Abstract
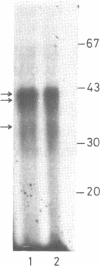
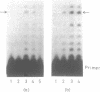
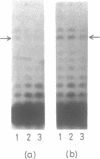
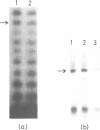
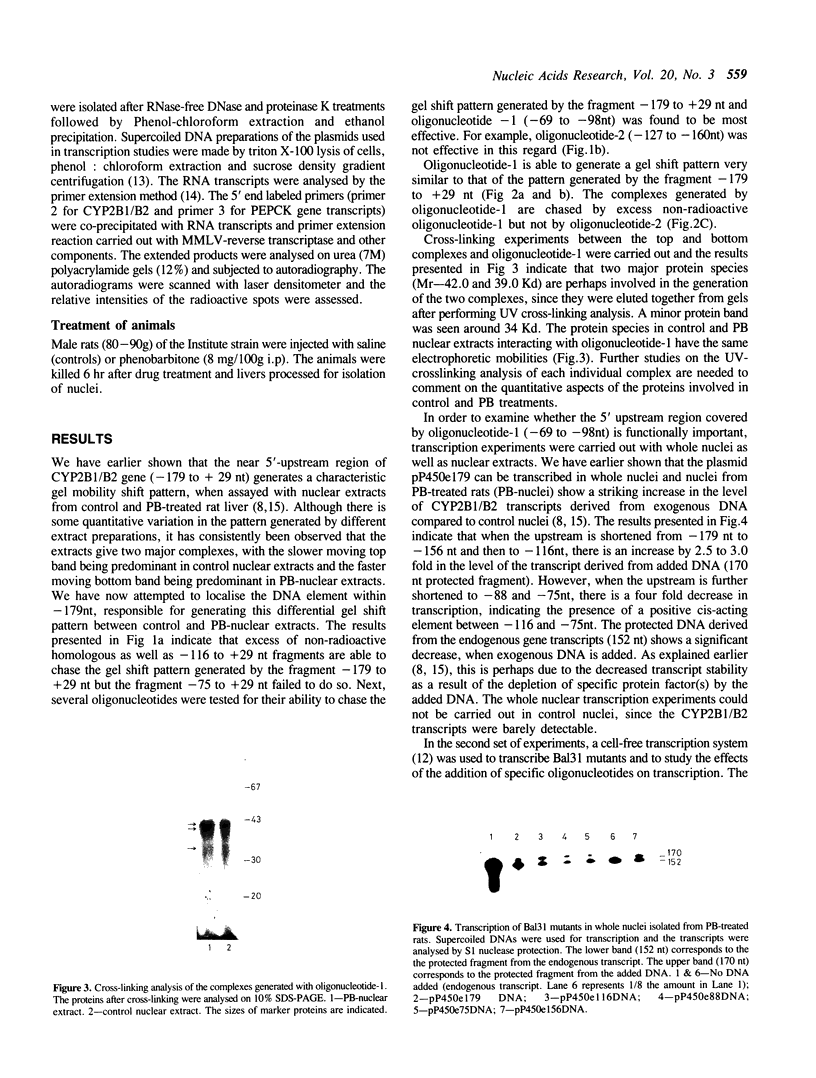
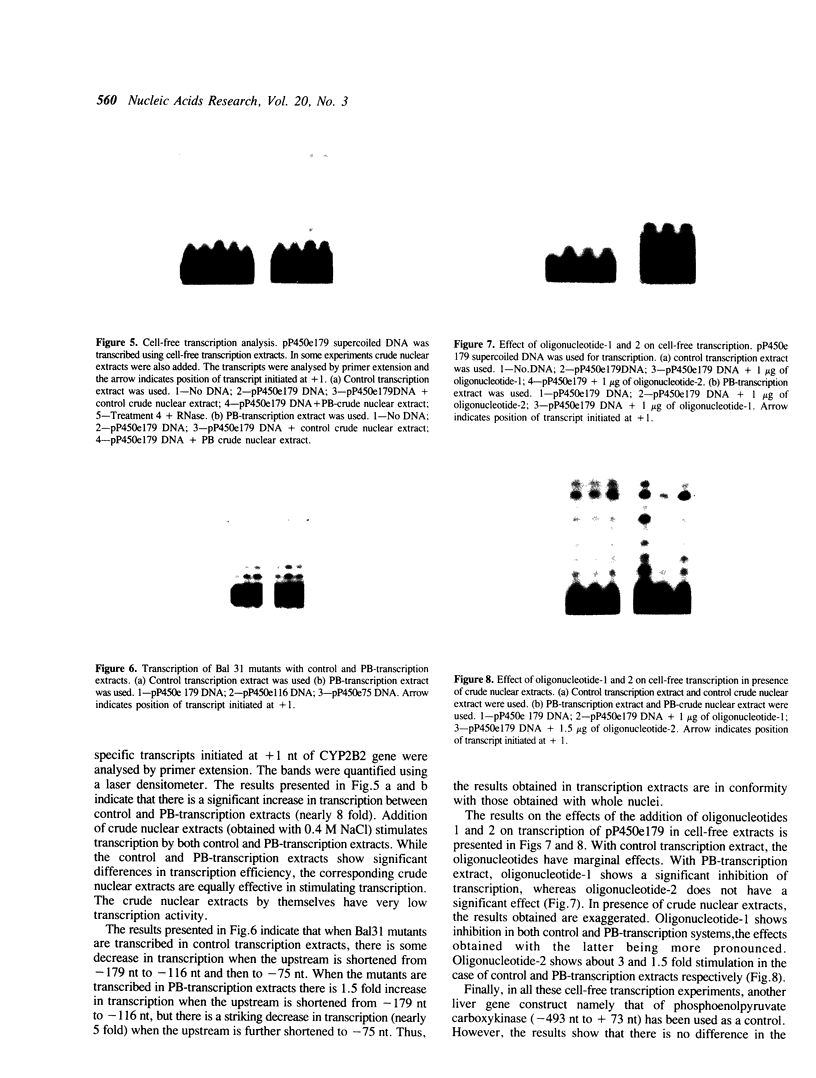
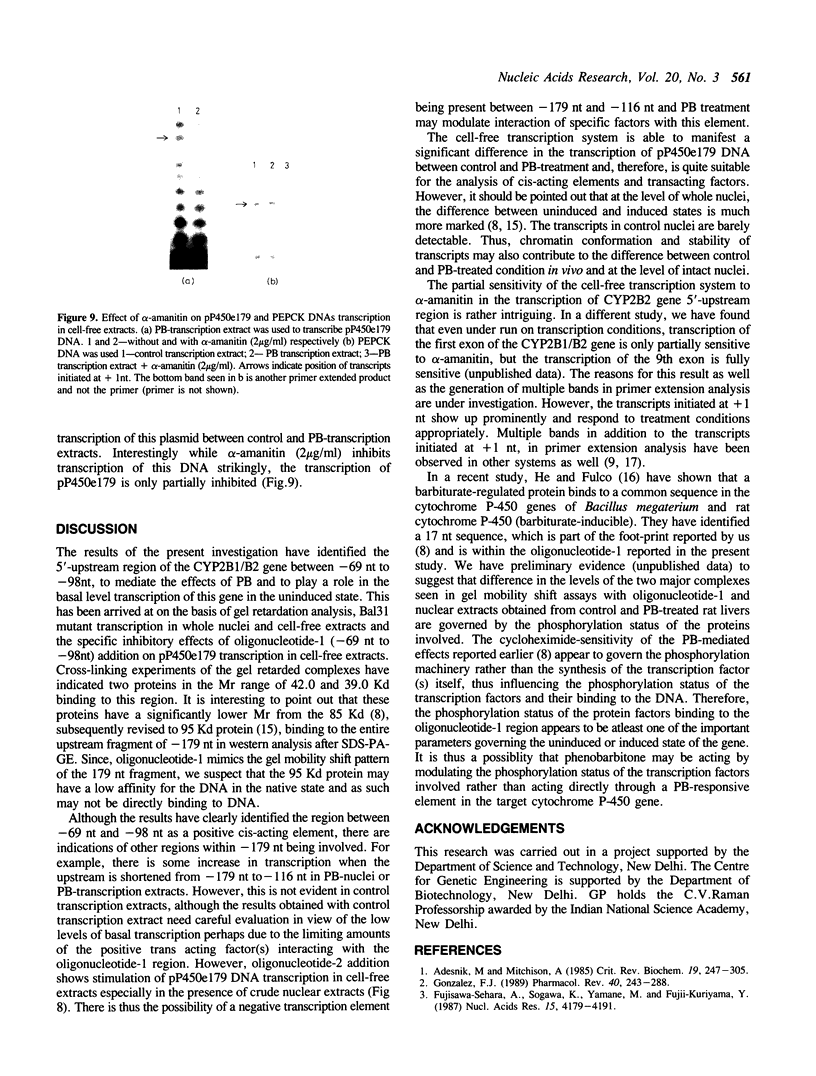
A positive cis-acting DNA element in the near 5'-upstream region of the CYP2B1/B2 genes in rat liver was found to play an important role in the transcription of these genes. An oligonucleotide covering -69 to -98 nt mimicked the gel mobility shift pattern given by the fragment -179 to +29 nt, which was earlier found adequate to confer the regulatory features of this gene. Two major complexes were seen, of which the slower and faster moving complexes became intense under uninduced and Phenobarbitone-induced conditions respectively. Minigene cloned DNA plasmid covering -179 to +181 nt in pUC 19 and Bal 31 mutants derived from this parent were transcribed in whole nuclei and cell free transcription extracts and mutants containing only upto -75 nt of the upstream were poorly transcribed. Transcription extracts from phenobarbitone-injected rat liver nuclei were significantly more active than extracts from uninduced rats in transcribing the minigene constructs. Addition of the oligonucleotide (-69 to -98nt) specifically inhibited the transcription of the minigene construct (-179 to +181 nt) in the cell free transcription system. It is therefore, concluded that the region -69 to -98 nt acts as a positive cis-acting element in the transcription of the CYP2B1/B2 genes and in mediating the inductive effects of phenobarbitone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., DasGupta C., Shibata T., Radding C. M. Homologous pairing in genetic recombination: recA protein makes joint molecules of gapped circular DNA and closed circular DNA. Cell. 1980 May;20(1):223–235. doi: 10.1016/0092-8674(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinari P., La Bella F., Heintz N. Characterization and purification of H1TF2, a novel CCAAT-binding protein that interacts with a histone H1 subtype-specific consensus element. Mol Cell Biol. 1989 Apr;9(4):1566–1575. doi: 10.1128/mcb.9.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- He J. S., Fulco A. J. A barbiturate-regulated protein binding to a common sequence in the cytochrome P450 genes of rodents and bacteria. J Biol Chem. 1991 Apr 25;266(12):7864–7869. [PubMed] [Google Scholar]
- Klemm D. J., Roesler W. J., Liu J. S., Park E. A., Hanson R. W. In vitro analysis of promoter elements regulating transcription of the phosphoenolpyruvate carboxykinase (GTP) gene. Mol Cell Biol. 1990 Feb;10(2):480–485. doi: 10.1128/mcb.10.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan P. N., Padmanaban G. Regulation of cytochrome P-450b/e gene expression by a heme- and phenobarbitone-modulated transcription factor. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3963–3967. doi: 10.1073/pnas.86.11.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Rangarajan P. N., Padmanaban G. Dexamethasone negatively regulates phenobarbitone-activated transcription but synergistically enhances cytoplasmic levels of cytochrome P-450b/e messenger RNA. J Biol Chem. 1990 Apr 5;265(10):5617–5622. [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Yeoman L. C., Daskal I., Busch H. Two-dimensional electrophoresis of proteins of citric acid nuclei prepared with aid of a Tissumizer. Exp Cell Res. 1973 Nov;82(1):215–226. doi: 10.1016/0014-4827(73)90264-4. [DOI] [PubMed] [Google Scholar]
- Wen L. P., Koeiman N., Whitlock J. P., Jr Dioxin-inducible, Ah receptor-dependent transcription in vitro. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8545–8549. doi: 10.1073/pnas.87.21.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Yanagida A., Sogawa K., Yasumoto K. I., Fujii-Kuriyama Y. A novel cis-acting DNA element required for a high level of inducible expression of the rat P-450c gene. Mol Cell Biol. 1990 Apr;10(4):1470–1475. doi: 10.1128/mcb.10.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]