Abstract
Objective
High rates of Substance Use Disorders (SUD) have been found in samples of adolescents and adults with Attention Deficit Hyperactivity Disorder (ADHD). Predictors of SUD among ADHD children who are at risk for the development of SUD remain understudied. The main aims of this study were to identify clinically meaningful characteristics of children that predicted the future development of SUD and to see whether the role of these characteristics varied by sex.
Method
Subjects were children and adolescents with (N=268; mean age ± SD = 10.9 ± 3.2 years) and without (N=229; mean age 11.9 ± 3.3 years) DSM-III-R ADHD followed prospectively and blindly over a ten-year follow-up period onto young adult years. Subjects were assessed with structured diagnostic interviews for psychopathology and SUDs.
Results
Over the ten-year follow-up period, ADHD was found to be a significant predictor of any SUD (Hazards Ratio, (95% Confidence Interval) = 1.47 (1.07, 2.02); p=0.01) and cigarette smoking (2.38 (1.61, 3.53); p<0.01). Within ADHD, comorbid conduct disorder (2.74 (1.66, 4.52); p<0.01) and oppositional defiant disorder (2.21 (1.40, 3.51); p<0.01) at baseline were also found to be significant predictors of SUD. We found similar results for cigarette, alcohol, and drug use disorders. There were few meaningful sex interaction effects. We did not find any clinically significant associations for any social or family environment factors nor cognitive functioning factors (all p values > 0.05).
Conclusions
These results indicate that ADHD is a significant risk factor for the development of SUD and cigarette smoking in both sexes.
Keywords: ADHD, substance-use disorders, longitudinal follow-up, conduct disorder
Introduction
Substance use disorders (SUD) are now conceptualized as having their developmental roots in childhood.1 Early-onset SUD has been associated with significant morbidity in multiple realms of functioning. Specifically, childhood-onset SUD predicts prolonged duration of SUD in adulthood,2 increased severity of SUD3 and reduced efforts to seek treatment. Early SUD is also associated with elevated rates of academic failure,4 suicidal behaviors, other dangerous behaviors,5 and high rates of psychiatric comorbidity.6
Psychiatric disorders have been observed in up to 85% of adolescents with SUD.7 One of the most common overlapping disorders in early-onset SUD is Attention Deficit Hyperactivity Disorder (ADHD). ADHD onsets in early childhood and affects 6–9% of juveniles8 and up to 5% of adults.9 Longitudinal data suggest that childhood ADHD persists into adolescence in 75% of cases10 and into adulthood in approximately one-half of cases.11
The overlap between ADHD and SUD in adolescents and adults has been an area of increasing clinical, research, and public health interest. The identification of specific risk factors of SUD within ADHD may permit more targeted treatments for both disorders at earlier stages of their expression, potentially reducing the morbidity, disability, and poor long-term prognosis in adolescents and adults with this comorbidity.12 Prospective studies of Children with ADHD have provided some evidence that the group with conduct and bipolar disorders co-occurring with ADHD have the highest risk for developing SUD.12–19 However, studies have questioned if conduct disorder mediates the relationship between ADHD and SUD. 10, 20, 21 For example, Brook et al. recently reported that ADHD was a risk factor for SUD, but when controlling for conduct disorder, the relationship was no longer significant.22 Nevertheless, other work has indicated that academic dysfunction,23 cognitive deficits,24 and neurobehavioral disinhibition,25 often observed in ADHD youth, increase the risk for SUD.
Despite a growing literature related to SUD many of the ADHD based studies are in younger adolescents and few examine early objective and subjective childhood risk factors within ADHD, excluding conduct disorder, that are associated with SUD. We previously examined issues relating to the relationship between ADHD and SUD in a series of retrospective analyses of adults with ADHD. The main aim of this study was to re-examine the risk factors for the development of SUDs in children with ADHD beyond that conferred by the comorbidity with conduct and bipolar disorders. To this end, we used data from longitudinal studies of two cohorts of boys and girls with and without ADHD who were followed for an average of 10 years examining predictors, influences, and sex effects on later SUD. We hypothesized that ADHD will be a risk factor for SUD. Within ADHD, we hypothesized that persistent ADHD, conduct and mood comorbidity, academic and cognitive dysfunction will predict later SUD in Children with ADHD of both sexes.
Method
Subjects
Detailed study methodology has been previously described.26, 27 Subjects were derived from two identically designed longitudinal case-control family studies of ADHD. These studies recruited male and female probands aged 6 to 17 years with (N=140 boys, N=140 girls) and without (N=120 boys, N=122 girls) DSM-III-R ADHD from pediatric and psychiatric clinics. The current sample includes the 10- and 11-year follow-up for the boys and girls studies, respectively. Of the 140 girls with ADHD and 122 comparison girls recruited at baseline, 96 (69%) and 91 (75%), respectively, were reassessed at the 11-year follow-up. The follow-up rates did not differ between groups.27 Of the 140 boys with ADHD and 120 comparison boys recruited at baseline, 112 (80%) and 105 (88%), respectively, were successfully reassessed at the 10-year follow-up and the rate of successful follow-up did not differ between groups. 26
As described previously we used a three-stage ascertainment of controls and probands including referral from clinic, a telephone screen, and structured interview.26, 27 Subjects with ADHD were derived from two major sources: a health maintenance organization and a child psychiatry practice. Controls were selected from outpatients who were referred for routine physical examinations to its pediatric medical clinics. There were no major baseline differences between either site in terms of severity of ADHD, psychosocial functioning, and comorbidity.28 Potential subjects were excluded if they had been adopted, their nuclear family was not available for study, if they had major sensorimotor handicaps, psychosis, autism, inadequate command of the English language, or a Full Scale IQ less than 80. Subjects with ADHD met full DSM-III-R diagnostic criteria for ADHD at the time of the clinic referral; at the time of recruitment they all had active symptoms of the disorder. Parents and adult offspring provided written informed consent to participate, and parents provided consent for offspring under the age of 18. Children and adolescents provided written assent to participate. The human research committee at Massachusetts General Hospital approved the initial assessments as well as all aspects of the follow up of this study.
Follow-up Assessment Procedures
Lifetime psychiatric assessment and SUD at the 10 and 11 year follow-ups relied on the Schedule for Affective Disorder and Schizophrenia for Children (K-SADS-E)29 for subjects younger than 18 years of age and the Structured Clinical Interview for the DSM-IV (SCID)30, 31 (supplemented with modules from the KSADS-E to assess childhood diagnoses) for subjects 18 years of age and older. We conducted interviews with subjects and indirect interviews with their mothers (i.e., mothers completed the interview about their offspring). We combined data from direct and indirect interviews by considering a diagnostic criterion positive if it was endorsed in either interview.
Board-certified child and adult psychiatrists who were blind to the subject’s ADHD status, referral source, and all other data resolved diagnostic uncertainties. All potential diagnoses of SUD were reviewed. To assess the reliability of our overall diagnostic procedures, we computed kappa coefficients of agreement by having experienced, blinded, board-certified child and adult psychiatrists diagnose subjects from audiotaped interviews made by the assessment staff. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98. Kappa coefficients for individual diagnoses included: major depression (1.0), mania (0.95), ADHD (0.88), conduct disorder (CD; 1.0), oppositional defiant disorder (ODD; 0.90), antisocial personality disorder (ASPD; 0.80), and SUD (1.0).
Interviewers assessed the degree of impairment on daily functioning associated with each disorder that subjects endorsed on a three-level ordinal scale: minimal, moderate, or severe. Because the diagnosis of mood disorders in youth is controversial, we analyzed major depression only if the depressive episode was associated with severe impairment, in order to avoid false positive diagnoses.32 As there is not a similar precedent for SUD or bipolar disorder, we adopted a less stringent approach and made the diagnoses only when associated with at least moderate impairment.
SUD and cigarette smoking were diagnosed on the basis of DSM-IV criteria using the KSADS-E and SCID. To meet a positive diagnosis of cigarette smoking, subjects under 18 needed to endorse any amount of smoking daily, whereas subjects over 18 needed to endorse smoking at least a pack of cigarettes per day. If either parent endorsed full criteria for a psychiatric disorder or SUD during his/her SCID, then the family history of the proband was considered positive.
Socioeconomic status (SES) was measured using the 5-point Hollingshead scale.33 Social functioning was assessed using the Social Adjustment Inventory for Children and Adolescents (SAICA), 34 a semi-structured interview administered to the child or parent. Family environment was assessed by the Family environment scale (FES), 35 a series of true or false statements administered following the structured interview.
Statistical Analysis
We compared the baseline characteristics of subjects using t-tests for continuous outcomes, the Wilcoxon rank-sum tests for SES, and Pearson χ2 tests for binary outcomes. We examined whether ADHD was a risk factor for each outcome using Cox proportional hazards, adjusting for SES, and parental history of SUD. Subjects who reported an onset of substance abuse/dependence (N=15), alcohol abuse/dependence (N=10), drug abuse/dependence (N=11), or cigarette smoking (N=16) at baseline were excluded from the analysis for each respective outcome. These analyses were repeated without subjects with CD at baseline. We also repeated the aforementioned analyses to assess the risk of developing both an alcohol and drug use disorder.
We used Cox models to assess the SUD risk associated with predictors within ADHD, adjusting for SES, and parental history of SUD. To reduce the number of statistical tests, we condensed the SAICA (we totaled the values of the 12 questions) and the FES (we standardized each scale, reversed the conflict scale to be in agreement with the other scales and totaled all three scales). If the collapsed totals were significant, we ran a second model examining the scale in detail. We derived proxies of DSM-IV subtypes from our DSM-III –R baseline interviews based on our previous work.36 For the proxies of ADHD subtype (hyperactive/impulsive, inattentive, combined), we primarily conducted an overall model and if this model was significant, we ran secondary analyses to compare the potential risks of each subtype. Due to small sample sizes and the transition from bipolar disorder to depression, we combined major depressive disorder and bipolar disorder into one category (mood disorders) if either diagnosis was positive.
Adjusting for age and SES, we used linear regression to assess the duration of SUD and logistic regression to assess the transition from abuse to dependence, the severity of SUD (moderate vs. severe), the endorsement of a type of drug, and persistent ADHD at the 10-year follow-up (subject reported full or substhreshold ADHD within the past 30 days).
Throughout, we assessed whether gender may predispose subjects to a different SUD risk profile. We included the sex-by-ADHD status interaction term when we studied the risk of SUD for ADHD and control subjects and the sex-by-predictor interaction term when we examined the risk among only Subjects with ADHD. At any point, if the interaction term was not significant, we collapsed across sex categories and if it was significant, we estimated the risk of SUD within strata of sex. The diagnoses of each outcome were defined as any positive response at any assessment. All tests were two-tailed, and our alpha level was set at 0.05. We calculated all statistics using STATA, version 10.0. Data are expressed as mean ± standard deviation (SD) unless otherwise specified.
Results
Overall, there were 497 probands available for study (268 cases of ADHD; mean baseline age ± SD: 10.91 ± 3.17 years) and 229 controls (mean baseline age ± SD: 11.88 ± 3.29 years). To test our first hypothesis, we stratified our subjects by the presence or absence of ADHD. We found that Subjects with ADHD without any SUD at baseline (N=257) were significantly younger at baseline, had a significantly lower socio-economic status, and were more likely to have a parental history of an alcohol use and/or drug use disorder compared to controls (N=225; Table 1). We did not find any differences between sexes or parental history of smoking (all p values > 0.05).
Table 1.
Demographics of Those Who Did Not Have Any Substance Use Disorders at Baseline (N=482)
| Demographic Features | Control (N=225) |
ADHD (N=257) |
Test statistic | p-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Baseline Age | 11.79 ± 3.24 | 10.70 ± 3.04 | t(480) = 3.82 | <0.001 |
| Age at Follow-up | 21.96 ± 3.86 | 20.19 ± 4.39 | t(480) = 4.70 | <0.001 |
| SES | 1.58 ± 0.76 | 1.90 ± 0.96 | z=−3.73 | <0.01 |
| N (%) | N (%) | |||
| Gender (% male) | 112 (50) | 133 (52) | χ2(1)=0.19 | 0.67 |
| Parental History of Alcohol Use Disorders | 64 (28) | 117 (46) | χ2(1)=14.93 | <0.01 |
| Parental History of Drug Use Disorders | 37 (16) | 71 (28) | χ2(1)=5.63 | <0.01 |
| Parental History of Smoking | 68 (30) | 86 (33) | χ2(1)=0.58 | 0.45 |
Note: SES = socioeconomic status.
At follow-up, among the subjects who were free of SUD at baseline (N=482), 25% (N=56) of controls and 32% (N=81) of Subjects with ADHD reported a substance use disorder; 22% (N=49) of controls and 27% (N=69) of Subjects with ADHD reported an alcohol use disorder; 10% (N=22) controls and 20 % (N=52) of Subjects with ADHD had a drug use disorder; and 12% (N=26) of controls and 27% (N=70) of Subjects with ADHD reported cigarette smoking.
Does ADHD predict SUD?
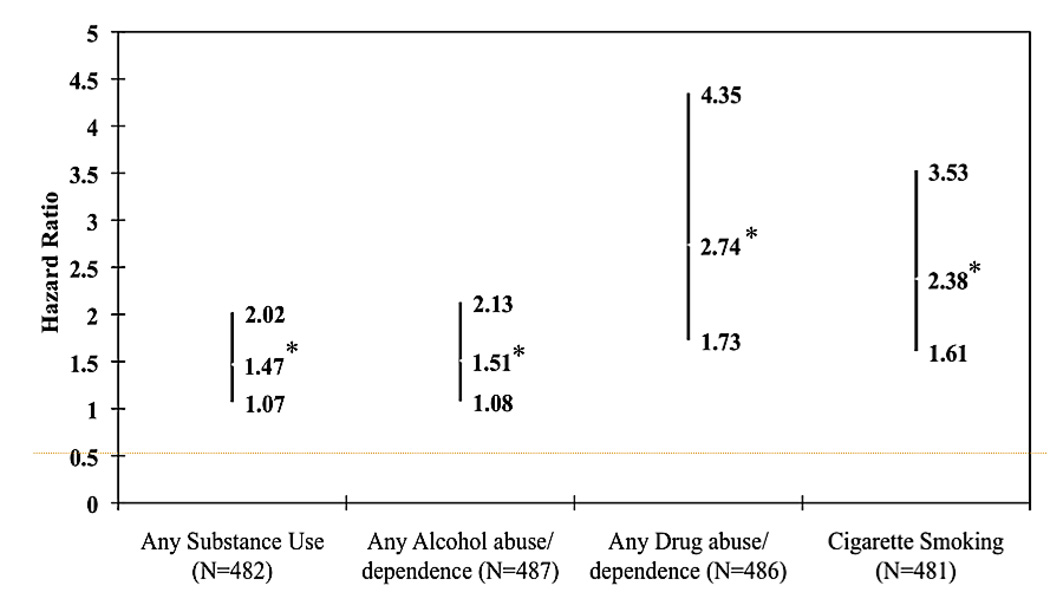
The coefficient examining the interaction between ADHD status and sex on the risk of SUD, adjusting for SES and parental history of SUD, was not significant (all p values > 0.05) and was removed from future analyses. We found that ADHD at baseline significantly predicted the development of any SUD with SES, sex, and parental SUD history in the model (Figure 1, p=0.02). Subjects with ADHD were 1.47 times more likely to develop a SUD compared to controls (95% Confidence Interval (CI): 1.07, 2.02). We found similar findings for alcohol use disorders (Hazard Ratio (HR): 1.51; 95% CI: 1.08, 2.13; p=0.02), drug use disorders (HR: 2.74; 95% CI: 1.73, 4.35; p<0.01) and cigarette smoking (HR: 2.38; 95% CI: 1.66, 3.63; p<0.01).
Figure 1.
Does Attention-Deficit/Hyperactivity Disorder (ADHD) Predict Substance Use Disorders and Cigarette Smoking? (N=497). Note: ADHD at baseline significantly predicted the development of any substance use disorders (SUD) while controlling for socioeconomic status (SES), sex, and parental history of any SUD (p=0.02), any alcohol use disorders (p=0.02), any drug use disorders (p<0.01), and cigarette smoking (p<0.01). Adjusted for SES and parental history of SUD. *=p<0.05
We repeated this analysis after removing subjects who reported lifetime history of conduct disorder at baseline. The interaction term between ADHD status and sex was not significant (all p values > 0.05) and was thus removed. We found that ADHD continued to be a significant risk factor for only any drug use disorders (HR: 2.54; 95% CI: 1.55; 4.17; p<0.01) and cigarette smoking (HR: 2.06; 95% CI: 1.36, 3.20; p<0.01). We did not find a significant association for overall substance use disorder (95% CI: 0.93, 1.83; p=0.13) or alcohol use disorders (95% CI: 0.94, 1.96; p=0.10).
We did not find a significant effect for the interaction between ADHD status and sex on SUD onset, alcohol use onset, or drug use onset (all p values > 0.05) and the term was subsequently removed. Subjects with ADHD had an earlier onset of SUD compared to controls (16.24 ± 2.64 years and 17.29 ± 2.69 years, respectively; beta=−1.04; 95% CI: −1.87, −0.21; p=0.02). For alcohol use disorders, we did not find a significant difference in onsets across ADHD status (beta: −0.68; 95% CI: −1.60, 0.23; p=0.14). For drug use, we found a significant difference in onset with Subjects with ADHD having an earlier onset than controls (16.02 ± 2.16 years and 17.12 ± 2.32 years, respectively; beta=−1.10; 95% CI: −2.11, −0.08; p=0.04). For cigarette smoking, there was a significant interaction between ADHD status and sex; therefore, we present the results within strata of sex. Among males, we found a significant difference in cigarette smoking onset, with Male subjects with ADHD having an earlier onset than male control subjects (14.62 ± 2.52 years and 17.32 ± 2.70 years, respectively; t=4.13; p=0.0001). Among females, we did not find a significant difference in onsets by ADHD status (ADHD: 14.47 ± 2.11 years and Control: 15.07 ± 3.10 years, respectively; t=0.78; p=0.44).
What predicts SUD in ADHD youth?
For comorbid disorders, no interaction term was significant (all p values > 0.05). Among Subjects with ADHD, comorbid oppositional defiant disorder and CD were significant predictors of SUD (or drug/alcohol/cigarette) while adjusting for sex and parental history of SUD (HR: 2.21, 95% CI: 1.40, 3.51; p<0.01; HR: 2.74, 95% CI: 1.66, 4.52; p<0.01, respectively; Table 2, Figure 2). We also found that for drug use disorders, comorbid mood disorder was a significant predictor (HR: 2.02; 95% CI: 1.22, 3.35; p<0.01).
Table 2.
Baseline Predictors Among Subjects With Attention-Deficit/Hyperactivity Disorder (ADHD) With and Without Subsequent Substance Use Disorders (SUD) (N=257)
| Substance Use Disorder + |
Substance Use Disorder − |
|
|---|---|---|
| N=93 | N=164 | |
| N (%) | N (%) | |
| Mood Disorders | 48 (52) | 69 (42) |
| Anxiety Disorders | 22 (24) | 37 (23) |
| Oppositional Defiant Disorder | 62 (68) | 68 (41) |
| Conduct Disorder | 22 (24) | 12 (7) |
| Extra Help (Male; N=133) | N=56; 26 (46) | N=77; 49 (64) |
| Extra Help (Female; N=124) | N=37; 29 (78) | N=87; 51(59) |
| Treatment: Medication | 71 (76) | 126 (77) |
| ADHD Persistence (at 10 year follow-up) | 60 (65) | 65 (40) |
| ADHD Subtypea | ||
| Inattentive | 13 (14) | 36 (22) |
| Hyperactive/Impulsive | 8 (9) | 8 (5) |
| Combined | 68 (73) | 112 (68) |
| Mean ± SD | Mean ± SD | |
| Social Adjustment Inventory for Children and Adolescents (SAICA) | 20.73 ± 7.08 | 19.84 ± 6.87 |
| Family Environment Scale (FES) | 0.02 ± 0.80 | 0.05 ± 0.69 |
| Full IQ | 108.83 ± 12.33 | 105.72 ± 13.76 |
| Digit Span | 9.41 ± 2.84 | 8.67 ± 3.10 |
| WRAT Arithmetic Scaled Score | 97.82 ± 14.71 | 95.10 ± 15.84 |
| Lifetime Sum Inattentive Symptoms | 5.74 ± 2.04 | 6.14 ± 2.04 |
| Lifetime Sum Hyperactive/Impulsive Symptoms | 6.00 ± 1.89 | 5.99 ± 1.83 |
Note: WRAT = Wide Range Achievement Test
2 subjects with any SUD did not have a subtype; 8 subjects without any SUD did not have a subtype
Figure 2.
Predictors of Substance Use Disorders (SUD) Among Young Adults with Attention-Deficit/Hyperactivity Disorder (ADHD) (N=257). Note: Comorbid oppositional defiant disorder and conduct disorder were significant predictors of any SUD while adjusting for sex and parental history of SUD (p<0.01; p<0.01, respectively). Sex predisposed subjects to a different risk profile for extra help (z=−2.19, p=0.03; all other p values > 0.05); extra help for boys was shown to be protective against the development of any SUD (p=0.03). SAICA = Social Adjustment Inventory for Children and Adolescents; WRAT Arithmetic S.S. = Wide Range Achievement Test Arithmetic Scaled Score. *=p<0.05
Family and School Environment
With the exception of a single significant interaction term (extra help-by-sex; z=−2.19, p=0.03) for any SUD, no other interaction effects were observed (all p values > 0.05). Extra help for boys (N=55; %) was shown to be protective against the development of any SUD (HR: 0.55; 95% CI: 0.32, 0.94; p=0.03); however, we did not find this significant association specifically for alcohol use disorders, drug use disorders, or cigarette smoking. We also found that those who repeated a grade were less likely to develop any drug use disorder (HR: 0.40; 95% CI: 0.20, 0.81; p=0.01). We found no other significant interaction terms for social or family environment factors or cognitive or any other school functioning factors.
We found a significant effect for parental history of smoking and cigarette smoking in young adults (HR: 3.74; 95% CI: 1.31, 10.68; p=0.01) while adjusting for sex. We did not find a significant effect of parental history of SUD on the development of SUD (HR: 1.09; 95% CI: 0.73, 1.66; p=0.22), alcohol use disorders (HR: 1.32; 95% CI: 0.85, 2.05; p=0.78), or drug use disorders (HR: 0.76; 95% CI: 0.41, 1.41; p=0.39). We found a significant effect of parental history of ADHD and alcohol use disorders (HR: 0.51; 95% CI: 0.29, 0.89; p=0.02) while adjusting for sex. We however did not find a significant effect of parental history of ADHD on the development of SUD (HR: 0.66; 95% CI: 0.40, 1.08; p=0.10), drug use disorders (HR: 0.72; 95% CI: 0.40, 1.29; p=0.27), or cigarette smoking (HR: 0.86; 95% CI: 0.52, 1.41; p=0.55).
Treatment
As previously reported 26, 27 we examined lifetime treatment reported at baseline: any medication (medication only, medication + therapy) vs. other types of treatment (therapy only, hospitalization, no treatment). There was not a significant interaction term between treatment and sex (p value > 0.05). Among Subjects with ADHD, any medication was not significantly associated with subsequent SUD (or alcohol/drug/cigarette) while adjusting for sex, SES, and parental history of SUD (HR: 0.83, 95% CI: 0.51, 1.35; p=0.45; HR: 0.85, 95% CI: 0.50, 1.45, p=0.56; HR: 0.70, 95% CI: 0.40, 1.25; p=0.23; HR: 1.12, 95% CI: 0.66, 1.91; p=0.67, respectively).
Persistent ADHD at the 10 year follow-up
For persistent ADHD (reported full or subthreshold ADHD within the past month) at the 10 year follow-up, we did not find a significant interaction between persistent ADHD and sex (all p values >0.05). Among Subjects with ADHD at the 10 year follow-up, we found that subjects with persistent ADHD were almost 3 times more likely to also be diagnosed with any SUD (alcohol/drug/cigarette) while adjusting for sex, SES, and parental history of SUD (OR: 2.83, 95% CI: 1.65, 4.87, p<0.001; OR: 2.66, 95% CI: 1.53, 4.62, p=0.001; OR: 2.01, 95% CI:1.12, 3.60, p=0.02; OR:1.91, 95% CI: 1.11, 3.30, p=0.02).
ADHD Subtype
We found no significant interaction terms between the ADHD subtypes and sex (all p values> 0.05). Among Subjects with ADHD, subtype (inattentive, hyperactive/impulsive, combined) reported at baseline was not a significant predictor of subsequent SUD (alcohol/drug/cigarette) while adjusting for sex, SES, and parental history of SUD (HR: 1.11, 95% CI: 0.84, 1.46; p=0.47; HR: 1.10, 95% CI: 0.82, 1.47, p=0.53; HR: 1.01, 95% CI: 0.73, 1.38; p=0.96; HR: 1.13, 95% CI: 0.85, 1.50; p=0.41, respectively).
We also examined substance use development using the total number of symptoms reported at baseline. We found no significant interaction terms between symptom count and sex (all p values>0.05). Among Subjects with ADHD, neither the inattentive symptom count nor the hyperactive/impulsive symptom count were predictive of subsequent SUD (alcohol/drug/cigarette) while adjusting for sex, SES, and parental history of SUD (inattentive: HR: 0.96, 95% CI: 0.81, 1.15; p=0.69, hyperactive/impulsive: HR: 1.04, 95% CI: 0.92, 1.17, p=0.56; inattentive: HR: 0.98, 95% CI: 0.82, 1.19, p=0.91, hyperactive/impulsive: HR: 1.02, 95% CI: 0.89, 1.15, p=0.82; inattentive: HR: 0.99, 95% CI: 0.80, 1.24; p=0.96, hyperactive/impulsive: HR: 1.02, 95% CI: 0.88, 1.19, p=0.74; inattentive: HR: 1.12, 95% CI: 0.91, 1.38; p=0.27, hyperactive/impulsive: HR: 1.04, 95% CI: 0.92, 1.19, p=0.52, respectively).
Presentation of SUD
Development of Both Alcohol and Drug Use Disorders
Among probands who did not have neither an alcohol nor a drug use disorder at baseline (N=482), 20% (N=51) of Subjects with ADHD (N=257) and 3% (N=17) of control subjects (N=225) developed both an alcohol and drug use disorder. The coefficient examining the interaction between ADHD status and sex, adjusting for the covariates of SES and parental history of SUD, was significant (z=2.73, p<0.01) and we present the results stratified by sex. Among females, those with ADHD at baseline were 3.49 times more likely to develop both an alcohol and drug use disorder compared to controls (95% CI: 1.27, 9.62, z=2.42, p=0.02). Among males, those with ADHD were 3.10 times more likely to develop both an alcohol and drug use disorder compared to controls (95% CI: 1.55, 6.20, z=3.21, p<0.01).
We repeated this analysis after removing subjects who reported lifetime history of conduct disorder at baseline. The interaction term between ADHD status and sex was not significant (z=1.71, p=0.09) and was thus removed. We found that ADHD continued to be a significant risk factor for the combination of an alcohol use and drug use disorder (HR: 3.34, 95 CI: 1.80, 6.20, p<0.01).
Transitions from Substance Abuse and Substance Dependence
For the transition from substance abuse to dependence, there was no interaction between sex and ADHD status (both p values>0.05); therefore, the interaction term was removed from both analyses. While controlling for age and SES, we did not find a significant difference in transition time for alcohol or drug abuse to dependence between Subjects with ADHD and controls (all p values >0.05).
Duration
Adjusting for age, sex, parental history, and SES, the coefficient examining the interaction between ADHD status and sex was not associated with the duration of SUD (all p values >0.05) and was thus removed. For alcohol use disorders, drug use disorders, and smoking, ADHD was not associated with the duration of subsequent SUD (t=0.14, p=0.89; t=1.80, 0.08; t=1.05, p=0.30; respectively).
Severity
The interaction term between ADHD and sex was not significant for any SUD, any alcohol use disorder nor for any drug use disorder (all p values >0.05); therefore, it was dropped from all analyses and we collapsed across sex status. We did not find a significant difference in impairment across ADHD status for any SUD (OR: 1.86; 95% CI: 0.94, 3.69; p=0.07), alcohol use disorders (OR: 1.39; 95% CI: 0.61, 3.17; p=0.43) or drug use disorders, (OR: 1.94; 95% CI: 0.75, 5.04; p=0.17).
Specific Drug
Because marijuana was the substance most endorsed for abuse (N=52) and for dependence (N=35), we collapsed all other drugs into one category. For abuse, people also endorsed hallucinogens (N=11), stimulants (N=8), sedatives (N=7), cocaine (N=6), ecstasy (N=4), and others including opioids, hash, and steroids (N=8). For dependence, people also endorsed cocaine (N=9), stimulants (N=4), sedatives (N=3), opiods (N=2) and others including hash, hallucinogens, and ecstasy (N=4).
For the endorsement of abuse and dependence, we did not find a significant interaction between ADHD and sex (both p values >.05). As a result, the interaction term was dropped from both analyses. We did not find any difference in the endorsement of marijuana versus other drugs across ADHD status for substance abuse (OR: 0.43; 95% CI: 0.16, 1.17; p=0.10) or substance dependence (OR: 0.25, 95% CI: 0.05, 1.26; p=0.09).
Discussion
The results of this follow-up (average 10 years) study of two cohorts of children to examine early predictors for later SUD partially support our hypotheses. Our results support the hypothesis that ADHD is a risk for later SUD and the major predictive importance of early CD on subsequent SUD in ADHD youth. However, we failed to demonstrate significant associations between familiality, baseline cognitive, non-CD psychiatric, treatment, subtype, or academic dysfunction and later SUD in our ADHD youth. Persistent ADHD was associated with later SUD. These longitudinal findings support and add to the literature the importance of ADHD as a risk factor for later cigarette smoking, drug, and alcohol use disorders.
Our findings support the hypothesis that ADHD itself is a risk for subsequent SUD. In our sample, ADHD was associated with both an earlier onset and a higher risk for SUD. These data support previous retrospective findings in adults with ADHD37 and prospective child studies.12, 14–18 For example, in 5- and 8-year follow-up studies, more alcohol use was shown among hyperactive and largely conduct disordered ADHD adolescents compared to non-ADHD controls.18 Molina et al.14 reported that comorbid disruptive symptoms and the severity of ADHD were associated specifically with increased SUD risk. Our data also show that when controlling for CD, ADHD continued to be significantly associated with SUD.
Our findings showing that CD with or without ADHD is a risk factor for SUD are well supported in the literature. For instance, Brook et al.5 and Crowley et al.38 have shown that independent of ADHD status, CD in children remains a major risk factor for subsequent SUD. Likewise, Mannuzza et al.12 have shown the importance of CD in ADHD and later SUD throughout adolescence and into adulthood. As part of the NY longitudinal study, Lampert and colleagues showed that delinquency in ADHD was related to SUD.39 Molina et al,17 as part of the multimodal treatment of ADHD three-year follow-up study reported that delinquent behavior in ADHD resulted in an increased risk for substance use. These aggregate studies are particularly noteworthy in that CD can be diagnosed early in life. In our sample, 30% of our Children with ADHD at their baseline assessment already manifested CD that subsequently increased their risk for SUD by almost three fold.
In the current data, we found that mood disorders predicted drug use disorders, but not overall SUD, alcohol use disorders, or cigarette smoking. These findings partially support our previous work in ADHD and controls in which major depression was not associated with SUD; yet, bipolar disorder was associated with SUD.13, 37, 40 Unlike our previous report that found a significant association between bipolar and SUD through the five-year follow-up of boys with ADHD,13 the current analysis used baseline data on combined mood disorders (due to our small sample size of bipolar subjects (N=26)) to predict later SUD. Emerging data strongly support the role of early bipolar disorder in later SUD41.
Contrary to our hypothesis, we also failed to find significant associations between indices of academic underachievement or cognitive dysfunction and later SUD in our ADHD group. These findings are at odds with an older literature indicating the importance of academic achievement and later substance use.23 Likewise, some studies have shown that adults with SUD manifest cognitive dysfunction at higher rates than those without SUD;42, 43 however, the directionality of causality remains unclear. Our findings are consistent with other studies that largely show cognitive deficits to be directly related to the pernicious effects of SUD. For instance, a number of studies have shown impaired cognitive or neuropsychological functioning in individuals with ongoing SUD; these deficits normalized with the cessation of SUD,43, 44 suggesting that these subjects had normal executive functioning prior to the effects of the substances of abuse. Moreover, we have recently shown that neuropsychologically defined executive functioning deficits in midadolescence do not predict SUD five years later.45 While the discrepancies between our findings and those above are not clear, it may be that our classification of academic and cognitive functioning may not have been sensitive enough to examine more subtle effects on later SUD. Alternatively, it may be that ADHD itself creates a risk factor for SUD that exceeds that of further academic and cognitive dysfunction (ceiling effect) or that neuropsychological/executive functioning deficits are not causal, but more a result of SUD.
We did not find that the subtype of ADHD nor ADHD symptom count predicted later SUD. However, we did find that the persistence of ADHD (e.g. presence of ADHD at 10 year follow-up) was associated with a robust 3 fold increased risk for SUD. Our findings are similar to Molina et al 14, who as part of a longitudinal study reported that persistent ADHD was associated with later SUD. We failed to find that medication (largely stimulant) or psychotherapy at baseline predicated SUD at the 10 year follow-up. These findings from this combined sample of males and females with ADHD extend previous findings in the 10 year sample of males 46 in which we reported no increase or decrease in SUD associated with stimulant treatment.16 As we previously speculated, the lack of protective effects of treatment on later SUD previously reported in mid adolescence 47, 48 may be related to the sample maturing through the age of risk for SUD, the inconsistent use of treatment (as evidenced by ADHD symptoms at follow-up related to SUD), offspring individuation and loss of parental monitoring, familial link of ADHD and SUD 49, or the combination. Further studies examining the developmental influence of treatment, treatment persistence and adequacy on later SUD is necessary.
As we have reported previously,49 we found higher rates of SUD in our ADHD families. However, among children with ADHD, family history of SUD was not a predictor of subsequent SUD. While a rich literature has demonstrated the familial nature of SUD,50 and that familial SUD is associated with early onset SUD in offspring,51–53 the current findings showed no effects of familial SUD on the development of SUD within ADHD. Although the reasons for this finding remain unclear, it may be that in our middle class sample, parents with SUD histories were sensitive to substance use and monitored emergent SUDs in their offspring. It may also be that ADHD and related comorbidity created a ceiling effect for the development of SUD, limiting the additive effects of family history. Alternatively, it may be that while providing follow-up of 10 to 11 years, our group of ADHD at a mean age of 20.38 ± 4.55 years is still not fully through the age of risk for SUD in ADHD.37
We also found that familial ADHD did not increase the risk for subsequent SUD. In fact, we found familial ADHD offered a protective effect against subsequent alcohol use disorder in our ADHD youth. Our findings are noteworthy given that we have previously shown that ADHD is linked to SUD in families.49 Our findings may be related to ADHD parents being attentive to the diagnosis and treatment of ADHD (and SUD) in their offspring. For instance, in these samples at 4- to 5-year follow-up, we have previously shown that treatment of ADHD may reduce or delay the onset of both cigarette smoking54 and SUD54 in adolescence. The aggregate family findings are reassuring in that neither ADHD nor SUD in a family member consistently increase the risk for SUD, although in the current study, we only examined baseline predictors of subsequent SUD.
Results from the current data have important clinical relevance when assessing subsequent SUD risk in children with a mean age of 11 years. For instance, children with ADHD, and in particular, those with comorbid CD, require enhanced monitoring for SUD and its antecedents. Family histories of either ADHD or SUD do not increase the risk for subsequent SUD. Similarly, ADHD subtype, social, cognitive, and academic dysfunction in Children with ADHD do not appear to further increase the risk for SUD. While early treatment of ADHD does not increase or decrease later SUD, persistent ADHD is associated with a substantially increased risk for SUD. Education for families of children with ADHD can help focus surveillance and early intervention that may reduce the subsequent development of SUD.
There are a number of methodological limitations to the current study. We utilized self and parent reports by structured interview for SUD instead of objective testing. We have previously shown that structured interview reports appear more sensitive and specific for establishing SUD in this population compared to urine toxicological testing.55 We may have missed important interval events as we were focused on examining baseline and subsequent SUD at the 10- to 11-year follow-up. Future studies should examine both moderators and mediators of subsequent SUD. Our sample of subjects with SUDs was relatively small, thereby limiting our power for sub-analyses and ability to adequately control for confounders. We chose not to control for multiple comparisons. Using the Bonferroni adjustment alters the statistical inference of a study from the testing of a number of specific hypotheses to a test of the universal null hypotheses.56, 57 This method increases the Type II error rate56, 57 and raises the issue of the amount of tests to be included in the adjustment.56 Our ADHD sample available for follow-up was largely middle class and Caucasian, thus limiting the generalization. Our sample at follow-up may not have been through the full age of risk for SUD.
Despite these limitations, our findings replicate previous work, highlighting that childhood ADHD is a risk factor for SUD, and CD within ADHD continues to be a very important determinant of subsequent SUD. Future studies examining the pathway to SUD in this age group and older samples are necessary to shed more light on potential mechanisms of SUD development in ADHD.
Acknowledgments
This study was funded by K24 DA016264 (T.W.) as well as in part by a grant from United States Public Health Service (National Institute on Child Health and Human Development), and grant 5R01 HD-36317-07 (J.B.), and in part by a grant from the Eli Lilly and Company Foundation, and the Pediatric Psychopharmacology Philanthropy Fund.
Dr. Wilens has received grant support from Abbott, McNeil, Eli Lilly and Co., the National Institutes of Health – National Institute on Drug Abuse, Merck, and Shire. He has served on the speakers’ bureau for Eli Lilly and Co., McNeil, Novartis, and Shire. He has served as a consultant to Abbott, AstraZeneca, McNeil, Eli Lilly and Co., the National Institutes of Health, Novartis, Merck, and Shire. He receives royalties from Guildford Press. Dr. Biederman has received research support from Abbott, Alza, AstraZeneca, Bristol Myers Squibb, Celltech, Cephalon, Eli Lilly and Co., Elminda, Esai, Forest, GlaxoSmithKline, Gliatech, Janssen, McNeil, Merck, the National Alliance for Research on Schizophrenia and Depression, the National Institute on Drug Abuse, New River, the National Institute of Child Health and Human Development, the National Institute of Mental Health, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, the Prechter Foundation, Shire, the Stanley Foundation, UCB Pharma, and Wyeth. He has served on the speakers’ bureau for Fundacion Areces, Medice Pharmaceuticals, and the Spanish Child Psychiatry Association. Dr. Joshi has recevied research support from Abbott, Bristol Myers Squibb, Cephalon, Eli Lilly and Co., GlaxoSmithKline, Johnson and Johnson, McNeil, Merck, the National Institute of Mental Health, New River, Novartis, Organon, Otsuka, Takeda, Pfizer, and Shire. He is a member of the National Institute of Mental Health Editorial Board. He has received the Ethel DuPont Fellowship Award. He has received the Pilot Research Award from the American Academy of Child and Adolescent Psychiatry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Ms. Martelon, Mr. Bateman, Ms. Fried, and Mr. Petty report no biomedical financial interests or potential conflicts of interest.
References
- 1.Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007 May;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 2.Brook JS, Whiteman M, Cohen P, Shapiro J, Balka E. Longitudinally predicting late adolescent and young adult drug use: Childhood and adolescent precursors. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(9):1230–1238. doi: 10.1097/00004583-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BA, Cloninger CR, Roache JD, Bordnick PS, Ruiz P. Age of onset as a discriminator between alcoholic subtypes in a treatment-seeking outpatient population. Am J Addict. 2000 Winter;9(1):17–27. doi: 10.1080/10550490050172191. [DOI] [PubMed] [Google Scholar]
- 4.Lewinsohn PM, Gotlib IH, Seeley JR. Adolescent psychopathology: IV. Specificity of psychosocial risk factors for depression and substance abuse in older adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(9):1221–1229. doi: 10.1097/00004583-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Brook JS, Whiteman M, Finch SJ, Cohen P. Young adult drug use and delinquency: Childhood antecedents and adolescent mediators. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(12):1584–1592. doi: 10.1097/00004583-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kandel DB, Johnson JG, Bird HR, et al. Psychiatric comorbidity among adolescents with substance use disorders: Findings from the MECA study. J Am Acad Child Adolesc Psychiatry. 1999;38(6):693–699. doi: 10.1097/00004583-199906000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Bukstein OG, Bernet W, Arnold V, et al. Practice parameter for the assessment and treatment of children and adolescents with substance use disorders. J Am Acad Child Adolesc Psychiatry. 2005 Jun;44(6):609–621. doi: 10.1097/01.chi.0000159135.33706.37. [DOI] [PubMed] [Google Scholar]
- 8.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. The American Journal of Psychiatry. 2007 Jun;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 9.Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. American Journal of Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective followup study. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29:546–557. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Mick E, Faraone SV, Biederman J, Spencer T. The course and outcome of ADHD. Primary Psychiatry. 2004;11(7):42–48. [Google Scholar]
- 12.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult outcome of hyperactive boys: Educational achievement, occupational rank, and psychiatric status. Archives of General Psychiatry. 1993;50:565–576. doi: 10.1001/archpsyc.1993.01820190067007. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J, Wilens T, Mick E, et al. Is ADHD a risk for psychoactive substance use disorder? Findings from a four year follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Molina B, Pelham W. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- 15.Hechtman L, Weiss G. Controlled prospective fifteen year follow-up of hyperactives as adults: Non-medical drug and alcohol use and anti-social behaviour. Canadian Journal of Psychiatry. 1986;31:557–567. doi: 10.1177/070674378603100614. [DOI] [PubMed] [Google Scholar]
- 16.Mannuzza S, Klein R, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. The American Journal of Psychiatry. 1998;155(4):493–498. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- 17.Molina BS, Flory K, Hinshaw SP, et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects. J Am Acad Child Adolesc Psychiatry. 2007 Aug;46(8):1028–1040. doi: 10.1097/chi.0b013e3180686d96. [DOI] [PubMed] [Google Scholar]
- 18.Satterfield JH, Hoppe CM, Schell AM. A prospective study of delinquency in 110 adolescent boys with attention deficit disorder and 88 normal adolescent boys. American Journal of Psychiatry. 1982;139:795–798. doi: 10.1176/ajp.139.6.795. [DOI] [PubMed] [Google Scholar]
- 19.Szobot CM, Rohde LA, Bukstein O, et al. Is attention-deficit/hyperactivity disorder associated with illicit substance use disorders in male adolescents? A community-based case-control study. Addiction. 2007 Jul;102(7):1122–1130. doi: 10.1111/j.1360-0443.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 20.Loeber R, Stouthamer-Loeber M, White HR. Developmental aspects of delinquency and internalizing problems and their association with persistent juvenile substance use between ages 7 and 18. Journal of Clinical Child Psychology. 1999;28(3):322–332. doi: 10.1207/S15374424jccp280304. [DOI] [PubMed] [Google Scholar]
- 21.Fergusson DM, Lynskey MT, Horwood LJ. Conduct problems and attention deficit behaviour in middle childhood and cannabis use by age 15. Australian and New Zealand Journal of Psychiatry. 1993;27(4):673–682. doi: 10.3109/00048679309075830. [DOI] [PubMed] [Google Scholar]
- 22.Brook DW, Brook JS, Zhang C, Koppel J. Association between attention-deficit/hyperactivity disorder in adolescence and substance use disorders in adulthood. Arch Pediatr Adolesc Med. 2010 Oct;164(10):930–934. doi: 10.1001/archpediatrics.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crum RM, Bucholz KK, Helzer JE, Anthony JC. The risk of alcohol abuse and dependence in adulthood: The association with educational level. American Journal of Epidemiology. 1992;135(9):989–999. doi: 10.1093/oxfordjournals.aje.a116411. [DOI] [PubMed] [Google Scholar]
- 24.Brook JS, Finch SJ, Whiteman M, Brook DW. Drug use and neurobehavioral, respiratory, and cognitive problems: precursors and mediators. J Adolesc Health. 2002;30(6):433–441. doi: 10.1016/s1054-139x(01)00395-0. [DOI] [PubMed] [Google Scholar]
- 25.Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003 Jun;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 26.Biederman J, Monuteaux M, Mick E, et al. Young Adult Outcome of Attention Deficit Hyperactivity Disorder: A Controlled 10 year Follow-Up Study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 27.Biederman J, Petty CR, Monuteaux MC, et al. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010 Apr;167(4):409–417. doi: 10.1176/appi.ajp.2009.09050736. [DOI] [PubMed] [Google Scholar]
- 28.Busch B, Biederman J, Cohen LG, et al. Correlates of ADHD among children in pediatric and psychiatric clinics. Psychiatric Services. 2002;53(9):1103–1111. doi: 10.1176/appi.ps.53.9.1103. [DOI] [PubMed] [Google Scholar]
- 29.Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- 30.First M, Spitzer R, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- 31.Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP, Version 1.0) Washington, D.C.: American Psychiatric Press; 1990. [Google Scholar]
- 32.Gershon ES, Hamovit J, Guroff JJ, et al. A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry. 1982 Oct;39(10):1157–1167. doi: 10.1001/archpsyc.1982.04290100031006. [DOI] [PubMed] [Google Scholar]
- 33.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 34.Orvaschel H, Walsh G. Assessment of Adaptive Functioning in Children: A Review of Existing Measures Suitable for Epidemiological and Clinical Services Research. Washington, DC: U.S. Department of Health and Human Services, National Institute of Mental Health, Division of Biometry and Epidemiology; 1984. [Google Scholar]
- 35.Moos RH, Moos BS. Manual for the Family Environment Scale. Palo Alto, CA: Consulting Psychologists Press; 1974. [Google Scholar]
- 36.Biederman J, Faraone SV, Weber W, Russell RL, Rater M, Park K. Correspondence between DSM-III-R and DSM-IV Attention Deficit Hyperactivity Disorder (ADHD) Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(12):1682–1687. doi: 10.1097/00004583-199712000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Wilens TE, Biederman J, Mick E, Faraone SV, Spencer T. Attention deficit hyperactivity disorder (ADHD) is associated with early onset substance use disorders. Journal of Nervous and Mental Disease. 1997;185(8):475–482. doi: 10.1097/00005053-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Crowley T, Mikulich S, MacDonald M, Young S, Zerbe G. Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome. Drug and Alcohol Dependence. 1998;49:225–237. doi: 10.1016/s0376-8716(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 39.Loney J. Substance abuse in adolescents: Diagnostic issues derived from studies of attention deficit disorder with hyperactivity. NIDA Research Monograph. 1988;77:19–26. [PubMed] [Google Scholar]
- 40.Biederman J, Wilens TE, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): Effects of ADHD and psychiatric comorbidity. American Journal of Psychiatry. 1995;152(11):1652–1658. doi: 10.1176/ajp.152.11.1652. [DOI] [PubMed] [Google Scholar]
- 41.Wilens TE, Biederman J, Adamson JJ, et al. Further evidence of an association between adolescent bipolar disorder with smoking and substance use disorders: a controlled study. Drug Alcohol Depend. 2008 Jun 1;95(3):188–198. doi: 10.1016/j.drugalcdep.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medina KL, Shear PK, Schafer J. Memory functioning in polysubstance dependent women. Drug Alcohol Depend. 2006 Oct 1;84(3):248–255. doi: 10.1016/j.drugalcdep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008 Jan;1(1):99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol Clin Exp Res. 2009 Mar;33(3):490–498. doi: 10.1111/j.1530-0277.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 45.Wilens T, Martelon M, Fried R, Petty C, Bateman C, Biederman J. Do Executive Function Deficits Predict Later Substance Use Disorders Among Adolescents and Young Adults? Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1016/j.jaac.2010.11.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. American Journal of Psychiatry. 2008 May;165(5):597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- 47.Wilens TE, Adamson J, Monuteaux MC, et al. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Archives of Pediatrics and Adolescent Medicine. 2008 Oct;162(10):916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biederman J, Wilens T, Mick E, Spencer T, Faraone S. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. 1999;104(2):e20. doi: 10.1542/peds.104.2.e20. [DOI] [PubMed] [Google Scholar]
- 49.Biederman J, Petty CR, Wilens TE, et al. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008 Jan;165(1):107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- 50.Tsuang MT, Lyons MJ, Eisen SA, et al. Genetic influences on DSM-III-R drug abuse and dependence: A study of 3,372 twin pairs. Am J Med Genet. 1996;67(5):473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Babor TF, Hofmann M, DelBoca FK, et al. Types of alcoholics, 1: Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- 52.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- 53.Irwin M, Schuckit M, Smith TL. Clinical importance of age at onset in type 1 and type 2 primary alcoholics. Archives of General Psychiatry. 1990;47:320–324. doi: 10.1001/archpsyc.1990.01810160020003. [DOI] [PubMed] [Google Scholar]
- 54.Wilens T, Adamson J. Impact of prior stimulant treatment for Attention-Deficit Hyperactivity Disorder in the subsequent risk for cigarette smoking, alcohol, and drug use disorders in adolescent girls. Arch Pediatr Adolesc Med. 2008 doi: 10.1001/archpedi.162.10.916. 7/29/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gignac M, Wilens TE, Biederman J, Kwon A, Mick E, Swezey A. Assessing cannabis use in adolescents and young adults: what do urine screen and parental report tell you? J Child Adolesc Psychopharmacol. 2005 Oct;15(5):742–750. doi: 10.1089/cap.2005.15.742. [DOI] [PubMed] [Google Scholar]
- 56.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998 Apr 18;316(7139):1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990 Jan;1(1):43–46. [PubMed] [Google Scholar]