor-alpha-induced cell death by suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation and activation of Akt/cyclic AMP response element-binding protein phosphorylation. J Pharmacol Exp Ther. 2003;306:1182–1190. doi: 10.1124/jpet.103.052365. [DOI] [PubMed] [Google Scholar]
- 13.Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002;300:787–793. doi: 10.1124/jpet.300.3.787. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Lee SW, Shin HK, Chung WT, Lee WS, Rhim BY, Hong KW, Kim CD. Cilostazol enhances apoptosis of synovial cells from rheumatoid arthritis patients with inhibition of cytokine formation via Nrf2-linked heme oxygenase 1 induction. Arthritis Rheum. 2010;62:732–741. doi: 10.1002/art.27291. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Liu H, Porvasnik SL, Terada N, Agarwal A, Cheng Y, Visner GA. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab Invest. 2006;86:62–71. doi: 10.1038/labinvest.3700361. [DOI] [PubMed] [Google Scholar]
- 16.Idriss NK, Blann AD, Lip GY. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Stanford SJ, Walters MJ, Hislop AA, Haworth SG, Evans TW, Mann BE, Motterlini R, Mitchell JA. Heme oxygenase is expressed in human pulmonary artery smooth muscle where carbon monoxide has an anti-proliferative role. Eur J Pharmacol. 2003;473:135–141. doi: 10.1016/s0014-2999(03)02001-6. [DOI] [PubMed] [Google Scholar]
- 18.Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 19.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1786–1795. doi: 10.1161/01.ATV.0000178169.95781.49. [DOI] [PubMed] [Google Scholar]
- 21.Hoekstra KA, Godin DV, Cheng KM. Protective role of heme oxygenase in the blood vessel wall during atherogenesis. Biochem Cell Biol. 2004;82:351–359. doi: 10.1139/o04-006. [DOI] [PubMed] [Google Scholar]
- 22.Risérus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L, Strum JC, Basu S, Cheeseman J, Fielding BA, Humphreys SM, Danoff T, Moore NR, Murgatroyd P, O'Rahilly S, Sutton P, Willson T, Hassall D, Frayn KN, Karpe F. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57:332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 23.Krönke G, Kadl A, Ikonomu E, Blüml S, Fürnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 24.Kintscher U, Lyon C, Wakino S, Bruemmer D, Feng X, Goetze S, Graf K, Moustakas A, Staels B, Fleck E, Hsueh WA, Law RE. PPARalpha inhibits TGF-beta-induced beta5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ Res. 2002;91:e35–e44. doi: 10.1161/01.res.0000046017.96083.34. [DOI] [PubMed] [Google Scholar]
- 25.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 26.Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat heme oxygenase-1 gene is transcriptionally induced via the protein kinase A signaling pathway in rat hepatocyte cultures. Mol Pharmacol. 1998;53:483–491. doi: 10.1124/mol.53.3.483. [DOI] [PubMed] [Google Scholar]
- 27.Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Kim KY, Lee YK, Park SY, Kim CD, Lee WS, Rhim BY, Hong KW. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 2004;308:896–903. doi: 10.1124/jpet.103.061853. [DOI] [PubMed] [Google Scholar]
- 29.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- 30.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPAR-gamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan CD, Zhao BX, Wei F, Zhang GH, Dong WL, Miao JY. Synthesis and discovery of autophagy inducers for A549 and H460 lung cancer cells, novel 1-(2'-hydroxy-3'-aroxypropyl)-3-aryl-1H-pyrazole-5-carbohydrazide derivatives. Bioorg Med Chem Lett. 2008;18:3860–3864. doi: 10.1016/j.bmcl.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302:453–457. doi: 10.1126/science.1087344. [DOI] [PubMed] [Google Scholar]
- 33.Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. doi: 10.1161/CIRCULATIONAHA.104.475673. [DOI] [PubMed] [Google Scholar]
- 34.Li MY, Yuan H, Ma LT, Kong AW, Hsin MK, Yip JH, Underwood MJ, Chen GG. Roles of peroxisome proliferator-activated receptor-alpha and -gamma in the development of non-small cell lung cancer. Am J Respir Cell Mol Biol. 2010;43:674–683. doi: 10.1165/rcmb.2009-0349OC. [DOI] [PubMed] [Google Scholar]
- 35.DiDonato M, Narindrasorasak S, Sarkar B. Expression, purification, and metal binding characteristics of the putative copper binding domain from the Wilson disease copper transporting ATPase (ATP7B) Adv Exp Med Biol. 1999;448:165–173. doi: 10.1007/978-1-4615-4859-1_14. [DOI] [PubMed] [Google Scholar]
- 36.Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–210. doi: 10.1016/s0002-9440(10)65266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S9–S12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- 38.Wu JS, Lin TN, Wu KK. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol. 2009;220:58–71. doi: 10.1002/jcp.21730. [DOI] [PubMed] [Google Scholar]
- 39.Mun L, Jun MS, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Son KH, Lee DH, Kim YS, Park K, Chang KC. 7,8-didehydrocimigenol from Cimicifugae rhizoma inhibits TNF-α-induced VCAM-1 but not ICAM-1expression through upregulation of PPAR-γ in human endothelial cells. Food Chem Toxicol. 2011;49:166–172. doi: 10.1016/j.fct.2010.10.012. [DOI] [PubMed] [Google Scholar]