Abstract
Fucoidan is a sulfated polysaccharide derived from brown algae that has been reported to perform multiple biological activities, including immunostimulation. In this study, we investigated whether fucoidan has beneficial effects on endotoxemia induced by LPS, a septic model in mice. The focus of this study was on survival rates and spleen function of the mice upon treatment. We found that fucoidan had prophylactic effects on the survival rate of mice with endotoxemia. Flow cytometric analysis using antibodies for subset-specific markers revealed that fucoidan profoundly reversed the depleted population of dendritic cells in mice with endotoxemia. According to Western blot analysis, the spleen cells of LPS/fucoidan-treated mice showed a higher expression of anti-apoptotic molecules compared to those of LPS-treated mice. Also, fucoidan-treated spleen cells were more responsive to mitogens. Taken together, these results demonstrate that fucoidan pre-treatment has beneficial effects on the survival rate and function of the spleen in mice with endotoxemia. This study may broaden the use of fucoidan in clinical fields, especially endotoxemia.
Keywords: Endotoxemia, Fucoidan, Survival, Spleen, Prophylactic effects
INTRODUCTION
Fucoidan is a sulfated polysaccharide of brown algae. Previous studies have shown that it protects bone marrow cells from radiation [1] and inhibits selectin-mediated adhesion of hematopoietic progenitor cells [2]. Also, fucoidan modulates the function of various immune cells such as lymphocytes [3], dendritic cells (DCs) [4], natural killer (NK) cells [5], and neutrophils [6].
A large amount of LPS in the blood, derived from Gram-negative bacteria, induces endotoxemia in hosts. LPS-induced endotoxemia has been classified as a type of sepsis. It is characterized by the excessive production of proinflammatory cytokines, reactive oxygen species, and nitric oxide, resulting in organ dysfunction, hypotension, septic shock, and death [7]. High mortality rates are closely related to immunosuppression [8]. Recent studies demonstrated that the number of DCs was decreased in both animal disease models [9] and in patients with sepsis [10]. Because DCs are crucial to both innate and adaptive immunity, it is postulated that a decrease in DCs is closely related to immunosuppression during sepsis. Based on our recent finding that fucoidan stimulates DCs [4], we hypothesized that fucoidan may prevent DCs from decreasing in an animal model of sepsis. In this study, we investigated the effects of fucoidan on several parameters, including survival rate and splenic DC number, in a mouse model of endotoxemia.
METHODS
Reagents
Fucoidan purified from Fucus vesiculosus, LPS purified from Escherichia coli O26, phorbol 12-myristate 13-acetate (PMA) and ionomycin were purchased (Sigma, USA). Fucoidan was dissolved in sterile phosphate buffered solution (PBS) and its endotoxin level was determined by QCL-1000® Chromogenic LAL endpoint assay (Lonza Walkersville, Inc., USA) according to the manufacturer's manual [4]. Oligodeoxynucleotide (ODN) 1826 was generously provided by Kangwon National University.
Animal and endotoxemia model
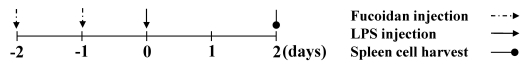
C57BL/6 and Balb/c mice were purchased (Orient Bio, Republic of Korea). They were maintained at our animal facility. 7- to 12-week old mice were used for all experiments. Animal experiments in this study were approved by Animal care and use committee of Jeju National University. For measuring the effects of fucoidan on the endotoxemia animal model, mice received PBS or 100 mg/kg fucoidan by intraperitoneal injection twice at an interval of 24 hours. At 24 hours after second fucoidan injection, mice were challenged with 10 mg/kg LPS, as a sub-lethal dose or 30 mg/kg LPS, as a lethal dose for the determination of mortality. 2 days later, the mice were killed and then spleens or blood were harvested (Fig. 1). The mice were weighed every day. Each group included 5 mice.
Fig. 1.
The injection schedule of fucoidan and LPS. Mice were injected with PBS, 100 mg/kg fucoidan and then 10 mg/kg or 30 mg/kg LPS intraperitoneally. After all injection procedure, the mice were killed and biological samples were harvested.
Preparation and treatment of spleen cells
Spleen cells were purified from control or endotoxemia-induced mice. At first, each spleen was weighed. The spleens were disrupted by mechanical force and red blood cells removed using hypotonic lysis buffer [11]. The cells were passed through 70 µm cell strainer to get single cells and used for staining or culture for following in vitro experiments. Harvested spleen cells were setup and treated with mitogens in 96-well culture plates. Cellular activity was measured by a MTT assay [12].
Flow cytometric analysis
To measure the expression levels of surface markers, the spleen cells were treated with biotin-labeled anti-CD4, anti-CD8, anti-CD19, anti-F4/80, anti-NK1.1 and anti-MHC II antibody and then streptavidin-FITC or anti-CD11c-PE (all from BD Biosciences, USA). For evaluating the antigen uptake capability of the spleen cells, the cells were incubated with 250 µg/ml FITC-dextran (Sigma, USA) for 1 hour and washed twice with PBS before analysis. All stained cells were analyzed by FACSCalibur® and CellQuest® (Beckton Dickinson, USA).
Western blot analysis
The spleen cells were dissolved in Western blot lysis buffer and the protein concentration of lysates was measured by Bio-Rad protein assay solution. 10 µg of protein was loaded onto each lane and electrophoretically separated in 12% sodium dodecyl sulfate polyacrylamide gels. The separated proteins were transferred onto a nitrocellulose membrane and detected by anti-Bcl-2, anti-Bcl-xL (Santa Cruz Biotechnology, USA), anti-cIAP-1 (Millipore, USA) or anti-beta actin antibody (Sigma, USA). Sequentially, the membrane was developed by horseradish peroxidase-labeled secondary antibody and SuperSignal® West Pico Chemiluminescent Substrate (Thermo Scientific, USA) to visualize specific protein bands.
Statistical analysis
All data were presented as means and standard deviations (mean±SD) and statistically analyzed by Tukey-Kramer multiple comparisons test. A p value of <0.05 was considered as significant.
RESULTS
The effect of fucoidan on the survival rate of mice with LPS-induced endotoxemia
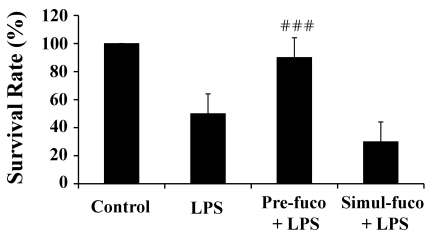
The mouse model of LPS-induced endotoxemia is well established as a sepsis animal model. To investigate whether fucoidan may have positive effects on the survival of mice with endotoxemia, we injected a lethal dose of LPS or fucoidan and then evaluated the mortality of the mice over the next 5 days. As expected, LPS significantly decreased survival rate. Interestingly, pre-treatment with fucoidan significantly reduced LPS-induced mortality (Fig. 2). To determine if fucoidan is therapeutically effective, it was injected simultaneously with (Fig. 2) or after (data not shown) LPS injection. In multiple experiments, fucoidan had only prophylactic effects on LPS-induced death of mice, and did not exhibit any therapeutic effects if administered with or after the LPS injection. In some preliminary experiments, the optimal dose of fucoidan was determined and used for this study (data not shown).
Fig. 2.
Prophylactic effect of fucoidan on the survival rate of mice with LPS-induced endotoxemia. Mice were intraperitoneally injected with 30 mg/kg LPS, a lethal dose and also with 100 mg/kg fucoidan before (Pre-) LPS injection, as described in the Methods. Other mice were injected with the same amount of LPS and fucoidan simultaneously (Simul-). The survival rate was determined 5 days after LPS injection. Each group included 5 mice. ###indicates p<0.001 compared to LPS-treated mice.
The effects of fucoidan on the spleen and splenocytes of mice
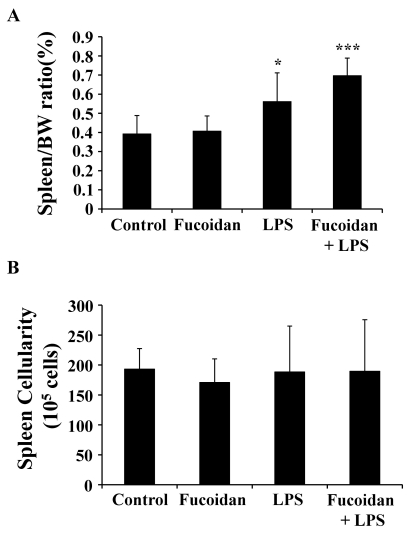
To investigate the effects of fucoidan on the spleen function of mice, we sacrificed mice treated with fucoidan, LPS, or both. The weight of the spleens was obtained and the ratio of spleen/body weight was calculated. Interestingly, the ratios of LPS-treated and LPS/fucoidan-treated mice were higher than those of control and fucoidan-treated mice (Fig. 3A). It is thus suggested that LPS plays a major role in increasing the ratio of spleen/body weight and fucoidan does support it in case of the combination with LPS. This result can be explained by inflammatory cytokines-mediated systemic toxicity or immunostimulatory activity of LPS and fucoidan. After harvesting spleen cells, the cellularity of the spleen was quantified based on cell number. There was no significant change in the number of spleen cells across the groups (Fig. 3B). These results suggest that fucoidan or LPS may enhance the weight, but not the cellularity, of spleens.
Fig. 3.
The effect of fucoidan on the spleen of mice with endotoxemia. Mice were injected with 100 mg/kg fucoidan and then 10 mg/kg LPS, a sub-lethal dose, as described in the Methods. The weight of the spleen (A) and the number of spleen cells (B) were measured. *, ***p<0.05, 0.001, respectively, compared to control mice.
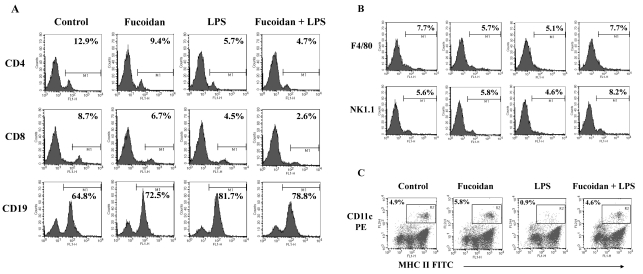
Recovery of LPS-induced decreases in DC ratio in spleen cells by fucoidan
The population of immune cells in the spleen was investigated using cell-specific markers. LPS-treated and LPS/fucoidan-treated mice had more CD19+ B cells than control and fucoidan-treated mice, respectively. Also, the proportions of CD4+ and CD8+ cells, mostly T cells, were marginally lower in LPS-treated and LPS/fucoidan-treated mice, respectively (Fig. 4A). The proportions of F4/80+ and NK1.1+ cells, mostly macrophages and NK cells respectively, were marginally lower in LPS-treated mice whereas slightly higher in LPS/fucoidan-treated mice compared to control mice (Fig. 4B). The levels of DCs, CD11c+ and MHC II+ cells were lower in LPS-treated mice, but the decrease was almost completely reversed by fucoidan treatment (Fig. 4C). Flow cytometry analysis using subset markers demonstrated that fucoidan specifically increased DCs in LPS-treated mice compared to other subsets of spleen cells.
Fig. 4.
Selective recovery of DCs in spleen cells of LPS/fucoidan-treated mice. Fucoidan followed by a sublethal dose of LPS were administrated to mice as described in the Methods. Spleen cells were then stained for subset-specific markers (A) CD4, CD8, CD19, (B) F4/80, NK1.1, (C) CD11c and MHCII and analyzed by flow cytometry. The numbers represent the percentages of a specific subset in the spleen cells.
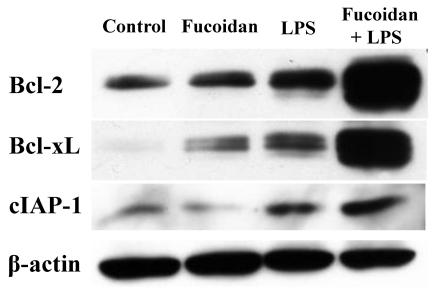
Increased expression of anti-apoptotic molecules in spleen cells by fucoidan
To analyze the ability of spleen cells to respond to apoptotic signals, we measured the expression levels of anti-apoptotic molecules in spleen cells (Fig. 5). Bcl-2, Bcl-xL, and cIAP-1 molecules are representative anti-apoptotic molecules. Western blot analysis revealed that the spleen cells of LPS/fucoidan-treated mice expressed the highest levels, whereas those of LPS-treated mice had higher levels than control or fucoidan-treated mice. This indicates that fucoidan and LPS have synergistic effects on the expression of anti-apoptotic molecules in mouse spleen cells and suggest that these two treatments may strengthen the survival potential of spleen cells against apoptotic signals. The related regulatory pathways, such as transcription factors and signal transductions need to be elucidated.
Fig. 5.
Fucoidan enhances anti-apoptotic molecules in the spleen cells of mice with LPS-induced endotoxemia. Fucoidan followed by a sub-lethal dose of LPS were administrated to mice, as described in the Methods. After harvesting the spleen cells and preparing cell lysates for Western blot analysis, the amount of protein was determined. An equal amount was loaded into each lane. Beta-actin was used as an internal control.
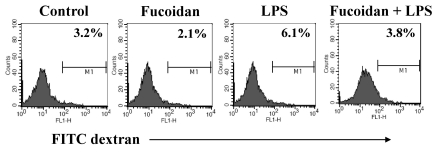
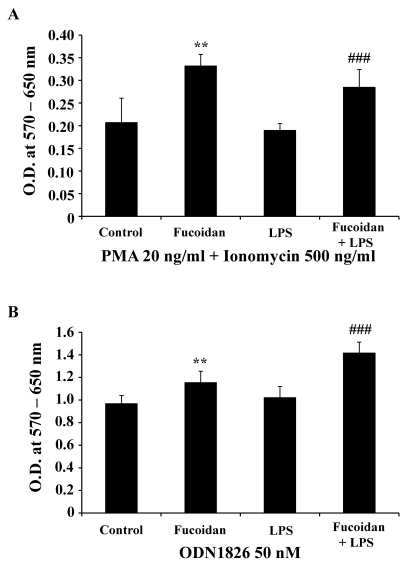
Enhanced function of spleen cells by fucoidan
Spleen cells are largely classified into two groups: lymphoid and myeloid cells. Lymphoid cells include T cells, B cells, and NK cells, whereas myeloid cells include macrophages and DCs. To detect the functional changes in spleen cells, antigen uptake and response to mitogens were measured in the spleen cells of treated mice. The ability of spleen cells to take up antigen reflects the maturation of myeloid cells such as DCs. In this assay, the cells of fucoidan-treated and LPS/fucoidan-treated mice showed lower antigen uptake than those of controls or LPS-treated mice (Fig. 6). This suggests that fucoidan treatment may decrease the antigen uptake ability of spleen cells via the maturation or activation of DCs in vivo. In addition, spleen cells of fucoidan-treated and LPS/fucoidan-treated mice showed a higher response to mitogens than those of control and LPS-treated mice (Fig. 7). PMA+ionomycin and ODN1826 treatment preferentially stimulated T cells and B cells, respectively. These results indicate that fucoidan treatment strengthens the responsiveness of spleen cells to mitogens in vivo.
Fig. 6.
Decreased antigen uptake ability of fucoidan-treated spleen cells. Fucoidan treatment followed by a sub-lethal dose of LPS was administrated to mice as described in the Methods. Harvested spleen cells were incubated with FITC-dextran, and stained cells were analyzed by flow cytometry.
Fig. 7.
Fucoidan increases the response of spleen cells to mitogens. Fucoidan treatment followed by a sub-lethal dose of LPS was administrated to mice as described in the Methods. Harvested spleen cells were cultured at a concentration of 4×105 cells/well in 96-well culture plates. Cells were treated with 20 ng/ml PMA + 500 ng/ml ionomycin (A) and 50 nM ODN1826 (B) for 2 days. After treatments, a MTT assay was used to measure cellular activity. Data are mean±SD from six individual wells. **, ###Indicate p<0.01 and 0.001 compared to the spleen cells of controls and LPS-treated mice, respectively.
DISCUSSION
The spleen is a major organ that provides critical immune cells, including lymphocytes and DCs. In this study, we demonstrated that LPS-induced endotoxemia depletes DCs in the spleen, and that treatment with fucoidan can recover this cell population. The effects of fucoidan on survival rate and spleen function were prophylactic, rather than therapeutic, suggesting that fucoidan requires time to protect target cells against the harmful effects of LPS. Based on previous studies, fucoidan blocks selectin-dependent leukocyte adhesion/rolling [13,14] and inhibit microvascular thrombus formation [15]. It is thus suggested that fucoidan may affect blood circulation dysfunction by LPS in mice. The two major effects of fucoidan, namely the changes in survival rate and spleen function, can be separated with respect to the mechanism of action. The immunostimulatory effects of fucoidan described in previous reports support the effects of fucoidan on spleen function, whereas the effect of fucoidan on the survival rate requires further investigation to determine if it functions by direct protection of immune cells or through other mechanisms, such as the attenuation of blood circulation dysfunction.
We demonstrated that a sub-lethal dose of LPS induces the depletion of DCs in mice. LPS-induced endotoxemia is a common mouse model of sepsis, and our results regarding DC depletion corroborate the model [9,10]. Interestingly, the weight and cellularity analyses of the spleen indicated that LPS increases the weight of the spleen but does not significantly alter the number of spleen cells. These results led us to speculate that LPS may increase the weight of the spleen by potentially triggering edema and also differentially influencing the number of specific subsets of spleen cells without altering the total cell number. Flow cytometry analysis revealed that LPS increases the number of B cells but decreases the number of DCs and importantly fucoidan sustain that of DCs. This result indicates a cytoprotective effect of fucoidan on DCs in LPS-mediated endotoxemia. To investigate the effects of fucoidan on spleen cells, we measured the expression of anti-apoptotic molecules, antigen uptake capability, and responsiveness to mitogens after in vivo treatments. Fucoidan increased the expression of anti-apoptotic molecules in spleen cells and had a synergistic effect with LPS. Although LPS treatment also increased the same molecules to a certain extent, fucoidan increased them to a much higher level. We therefore suggest that reversal of DC depletion by fucoidan may be partially related to the increase in anti-apoptotic molecules. The increase of anti-apoptotic molecules in spleen cells of LPS-treated mice can be attributed to the mitogenic effect of LPS for B lymphocytes [16].
To measure the activation status of antigen presenting cells (APCs), we measured the antigen uptake ability of spleen cells using FITC-dextran. LPS increased the antigen uptake of spleen cells whereas fucoidan decreased it (Fig. 6). Previous studies demonstrated that LPS increases the antigen uptake ability of APCs [17,18] whereas fucoidan decreases it [4,19]. At this time, there are two potential explanations for this data: the blocking of scavenger receptor A, of which fucoidan is a major antagonist [20,21] and the maturation of DCs. To determine cell responsiveness, we treated harvested spleen cells of mice with PMA + ionomycin or ODN1826, which can preferentially stimulate T or B lymphocytes respectively. Fucoidan increased the responses of spleen cells to these mitogens. This would suggest that fucoidan increases the activity of spleen cells, including T and B lymphocytes, during endotoxemia.
This study demonstrated that fucoidan reverses DC depletion by LPS, but that the detailed mechanisms by which fucoidan acts need to be further investigated. And also, the protective effects of fucoidan on antigen-specific and memory immune responses in hosts with endotoxemia should be a good topic for a future study. Because DCs are a major type of APC that play critical roles in immunity, the ability of fucoidan to protect DCs and spleen cells from endotoxemia may broaden its use both clinically and in future research of endotoxemia.
ACKNOWLEDGEMENTS
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (KRF-2008-313-E00609) and Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
ABBREVIATIONS
- IL
interleukin
- LPS
lipopolysaccharide
- PE
phycoerythrin
- FITC
fluorescein isothiocyanate
References
- 1.Byon YY, Kim MH, Yoo ES, Hwang KK, Jee Y, Shin T, Joo HG. Radioprotective effects of fucoidan on bone marrow cells: improvement of the cell survival and immunoreactivity. J Vet Sci. 2008;9:359–365. doi: 10.4142/jvs.2008.9.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frenette PS, Weiss L. Sulfated glycans induce rapid hematopoietic progenitor cell mobilization: evidence for selectin-dependent and independent mechanisms. Blood. 2000;96:2460–2468. [PubMed] [Google Scholar]
- 3.Oomizu S, Yanase Y, Suzuki H, Kameyoshi Y, Hide M. Fucoidan prevents C epsilon germline transcription and NFkappaB p52 translocation for IgE production in B cells. Biochem Biophys Res Commun. 2006;350:501–507. doi: 10.1016/j.bbrc.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama H, Tamauchi H, Iizuka M, Nakano T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu) Planta Med. 2006;72:1415–1417. doi: 10.1055/s-2006-951703. [DOI] [PubMed] [Google Scholar]
- 6.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–5278. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 8.Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr Pharm Des. 2008;14:1870–1881. doi: 10.2174/138161208784980545. [DOI] [PubMed] [Google Scholar]
- 9.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 11.Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- 12.Kim MH, Byon YY, Ko EJ, Song JY, Yun YS, Shin T, Joo HG. Immunomodulatory activity of ginsan, a polysaccharide of Panax ginseng, on dendritic cells. Korean J Physiol Pharmacol. 2009;13:169–173. doi: 10.4196/kjpp.2009.13.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ley K, Linnemann G, Meinen M, Stoolman LM, Gaehtgens P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993;81:177–185. [PubMed] [Google Scholar]
- 14.Granert C, Raud J, Xie X, Lindquist L, Lindbom L. Inhibition of leukocyte rolling with polysaccharide fucoidin prevents pleocytosis in experimental meningitis in the rabbit. J Clin Invest. 1994;93:929–936. doi: 10.1172/JCI117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorlacius H, Vollmar B, Seyfert UT, Vestweber D, Menger MD. The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and L-selectin function in vivo. Eur J Clin Invest. 2000;30:804–810. doi: 10.1046/j.1365-2362.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- 16.Hofstad T, Skaug N, Sveen K. Stimulation of B lymphocytes by lipopolysaccharides from anaerobic bacteria. Clin Infect Dis. 1993;16(Suppl 4):S200–S202. doi: 10.1093/clinids/16.supplement_4.s200. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Qu JM, He LX. IL-12 suppression, enhanced endocytosis and up-regulation of MHC-II and CD80 in dendritic cells during experimental endotoxin tolerance. Acta Pharmacol Sin. 2009;30:582–588. doi: 10.1038/aps.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30:267–273. doi: 10.1097/shk.0b013e318162c190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa K, Arai H, Inoue K. Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages. J Biol Chem. 1990;265:5226–5231. [PubMed] [Google Scholar]
- 20.Bochkov VN, Tkachuk VA, Philippova MP, Stambolsky DV, Bühler FR, Resink TJ. Ligand selectivity of 105 kDa and 130 kDa lipoprotein-binding proteins in vascular-smooth-muscle-cell membranes is unique. Biochem J. 1996;317:297–304. doi: 10.1042/bj3170297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006;8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]