Abstract
Objective
Studies of healthy individuals and those with cerebellar damage have implicated the cerebellum in a variety of cognitive and behavioral processes. Reduced cerebellar volume has been found in children with ADHD and differentially related to behavioral outcomes. In the current study, we sought to determine if reduced cerebellar vermis volume was present in children with ADHD-Combined type (ADHD-C) compared to controls and whether volume related to parent and teacher reported levels of ADHD symptomatology.
Method
2T MRI images and parent and teacher reported ADHD symptoms were acquired for 32 children diagnosed with ADHD-C and 15 typically-developing controls. Participants were right-handed, had no comorbid diagnoses of learning disabilities, conduct disorder, or affective/mood disorder, and were between the ages of 9 and 15.
Results
Participants with ADHD-C showed significantly reduced volume in the posterior inferior vermis compared to controls. No statistically significant differences were observed for cerebral volume, anterior vermis volume, posterior superior volume, or total cerebellar volume. Regression analyses indicated that a significant amount of the variance in parent-reported BASC-II Hyperactivity and Attention and Conners’ Restless/Impulsive ratings was explained by volume of the posterior inferior vermis.
Conclusions
Consistent with previous studies, children with ADHD had reduced volume in the posterior inferior vermis. New findings emerged with reduced volume of the posterior inferior vermis predicting significant amount of the variance in parent-reported hyperactivity, attention, and restlessness/impulsivity. Thus, symptoms of hyperactivity and inattention in ADHD may be partially explained by reduced volume of the cerebellar vermis and its connections within the cerebrum.
Keywords: ADHD, cerebellum, vermis, hyperactivity, attention
BACKGROUND
Attention-Deficit/Hyperactivity Disorder (ADHD) is one of the most commonly diagnosed neuropsychiatric disorders in children and adolescents. It is estimated that between 3% and 7% of all school-aged children and nearly 5% of adults in the United States meet diagnostic criteria for ADHD.1,2 Worldwide prevalence estimations of ADHD are around 5.3%.3 ADHD is characterized by developmentally inappropriate levels of hyperactivity, impulsivity, and/or inattention that cause significant difficulty in social, academic, or work-related settings. Symptoms are often explained due to impairments in neuropsychological executive functions such as response inhibition, working memory, and inhibitory control.4–7
While many theories have attempted to explain how ADHD develops, the cause remains unknown. There is some evidence, however, that symptoms of ADHD are related to structural and functional brain differences and delayed development of the neocortex.8 For example, children with ADHD have been found to have reduced volume of the total cortex, cortical gray matter, frontal cortex, caudate nucleus, cerebellum, and anterior cingulate cortex compared to age-mates without ADHD.8–17 Furthermore, functional MRI studies revealed reduced activation and slower processing of the ventrolateral and dorsolateral prefrontal cortex in children with ADHD while performing working memory and response inhibition tasks.18–21 These studies suggest that the causal mechanism of ADHD symptomotology is likely related to a broad range of structural and functional abnormalities of the central nervous system.
Most studies on ADHD etiology attempt to identify one or more neural mechanisms for inattention or impulsivity. For years, this body of research focused on the ventrolateral, dorsolateral, prefrontal, and anterior cingulate cortices due to their involvement in attention and executive functions. New theories and research, however, implicate disruptions in attention and symptoms of ADHD through disruptions in parietal-frontal and cerebellar pathways along with frontal-striatal networks.22,23 Both studies in healthy individuals and those with cerebellar lesions suggest the cerebellum is involved in attention processes, making it an important area of research to those studying ADHD.
While the cerebellum is often described as a structure involved in motor coordination, it is also involved in numerous facets of cognitive and behavioral functioning. For example, patients with cerebellar lesions or atrophy have been found to show deficits in the ability to shift attention, with visuospatial processing, and with planning.24–27 Consistent with this clinical finding, neuroimaging studies in patients with cerebellar damage have indicated the cerebellum is involved in temporal information processing, working memory, and executive functioning abilities.28–30 In a classic study on the neural correlates of motor and non-motor cerebellar function, Allen, Buxton, Wong, and Courchesne 31 demonstrated differential activation within the cerebellum for visual attention and motor performance in healthy participants. This double dissociation suggested the cerebellum contains distinct regions for attention that are independent of motor movement regions.
The extant research suggests that the cerebellum, given its dense connections with the frontal cortex and basal ganglia,32 and high concentration of dopamine transporters in the posterior inferior vermis,33 might be disrupted in those with ADHD. Indeed, one of the most common regions investigated and consistently found to be abnormal in children with ADHD is the cerebellum with at least seven neuroimaging studies reporting abnormalities in the cerebellum or cerebellar vermis in ADHD.11,14,15,17,34–36 Many of these studies have reported reduced volume in the posterior inferior lobule of the cerebellar vermis (Lobules VIII-X, see Figure 1). Other studies, using diffusion tensor imaging (DTI), reported reduced fractional anisotropy in cerebellar white matter that accounted for attentional difficulties in participants with ADHD.37 Reduced fractional anisotropy in the right middle cerebellar peduncle has also been reported in children with ADHD, suggesting deficient cerebellar connectivity may be involved in the pathophysiology of ADHD.38 Some have found cerebellar vermis volume to be predictive of behavioral outcomes. For example, when ADHD participants were divided into better or worse outcome groups (Children’s Global Assessment Scale39 (CGAS) cutoff scores, based on a scale of 1 to 100) in a longitudinal study, those in the worse outcome group evinced thinning of the right and left inferior posterior cerebellar vermis compared to both controls and ADHD participants in the better outcome group. The CGAS, however, is a unidimensional assessment of functioning and does not provide estimates of domain-specific behavior (e.g., inattention, hyperactivity, impulsivity, aggressiveness, mood, or affective symptoms), making the interpretation of the outcome groups relatively broad. Nonetheless, it was therefore hypothesized that the development and morphology of the cerebellar vermis may be involved in the development and/or recovery from ADHD. While these hypotheses are intriguing, it is unknown if reduced cerebellar volume in ADHD is related to specific domains of functioning such as hyperactivity or inattention, cardinal difficulties in those with ADHD.
Figure 1.
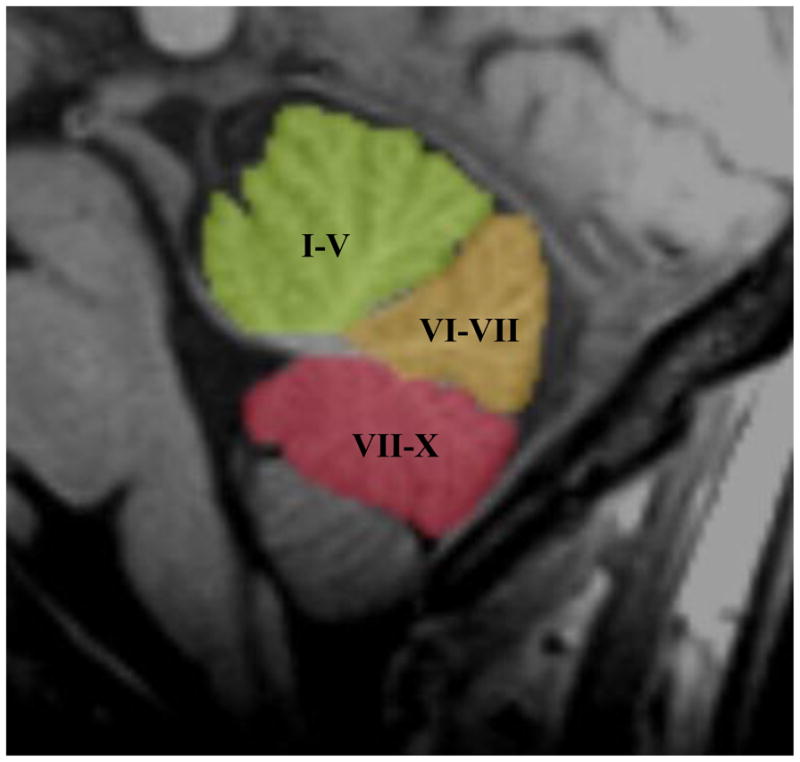
Mid-Sagittal Image Depicting the Lobules of the Cerebellar Vermis
Note: I–V = Lobules I–V of the Anterior Vermis; VI–VII = Lobules VI–VII of the Posterior Superior Vermis; VII–X = Lobules VII–X of the Posterior Inferior Vermis
The purpose of the current study was two-fold. First, we sought to determine if cerebellar vermis volume differed between a carefully diagnosed sample of children with ADHD-Combined Subtype (ADHD-C) and a sample of typically-developing control participants in three lobules of the cerebellar vermis (see Figure 1). Based on other work on the cerebellum and ADHD, it was hypothesized that volume of the posterior inferior cerebellar vermis (lobules VIII-X) would be significantly smaller in participants with ADHD-C. Second, we used regression models to determine if vermal volume was related to neuropsychological behavioral outcomes. It was hypothesized that volume of the posterior inferior vermis would predict a significant amount of variance in parent and teacher reported levels of hyperactivity, inattention, and restless/impulsive behavior.
METHOD
Participants
Approval for the current study was granted through the Institutional Review Board at large Southwestern and Midwestern medical schools. Participants were recruited through local schools, organizations, and psychological and psychiatric outpatient clinics and hospitals. The current study consisted of 32 boys and 15 girls with a mean age of 11.5 ± 1.90 years. There were two groups: ADHD-C (n = 32), and typically-developing controls (n = 15). All 47 participants completed neuropsychological testing and MRI scanning. Participants in the current study represent a subsample from a previous study.11 Nine ADHD participants and three control participants were excluded in the current study due to incomplete neuropsychological data (not an objective in the previous study). All participants were right-handed. Participants were recruited from the community as well as from a large Southwestern medical school.
ADHD-C Group
Children in the ADHD-C group were diagnosed according to the DSM-IV-TR with the Diagnostic Interview Schedule for Children-IV Parent Edition (DISC-IV-P)40 administered under the supervision of a licensed psychiatrist. Medical and developmental family history was reviewed with each participant’s parent or primary caregiver by the second or third author. ADHD participants did not meet past or current DSM-IV-TR diagnoses for any other psychological or psychiatric disorder including Oppositional Defiant Disorder, Conduct Disorder, Learning Disability, Tic Disorder, or other affective illness. In addition, parents or caregivers reported no history of neurological and/or developmental motor problems. To confirm the ADHD diagnosis, children with ADHD were evaluated with the Conners’ Global Index –Restless/Impulsive composite (CGI-R/I).41 ADHD participants fell at least 1.5 standard deviations above the mean on the Restless/Impulsive composite for their age and gender according to parent reporting. General Conceptual Ability from the Differential Ability Scales (DAS-GCA) was used to determine participants’ current level intellectual ability and the Wechsler Individual Achievement Test – Second Edition (WIAT-II) was used to determine current mathematics and reading ability.42,43 ADHD participants demonstrated DAS-GCA scores above 85 and did not meet criteria for learning disabilities according to the DAS-GCA and WIAT-II. Of the 32 children with ADHD, 16 were chronically-treated with stimulant medication at the time of study. No child was on medication for 24 to 48 hours prior to the MRI.
Control Group
Participants in the control group were matched on age and gender with ADHD participants. Children that served in the control group did not meet criteria for any DSM-IV-TR disorder according to DISC-IV-P interview. In addition, medical and developmental family history was reviewed with each participant’s primary caregiver. Control participants all fell within one standard deviation of average for their age and gender according to parent and teacher report on the CGI-R/I. DAS-GCA was used to determine control participants’ current level intellectual ability and the WIAT-II was used to determine current mathematics and reading ability 42,43. Control participants demonstrated DAS-GCA scores above 85 and did not meet criteria for learning disabilities according to the DAS-GCA and WIAT-II. DAS-GCA and WIAT-II scores are presented in Table 1.
Table 1.
Demographics and Neuropsychological Characteristics of Participants
| Measure | Group
|
p Value | |
|---|---|---|---|
| ADHD (n = 32) | Control (n = 15) | ||
| Gender | 21m/11f | 11m/4f | |
| Age (Years) | 11.11 (2.01) | 11.71 (1.80) | .307 |
| WIAT-II Word Reading | 101.53 (10.18) | 107.20 (10.35) | .083 |
| DAS-GCA | 103.65 (12.73) | 113.86 (12.21) | 0.13 |
| BASC-II Hyperactivity | 64.62 (24.58) | 36.53 (6.91) | <.000 |
| BASC-II Attention | 62.17 (11.05) | 39.47 (7.26) | <.000 |
| CGI-R/I Parent | 79.62 (12.27) | 46.73 (5.82) | <.000 |
| CGI-R/I Teacher | 76.06 (16.03) | 55.80 (14.87) | <.000 |
Note: ADHD = Attention-Deficit/Hyperactivity Disorder; BASC-II Attention = Behavioral Assessment Scale for Children 2nd Edition Attention Scale T-Score; BASC-II Hyperactivity = Behavioral Assessment Scale for Children 2nd Edition Hyperactivity Scale T-Score; CGI-R/I Parent = Conners’ Global Index – Restless Impulsive Parent Ratings Scale T-Score; CGI-R/I Teacher = Conners’ Global Index – Restless Impulsive Teacher Ratings Scale T-Score; DAS-GCA = Differential Ability Scales General Conceptual Ability; WIAT-II Word Reading = Wechsler Individual Achievement Test 2nd Edition Word Reading Subtest – Standard Score.
Neuropsychological Outcome Measures
For the behavioral rating scales, parents were asked to rate their child off of medication. The Behavior Assessment System for Children, 2nd Edition (BASC-II) was used to assess parent reported levels of hyperactivity and attention problems for both ADHD and control participants.44 The BASC-II is a comprehensive and multidimensional behavioral questionnaire designed to assess a wide range of internalizing and externalizing behaviors in children aged 2 to 18 and consists of self, parent, and teacher report forms allowing for the assessment of behaviors in different environmental contexts. The BASC-II is among the most widely used assessment questionnaires of childhood behavior in the United States.44 For the purposes of this study the parent Hyperactivity and Attention Problems scales were used. Reliability and validity estimations of the BASC-II suggest excellent internal consistency (.90 for composite and .80 for individual scales), test-retest reliability (.70 for composite scores and .80 for individual scales). Sensitivity and specificity of the BASC-II for differentiating ADHD from typically-developing control participants is high (93.3% and 93.5%, respectively).45 The Hyperactivity and Attention Problems scales, in particular, have demonstrated good sensitivity, specificity, and predictive validity in studies of ADHD.46,47
The Conners’ Ratings Scales-Revised (CRS-R) were used to assess parent and teacher reported levels of inattention and impulsivity for both ADHD and control participants.41 The CRS-R is a multidimensional assessment questionnaire of both internalizing and externalizing symptoms and DSM-IV disorders for children ages 3–17. The CRS-R features numerous composite scores used to assess ADHD symptoms including Restless/Impulsive, Hyperactive/Impulsive, and inattentive symptoms. Many studies have utilized the CRS-R in the assessment of ADHD and associated internalizing and externalizing behaviors as well as in the assessment of treatment outcomes.48,49 For the purposes of this study the parent and teacher Conners’ Global Index – Restless/Impulsive composite (CGI-R/I) was used as a measure of ADHD symptoms.
Neuroanatomical Acquisition and Analysis
T1-weighted magnetic resonance imaging (MRI) images were obtained at the Research Imaging Center at the UTHSCSA on a General Electric – Elscint 2T Prestige Scanner (Milwaukee, Wisconsin). Images were produced via three-dimensional gradient-recalled acquisitions in the steady state (3-D GRASS), with a repetition time (RT) of 33ms, time of echo (TE) of 12ms, and a flip angle of 60 degrees, rendering a 256 × 192 × 192 volume of data, and a spatial resolution of 1mm X 1mm X 1mm. Head movement was minimized with the use of single-sided tape and foam ear pads placed within the head coil. Raw data was analyzed on a Silicon Graphics (Sunnyvale, California) workstation and converted to MINC (Medical Image NetCDF) files. All T1 images were oriented to the anterior commissure and posterior commissure boundaries and spatially normalized to a standardized template to control for differences in brain volume. Cerebellar vermis measurements were analyzed using Analysis of Functional Neuroimages (AFNI) software.39 Total cerebral volume was calculated using the FreeSurfer neuroimaging analysis package.50
All cerebellum measurements were started in the midsaggital plane. The midsaggital plane was carefully identified by identification of the cerebral aqueduct, 4th ventricle, and anatomy of the cerebellar vermis. These methods for identifying the cerebellar vermis have been useful in other studies.15,17 The midsaggital plane was also confirmed in the coronal and axial planes due to slight differences in the alignment of the vermis within the cerebellum and cerebrum. The cerebellar vermis was parcellated into the anterior vermis (Lobules I–V), posterior superior vermis (Lobules VI–VII), and posterior inferior vermis (Lobules VIII–X) based on guidelines and anatomical landmarks from an MRI atlas of the cerebellum,51 as well as with detailed descriptions of manual and semiautomated methods. 52 All volume measurements were individually hand-traced and started at the apex of the fourth ventricle on the anterior vermis. Total vermis volume was calculated as the sum of Lobules I–X. AFNI paint opacity was adjusted to .3 for all participants in order to provide clear contrast between the vermal subregions, cerebrospinal fluid, and cerebellar peduncles. All volumetric measurements were obtained by a researcher blind to participant diagnosis. One third of the vermal lobules were remeasured to ensure reliability. Intrarater reliability was measured using intraclass correlational coefficients (ICC). ICC = .83 for the anterior vermis, .88 for the superior vermis, and .85 for the inferior vermis. Midsaggital images illustrating cerebellar vermal lobules (lobules I–V, lobules IV–VII, and lobules VIII–X) are presented in Figure 1. Figure 2 shows coronal segmentation of each vermal lobule.
Figure 2.
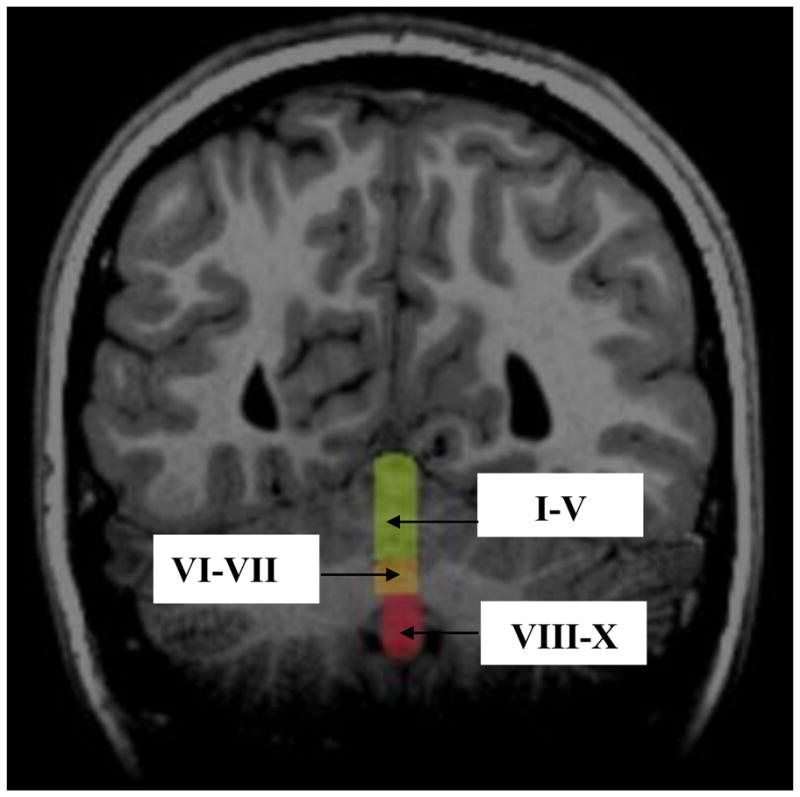
Coronal Image Depicting the Lobules of the Cerebellar Vermis
Note: I–V = Lobules I–V of the Anterior Vermis; VI–VII = Lobules VI–VII of the Posterior Superior Vermis; VII–X = Lobules VII–X of the Posterior Inferior Vermis
RESULTS
Demographic and Neuropsychological Characteristics
Analysis of Variance (ANOVA) was used to determine group differences in age, conceptual ability, and reading ability. There were no main effects between the groups on age, [F(1, 46) = 1.07, p = .307, Cohen’s d = .314] or word reading ability [F(1,46) = 1, 46, p = .083, Cohen’s d = .552]. Control participants demonstrated higher General Conceptual Ability (DAS-GCA) than ADHD participants [F(1, 46) = 6.42, p = .013, Cohen’s d = .818].
A 2 group by 4 measure multivariate analysis of covariance (MANCOVA) was used to determine differences in clinical/neuropsychological measures. Because DAS-GCA was significantly different between groups, it was controlled for in the analysis of clinical/neuropsychological tests. Multivariate analyses revealed a statistically significant group by neuropsychological functioning interaction [Wilks’ Λ = .274, F(4, 41) = 27.134, p < .000, η2 = .726]. Follow-up ANOVA’s resulted in statistically significant group differences in BASC-II/Hyperactivity [F(1, 46) = 75.002, p = < .000, Cohen’s d = 1.555], BASC-II/Attention [F(1, 46) = 112.993, p < .000, Cohen’s d = 2.42], Conners’ Global Index – Restless/Impulsive Parent report [F(1, 46) = 96.723, p < .000, Cohen’s d = 3.425], and Conners’ Global Index – Restless/Impulsive Teacher report [F(1, 46) = 17.054, p = .245, Cohen’s d = 1.310]. Demographic and clinical/neuropsychological characteristics are summarized in Table 1 below.
Cerebellar Vermis
ANOVA was used to determine group differences in cerebral volume and total cerebellar vermis volume. ADHD and control participants did not differ in total cerebral volume [F(1, 46) = .448, p = .507, Cohen’s d = .213] or total cerebellar vermis volume [F(1, 46) = 1.129, p = .294, Cohen’s d = .348]. Children with ADHD differed from control children with respect to volume of the vermis according to a 2 (group) by 3 (measure) MANOVA [Wilks’ Λ = .727, F(3, 43) = 5.378, p = .002, η2 = .273]. Follow-up ANOVA’s resulted in statistically significant group differences in the posterior inferior vermis (Lobules VIII–X) [F(1, 46) = 12.705, p = .001, Cohen’s d = .353]. No group differences emerged for the anterior vermis (Lobules I–V) [F(1, 46) = .101, p = .752, Cohen’s d = .100] or posterior superior vermis (Lobules VI–VII) [F(1, 46) = .096, p = .758, Cohen’s d = .104]. Cerebellar vermis analysis and measurements are presented in Table 2.
Table 2.
Cerebellar Vermis Measurements (mL) and Total Cerebral Volume (mm3)
| Region | ADHD (n = 32) | Control (n = 15) | p Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Anterior Vermis | 5.87 | 1.18 | 5.77 | .76 | .752 |
| Posterior Superior | 4.14 | 1.10 | 4.24 | .79 | .758 |
| Posterior Inferior Vermis | 3.14 | .71 | 3.95 | .73 | .001 |
| Total Vermis | 13.16 | 2.53 | 13.96 | 2.04 | .294 |
| Total Cerebral Volume | 109.04 | 12.26 | 106.51 | 11.52 | .507 |
Note: ADHD = Attention-Deficit/Hyperactivity Disorder; Anterior Vermis = Lobules I–V; Posterior Inferior Vermis = Lobules VIII–X; Posterior Superior Vermis = Lobules VI–VII; Total Cerebral Volume = Total Right and Left Gray Matter + Total Right and Left White Matter.
Brain – Behavior Correlates
Linear regression models were used to determine if vermal volume was related to behavioral measures. In order to ensure that DAS-GCA (intellectual functioning) was not accounting for the relationship between posterior inferior vermis and neuropsychological measures, a linear regression model with DAS-GCA held constant was run for each neuropsychological measure. Regressions indicated that volume of the posterior inferior vermis (Lobules VIII-X) predicted a significant amount of the variance in BASC-II Hyperactivity ratings (B = −.013, β = −.425, p = .000), BASC-II Attention ratings (B = −012, β = −.511, p = .000), and CGI-R/I parent ratings (B = −.012, β = −.495, p = .000). CGI-R/I teacher ratings did not reach significance. Regression analyses are presented in Table 3.
Table 3.
Regression Analyses for Posterior Inferior Vermis Volumes (Lobules VIII–X)
| Variable | R2 | β | t | p Value |
|---|---|---|---|---|
| BASC-II Attention | .318 | −.511 | −4.10 | <.000 |
| BASC-II Hyperactivity | .320 | −.425 | −3.41 | <.000 |
| CGI-R/I Parent | .316 | −.495 | −3.97 | <.000 |
| CGI-R/I Teacher | .085 | −.211 | −1.46 | .143 |
Note: BASC-II Attention = Behavioral Assessment Scale for Children 2nd Edition Attention Scale T-Score; BASC-II Hyperactivity = Behavioral Assessment Scale for Children 2nd Edition Hyperactivity Scale T-Score; CGI-R/I Parent = Conners’ Global Index – Restless Impulsive Parent Ratings Scale; CGI-R/I Teacher = Conners’ Global Index – Restless Impulsive Teacher Ratings Scale.
DISCUSSION
Consistent with previous work, we found reduced volume of the posterior inferior cerebellar vermis in children with ADHD.11,14,15,17,34–36 In addition, we hypothesized that vermal volume would be related to parent and/or teacher reported levels of hyperactivity and attention. Results suggest one lobule of the vermis (posterior inferior vermis) explained a significant amount of variance in parent reported levels of hyperactivity, attention, and restlessness/impulsivity but was not associated with teacher reported levels of restlessness/impulsivity. This study is among the first to connect cerebellar vermis volume with behavioral outcomes, in a small sample of children with ADHD-C. These findings are consistent with other studies which have implicated the cerebellum in impulse control processes, attention shifting, and motor-coordination in healthy individuals and those with damage to the cerebellum. 14,24,25,31
Atypical cerebellar volume is one of the most consistent findings in the neuroimaging literature on ADHD. New research, however, suggests the cerebellum may be functionally underactive compared to those without ADHD, when estimating time, location, and identity of stimuli. Reduced functional activation of the cerebellum was found to be similar among children with ADHD and their unaffected siblings: unaffected siblings of children with ADHD showed a significant decrease in activation of the inferior cerebellum compared to controls on a modified (e.g., expected and unexpected stimuli at expected and unexpected times) (go/no-go) fMRI task.53 In a study using the same modified go/no-go fMRI task, children with ADHD demonstrated reduced activation of the cerebellum during stimulus timing violations as well as reduced activation of the anterior cingulate and ventral prefrontal cortex during stimulus timing (i.e., when an event will occur) and identify violations (i.e., what event will occur, using cues) compared to controls.54
These studies demonstrated that children with ADHD experience more difficulties with inhibiting their behavior and that these problems with inhibition may be related to hypofunctioning of cerebellar-frontal neural circuits. Symptoms of ADHD, such as inhibiting disruptive behaviors, poor impulse control, an inability to adjust behavior to fit contextual/environmental demands, and the difficulty in predicting and adapting to changes in the environment, may be partially explained by deficits in frontal-striatal-cerebellar neural circuitry.54 Because the current study found reduced volume in the posterior inferior vermis, it may be that these functional deficits are related to an underdeveloped cerebellar vermis in ADHD-C. Furthermore, a longitudinal brain development study in children with ADHD reported fixed and non-progressive thinning of the right and left posterior inferior vermis in children in a worse outcome group (i.e., cutoff score < 62 on the CGAS) compared to better (i.e., cutoff score ≥ 62 on the Children’s Global Assessment Scale, CGAS) outcome group.14
Our finding, that BASC-II Hyperactivity scores were related to volume of the posterior inferior vermis, also implicates an impaired cerebellar-frontal pathway in ADHD. Given we found a connection between levels of hyperactivity and one lobule of the vermis in a regression model that included children with ADHD and controls indicates not only a potential relationship between cerebellar development and ADHD symptoms, but more broadly, a brain-behavior relationship between posterior inferior vermal volume and hyperactive and inattentive behavior.
There was also an observed relationship between posterior inferior vermal volume and parent reported levels of attention. This finding suggests the vermis of the cerebellum may be a structure related to symptoms of hyperactivity and impulsivity as well as in the modulation of attention. Such hypotheses regarding behavioral disinhibition and inattention as a core deficit in ADHD are supported by landmark theories of ADHD etiology4 as well as fMRI research findings of reduced brain activation during response inhibition tasks. 18,20,21 The current study, however, connects these symptoms with cerebellar volume which in turn further implicates a cerebellar-frontal-striatal network in symptoms of ADHD. Further studies following children at relatively young ages through adolescence are needed to support this hypothesis as the cerebellum changes quite drastically from ages 5 to 20 and is protracted in development as much as 3.8 years later for males compared to females.55 These studies provide further support for a neuronal developmental phenotype in ADHD, with cerebellar-frontal-striatal circuits playing a prominent role.23,56,57
Also of clinical relevance and discussion is the use of chronic stimulant medication and the effects on brain volume. There are previous reports of reductions in the posterior inferior vermis in children with ADHD-C who had never taken stimulant medication.11 There were no differences, however, between chronically-treated children and controls, suggesting there may be a neuro-protective factor associated with chronic medication use. The effects of chronic stimulant treatment on brain volume, however, are inconclusive; one large study reported no differences in chronically-treated and treatment-naïve children with ADHD,12 whereas other studies have found reductions in the right anterior cingulate cortex,13 decreased metabolism in the prefrontal cortex,58 increased rate of cortical thinning of the left middle, inferior frontal, and right parieto-occipital region in children with ADHD who were treatment-naïve.59 There also appear to be functional differences in the vermis in treated boys with ADHD. Moderate to high doses of methylphenidate were found to increase T2 relaxation time (i.e., an estimation of localized cerebral blood perfusion) in the cerebellar vermis in children with ADHD.60 Relaxation time, however, was moderated by basal levels of hyperactivity: children with ADHD who were more hyperactive evinced greater relaxation time compared to those with ADHD who were rated as less hyperactive. Thus, these studies support our tentative finding that the posterior inferior vermis is involved in symptoms of hyperactivity and attention disorders. Emphasis on the involvement of the posterior inferior vermis is important due to this region containing the highest concentration of dopamine transporters within the vermis.33
While this study is one of the first to connect brain volume and behavior in ADHD-C, future studies with larger sample sizes are needed to confirm conclusions on the relationship between cerebellar volume and behavioral outcomes. This is especially true in light of the wide behavioral and neuronal heterogeneity in ADHD. Such studies would provide evidence for or against the purported relationship between cerebellar vermis abnormalities and the pathophysiology of ADHD symptoms. The cerebellum has been implicated in motor coordination and movement and so is important for continued study in ADHD. Our study found a relationship between hyperactivity and inattention behaviors and cerebellar abnormality; however, it is unclear to what extent extracerebellar abnormalities might also be related to symptoms of ADHD. Our study was limited in that we focused on the cerebellar vermis and a limited set of ADHD symptoms (e.g., hyperactivity and attention). Future studies both in structural and functional neuroimaging and in neuropsychology should seek to test hypotheses about the brain-behavior relationships of extracerebellar structures and ADHD symptoms. There is evidence for differential motor coordination problems between DSM-IV ADHD subtypes,61 thus future studies should also attempt to understand the relative influence of cerebellar abnormality as it pertains to behavioral differences between ADHD subtypes. The current study was also limited in the number of behavioral outcome variables used to contrast those with ADHD and those without. The BASC-II and Conners’ Ratings Scales are commonly used questionnaires both in research and clinical settings for ADHD, however, studies that utilize neuropsychological and cognitive measures (e.g., comprehensive executive function batteries such as the Delis-Kaplan Executive Functioning System; Delis, Kaplan, & Kramer)62 that cover specific domains of functioning (e.g., planning, verbal fluency, response inhibition) would provide more precise information regarding neurobehavioral impairments.
Acknowledgments
This work was supported by National Institutes of Health Grant ROI-H63986.
We would like to thank the families that participated in the study.
Footnotes
Disclosure: Dr. Semrud-Clikeman has received funding from the Broitman Foundation. Dr. Pliszka has served as a consultant for Shire and Ortho McNeil. He has received research support from Ortho McNeil. He has served as an expert witness for Eli Lilly and Co. He has received honoraria from Janssen K.K. Mr. Bledsoe reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mr. Jesse C. Bledsoe, Michigan State University and the Consortium for Neurodevelopmental Study
Dr. Margaret Semrud-Clikeman, Michigan State University and the Consortium for Neurodevelopmental Study
Dr. Steven R. Pliszka, University of Texas Health Science Center at San Antonio
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4 Text Revision. American Psychiatric Association; 2000. [Google Scholar]
- 2.Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, OD The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. American Journal of Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polanczyk G, de Lima M, Horta B, Biederman J, Rohde L. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. The American Journal of Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA. Behavioral Inhibition, Sustained Attention, and Executive Functions: Constructing a Unifying Theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 5.Sonuga-Barke EJS. Psychological heterogeneity in AD/HD - A dual pathway model of behaviour and cognition. Behavioral Brain Research. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 6.Willcutt E, Doyle A, Nigg J, Faraone S, Pennington B. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 8.Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Science USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw P, Lalonde F, Lepage C, et al. Development of cortical asymmetry in typically developing children and its disruption in Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry. 2009;66(8):888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellanos FX, Giedd JN, Eckburg WL, et al. Quantitative morphology of the caudate nucleus in attention deficit hyperactivity disorder. American Journal of Psychiatry. 1994;151(1212):1791–1796. doi: 10.1176/ajp.151.12.1791. [DOI] [PubMed] [Google Scholar]
- 11.Bledsoe J, Semrud-Clikeman M, Pliszka S. An MRI Study of the Cerebellar Vermis in Chronically-Treated and Treatment-Naive Children with ADHD-Combined Type. Biological Psychiatry. 2009;65(7):620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in childrne with adolescents with attention-deficit/hyperactivity disorder. The Journal of the American Medical Association. 2002;28(4):1740–1749. [Google Scholar]
- 13.Semrud-Clikeman M, Pliszka SR, Lancaster J, Liotti M. Volumetric MRI differences in treatment-naïve vs chornically treated children with ADHD. Neurology. 2006;67:1023–1027. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- 14.Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention-deficit/hyperactivity disorder. American Journal of Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- 15.Berquin PC, Giedd JN, Jacobsen LK, Hamburger SD, Krain AL, Rapoport J. Cerebellum in attention-deficit/hyperactivity disorder: A morphometric MRI study. Neurology. 1998;50:1087–1093. doi: 10.1212/wnl.50.4.1087. [DOI] [PubMed] [Google Scholar]
- 16.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufman WE. Smaller Prefrontal and Premotor Volumes in Boys with Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2002;52(8):785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 17.Mostofsky SH, Reiss AL, Lockhart P, Denckla MB. Evaluation of cerebellar size in attention-deficit/hyperactivity disorder. Neurology. 1998;13:434–439. doi: 10.1177/088307389801300904. [DOI] [PubMed] [Google Scholar]
- 18.Bush G, Frazier JA, Rauch SL, et al. Anterior cingulate cortex dysfunction in Attention-Deficit/Hyperactivity Disorder revealed by fMRI and the counting Stroop. Biological Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 19.Sheridan MA, Hinshaw S, D’Esposito M. Efficiency of the prefrontal cortex during working memory in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(10):1357–1366. doi: 10.1097/chi.0b013e31812eecf7. [DOI] [PubMed] [Google Scholar]
- 20.Schulz K, Newcorn J, Fan J, Tang C, Halperin J. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- 21.Durston S. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53(10):871. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 22.Castellanos FX, Marguiles DS, Kelly C, et al. Cingulate-precuneus interactions: A new locus of dysfunction in adults Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigg JT, Casey BJ. An Integrative Theory of Attention-Deficit/Hyperactivity Disorder Based on the Cognitive and Affective Neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 24.Akshoomoff NA, Courchesne E. ERP evidence for a shifting attention deficit in patients with damage to the cerebellum. Journal of Cognitive Neuroscience. 1994;6(4):388–399. doi: 10.1162/jocn.1994.6.4.388. [DOI] [PubMed] [Google Scholar]
- 25.Botez MI, Botez T, Elie R, Attig E. Role of the cerebellum in complex human behavior. Italian Journal of Neurological Sciences. 1989;10:291–300. doi: 10.1007/BF02333774. [DOI] [PubMed] [Google Scholar]
- 26.Courchesne E, Townsend J, Akshoomoff NA, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- 27.Golla H, Thier P, Haarmeier T. Disturbed overt but normal covert shifts of attention in adult cerebellar patients. Brain. 2005;128:1525–1535. doi: 10.1093/brain/awh523. [DOI] [PubMed] [Google Scholar]
- 28.Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Annal of the New York Academy of Science. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 30.Ivry RB. Cerebellar timing systems. International Review of Neurobiology. 1997;41:555–573. [PubMed] [Google Scholar]
- 31.Allen G, Buxton RB, Wong EC, Courchesne E. Attentional Activation of the Cerebellum Independent of Motor Involvement. Science. 1997;275(28):1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 32.Williams RW, Herrup K. The control of neuron number. Annual Review of Neuroscience. 1988;11:423–453. doi: 10.1146/annurev.ne.11.030188.002231. [DOI] [PubMed] [Google Scholar]
- 33.Melchitzky DS, Lewis DA. Tyrosine Hydrolase- and Dopamine Transporter-Immunoreactive Axons in the Primate Cerebellum: Evidence for a Lobular- and Laminar-Specific Dopamine Innervation. Neuropsychopharmacology. 2000;22:466–472. doi: 10.1016/S0893-133X(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 34.Bussing R, Grudnick J, Mason D, Wasiak M, Leonard C. ADHD and conduct disorder: An MRI study in a community sample. World Journal of Biological Psychiatry. 2002;3:216–220. doi: 10.3109/15622970209150624. [DOI] [PubMed] [Google Scholar]
- 35.Castellanos FX, Giedd JN, Berquin PC, Walter JM, Sharp W, Tran T. Quantification brain magnetic resonance imaging in girls with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 36.Hill DE, Yeo RA, Compbell RA, Hart B, Vigil J, Brooks W. Magnetic resonance imaging correlates of attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2003;58:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 37.Ashtari M, Kumra S, Bhaskar SL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biological Psychiatry. 2005 Mar 1;57(5):448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 38.Bechtel N, Kobel M, Penner IK, et al. Decreased fractional anisotropy in the middle cerebellar peduncle in children with epilepsy and/or attention-deficit/hyperactivity disorder: A preliminary study. Epilepsy & Behavior. 2009;15:294–298. doi: 10.1016/j.yebeh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 40.Shaffer D, Fisher P, Lucas C, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH-DISC-IV), description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Conners CK. Conners’ Rating Scales-Revised Technical Manual. New York: Multi-Health Systems; 2000. [Google Scholar]
- 42.Elliott CD. Differential Ability Scales. San Antonia, TX: Psychological Corporation; 1990. [Google Scholar]
- 43.Wechsler D. Wechsler Individual Achievement Test. 2. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]
- 44.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children-2. Circle Pines, MN: Pearson Assessments; 2004. [Google Scholar]
- 45.Ostrander R, Weinfurt KP, Yarnold PR, August GJ. Diagnosing attention deficit disorders with the Behavioral Assessment System for Children and the Child Behavior Checklist: Test and construct validity analyses using optimal discriminant classification trees. Journal of Consulting and Clinical Psychology. 1998;66:660–672. doi: 10.1037/0022-006X.66.4.660. [DOI] [PubMed] [Google Scholar]
- 46.Pineda DA, Aguirre DC, Garcia MA, Lopera FJ, Palacio LG, Kamphaus RW. Validation of two ratings scales for Attention-Deficit/Hyperactivity Disorder diagnosis in Columbian children. Pediatric Neurology. 2005;33(1):15–25. doi: 10.1016/j.pediatrneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Scahill L, Schwab-Stone ME, Merikangas KR, Leckman JF, Zhang H, Kasl S. Psychosocial and clinical correlates of ADHD in a community sample of school-age children. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(8):976–984. doi: 10.1097/00004583-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long-term methylphenidate therapy in children with comorbid Attention-Deficit Hyperactivity Disorder and Chronic Multiple Tic Disorder. Archives of General Psychiatry. 1999;56(4):330–336. doi: 10.1001/archpsyc.56.4.330. [DOI] [PubMed] [Google Scholar]
- 49.Rucklidge JJ, Tannock R. Psychiatric, psychosocial, and cognitive functioning of female adolesecents with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(5):530–540. doi: 10.1097/00004583-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Fischl B, Dale AM. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proceedings of the National Academy of Science USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmahmann JD, Doyon J, Petrides M, Evans A, Toga AW. MRI Atlas of the Human Cerebellum. Boston: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- 52.Pierson R, Corson PW, Sears LL, et al. Manual and Semiautomated Measurement of Cerebellar Subregions on MR Images. NeuroImage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- 53.Mulder MJ, Baeyens D, Davidson MC, et al. Familial Vulnerability to ADHD Affects Activity in the Cerebellum in Addition to the Prefrontal System. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):68–75. doi: 10.1097/chi.0b013e31815a56dc. [DOI] [PubMed] [Google Scholar]
- 54.Durston S, Davidson MC, Mulder MJ, et al. Neural and Behavioral Correlates of Expectancy Violations in Attention-Deficit/Hyperactivity Disorder. Journal of Child Psychology and Psychiatry. 2007;48(9):881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 55.Tiemeier H, Lenroot RK, Greenstein D, Tran L, Pierson R, Giedd JN. Cerebellum Development During Childhood and Adolescence: A Longitudinal Morphometric MRI Study. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonuga-Barke EJS. Disambiguating Inhibitory Dysfunction in Attention-Deficit/Hyperactivity Disorder: Toward the Decomposition of Developmental Brain Phenotypes. Biologial Psychiatry. 2010;67:599–601. doi: 10.1016/j.biopsych.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Castellanos FX, Tannock R. Neuroscience of Attention-Deficit/Hyperactivity Disorder: The Search for Endophenotypes. Nature Neuroscience. 2002;3:614–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 58.Stanley JA, Kipp H, Greisenegger E, et al. Evidence of Developmental Alterations in Cortical and Subcortical Regions of Children With Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry. 2008;65(12):1419–1428. doi: 10.1001/archgenpsychiatry.2008.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw P, Sharp WS, Morrison M, et al. Psychostimulant Treatment and the Developing Cortex in Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2009;166:58–69. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson CM, Polcari A, Lowen SB, Renshaw PF, Teicher MH. Effects of Methylphenidate on Functional Magnetic Resonance Relaxometry of the of the Cerebellar Vermis in Boys with ADHD. American Journal of Psychiatry. 2002;159(8):1322–1328. doi: 10.1176/appi.ajp.159.8.1322. [DOI] [PubMed] [Google Scholar]
- 61.Piek JP, Pitcher TM, Hay DA. Motor Coordination and Kinaesthesis in Boys with Attention Deficit-Hyperactivity Disorder. Developmental Medicine and Child Neurology. 1999;41:159–165. doi: 10.1017/s0012162299000341. [DOI] [PubMed] [Google Scholar]
- 62.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Functioning System: Examiner’s Manual. San Antonio, TX: the Psychological Corporation; 2001. [Google Scholar]


