Abstract
Objectives
Impaired attentional control and behavioral control are implicated in adult suicidal behavior. Little is known about the functional integrity of neural circuitry supporting these processes in suicidal behavior in adolescence.
Method
We employed fMRI in 15 adolescent suicide attempters with history of major depressive disorder (MDD; ATT), 15 adolescents with history of MDD but not suicide attempt (NAT), and 14 healthy controls (HC), during performance of a well-validated GoNoGo response inhibition and motor control task that measures attentional and behavioral control, and has been shown to activate prefrontal, anterior cingulate and parietal cortical circuitry. Questionnaires assessed symptoms and standardized interviews characterized suicide attempt.
Results
A 3 group × 2 condition (“GoNoGo” response inhibition versus “Go” motor control blocks) block-design whole-brain analysis (p<0.05, corrected) revealed that NAT showed greater activity than ATT in right anterior cingulate gyrus (p=0.008), and that NAT, but not ATT, showed significantly greater activity than HC in the left insula (p=0.004) to GoNoGo response inhibition blocks.
Conclusions
While ATT did not show differential patterns of neural activity from HC during GoNoGo response inhibition blocks, ATT and NAT showed differential activation of the right anterior cingulate gyrus during response inhibition. Our findings indicate that suicide attempt during adolescence is not associated with abnormal activity in response inhibition neural circuitry. The differential patterns of activity in response inhibition neural circuitry in ATT and NAT, however, suggest different neural mechanisms for suicide attempt versus MDD in general in adolescence that should be a focus of further study.
Keywords: adolescence, suicide, response inhibition, cingulate, prefrontal cortex
Introduction
Suicide and suicidal behavior remain leading causes of morbidity and mortality in adolescence1, and prevention of early-onset suicide attempt is identified as a mission of NIMH. In order to prevent early-onset suicide, it is necessary to identify biomarkers associated with suicidal risk, and also to better understand the pathogenesis of suicidal behavior in order to target and reverse this pathogenic process. Despite progress in understanding the epidemiology and risk factors for suicidal behavior,2–5 however, the pathophysiologic processes that may predispose to suicidal behavior, including alterations in neural circuitry, remain poorly understood.
In adults, impaired cognitive control and abnormal risky decision-making6–7 have been reported in suicide attempters relative to healthy and affected controls, and have been associated with impulsivity in some8–10 but not all11–12 previous studies. Cognitive inflexibility and impaired executive function have also been shown to be more prominent in high lethality attempters13. One measure of cognitive control is prepotent response inhibition, a measure of both attentional and behavioral control14, which is subserved by cingulo-frontal-parietal neural circuitry in healthy adults.15 Three independent aspects of attentional control have been linked to separate brain regions. These include “alerting” in fronto-parietal cortex and thalamus, “orienting” in bilateral parietal cortex, and “executive control” in the bilateral anterior cingulate, frontal and fusiform gyri.16 While one neuroimaging study in adult suicide attempters17 reported lower glucose uptake in the prefrontal cortex and anterior cingulate gyrus in high versus low lethality suicide attempters, no neuroimaging study to date has focused on examination of neural activity associated with cognitive control in general, or response inhibition in particular, in suicide attempters. The development of the prefrontal cortex during adolescence has been well-documented, and maturation has been associated with greater ability to perform higher-order cognitive tasks.1, 18–20 It is therefore possible that vulnerability to suicidal behavior in adolescence may reflect abnormal prefrontal cortical development associated with prefrontal cortical dysfunction during cognitive control, but this hypothesis remains unexamined.
The GoNoGo task is a well-validated, block design, response inhibition and motor control task that has been utilized extensively to measure neural activity during response inhibition and motor control in adolescents with a variety of different psychiatric disorders, including Attention Deficit Disorder (ADHD) and obsessive-compulsive disorder (OCD).21–23 In this task, participants are shown a series of letters, and press a button in response to visually presented letter stimuli (“Go” trials), but avoid responding to a rare non-target (“NoGo” trials, the letter “V”). In healthy children (and adults), the GoNoGo task has been associated with activity in cingulo-frontal-parietal neural circuitry, specifically within inferior frontal, middle frontal, orbital frontal, and anterior cingulate gyri24, while abnormally reduced ventral prefrontal and anterior cingulate gyral activity has been reported in youth with ADHD during performance of this task.25 One recent study demonstrated that adult suicide attempters show abnormal behavioral performance on the GoNoGo task.26
Using different tasks of attention, one study examined 21 medication naïve adolescents with first episode MDD using fMRI, including selective attention, attentional switching, and response inhibition with error detection (Stop Task). Adolescents with MDD showed reduced activation in right dorsolateral prefrontal cortex, inferior prefrontal cortex, and anterior cingulate gyrus on all three tasks.27 No studies to date, however, have examined neural activity in adolescent (or adult) suicide attempters during performance of the GoNoGo task. The extent to which abnormal activity in neural circuitry supporting response inhibition is present specifically in adolescent suicide attempters, or is present in depressed adolescents in general, therefore remains unknown.
The aim of the present study was to measure neural activity during performance of the GoNoGo task in adolescents with a history of suicide attempt versus age-matched healthy controls and age-matched adolescents with a history of depression but not suicide attempt, using fMRI. Based on previous findings of abnormal GoNoGo task performance in adult suicide attempters, reduced activity in prefrontal cortex and anterior cingulate gyrus during the task in adolescents with ADHD, and the potential link between abnormal prefrontal cortical development in adolescent suicide attempters, we hypothesized that during the task, adolescent suicide attempters would show reduced activity relative to healthy control adolescents in response inhibition neural circuitry, especially within prefrontal cortex and anterior cingulate gyrus. The absence of existing data comparing adolescent suicide attempters and non-attempters on response inhibition and motor control tasks did not allow us to have specific hypotheses regarding the differential pattern of abnormal neural activity during the GoNoGo task in these two adolescent groups.
Method
Participants
44 adolescents completed the study, including: 1)15 attempters (ATT) with a lifetime history of suicide attempt and Major Depressive Disorder (MDD); 2) 15 non-attempters (NAT) with a history of MDD, but no history of suicide attempt; 3)14 healthy controls (HC), without psychiatric disorder or suicide attempt.
ATT and NAT were recruited from existing studies of familial inheritance of suicidal behavior and a clinic registry for depressed and suicidal youth. HC were recruited from existing healthy control groups and advertisement in pediatric practices. Inclusion criteria for NAT and ATT included a lifetime history of MDD according to DSM-IV criteria. ATT had a history of at least one suicide attempt as defined by the Columbia Classification Algorithm of Suicide Assessment (C-CASA). 28 Participants were without intent for self-harm at the time of the study. All participants were right-handed as determined by patient interview and parent confirmation.
Exclusion criteria included a lifetime history of: a) physical, neuromuscular, or neurological problems; b) mild mental retardation as assessed using the Verbal IQ of the Wechsler Abbreviated Intelligence Scale29 (cut off 80); c) use of sedative or narcotic medication; d) drug or alcohol dependence, intoxication and/or positive urine drug screen; e) pregnancy; e)claustrophobia; f)any metal objects in the body. Participants could not have bipolar disorder or symptoms of psychosis. Any ATT whose suicide attempt had been associated with anoxia or head injury was excluded.
DSM-IV criteria were assessed using the K-SADS diagnostic interview. Suicide attempt was assessed using the Suicide Intent Scale (SIS) 30, Columbia Suicide History Form and Lethality Rating Scale (SHF) 31. Depression, anxiety and suicidal ideation were assessed with The Beck Depression Inventory (BDI) 32, Screen for Childhood Anxiety Related Emotional Disorders (SCARED) parent and child versions33, and Suicidal Ideation Questionnaire (SIQ) 34, respectively. Pubertal status was assessed using the Petersen Pubertal Development Scale. 35
The University of Pittsburgh Institutional Review Board approved the study protocol. Parents and participants provided informed written consent and assent prior to the study. Participants were reimbursed $100.
Paradigm
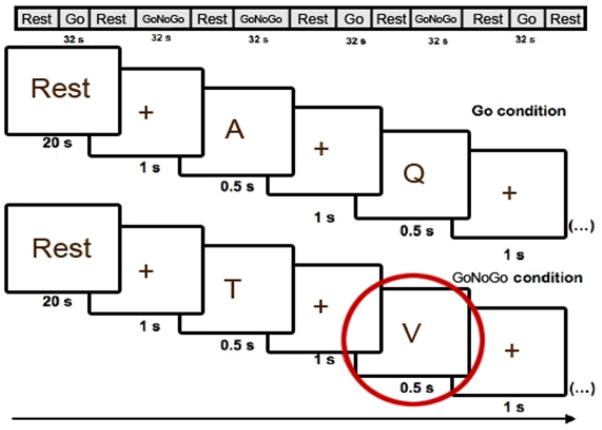
The GoNoGo task21–24 comprised a five minute and 38 second blocked-design paradigm in which participants were shown a total of 120 letters. In Go trials, participants pressed a button to a visually-presented letter stimulus. In NoGo trials, participants avoided response to a non-target letter stimulus (the letter “V”). Trials were segregated into two block types: Block A, “Go”, blocks, each with 20 “Go” trials, and Block B, “GoNoGo”, blocks, each with 10 randomly distributed “Go” and 10 “NoGo” trials. Seventy-five percent of the 120 trials over the entire task were therefore “Go” trials. Blocks were presented in the order of ABBABA, with 20 second interleaved rest periods (blank screen). Blocks began with a one second black screen with the word “Start” and closed with a one second fixation cross. Letter presentation for all trials comprised white letter stimuli being projected on a black background for 500 ms, with an inter-stimulus interval of 1000ms, during which a cross-hair was shown. During the 20 second interleaved rest periods participants viewed a black screen with the word “Rest” presented centrally (Figure 1).
Figure 1.
Paradigm presentation for the Go and GoNoGo Blocks. Note: Participants were shown a series of 120 letters, and pressed a button in response to visually presented letter stimuli (“Go” trials), but avoided responding to a rare non-target (“NoGo” trials, the letter “V”). Letters were presented for 0.5 sec with a 1 sec crosshair between stimuli. Blocks began with a one second black screen with the word “Start” and closed with a one second fixation cross. During the 20 sec interleaved rest periods subjects viewed a black screen with the word “Rest” presented centrally. A, Q, T, and V represent stimuli presented to the participant.
Data acquisition
Scans were acquired on a 3.0 T Siemens Allegra magnetic resonance imaging (MRI) scanner. Functional scans of 34 contiguous 3 mm axial slices were acquired with a T2-weighted gradient echo epi sequence (repetition time: 2000ms; echo time: 30ms; field of view: 205 mm; matrix: 64×64; in-plane resolution: 3 mm×3 mm). Stimuli were projected onto a screen approximately 55cm from the subject with a rear screen projector. High-resolution T1-weighted magnetization prepared rapid gradient echo (MPRAGE) structural images of 240 0.8 mm slices were acquired (repetition time: 1.630ms; echo time: 2.48ms; inversion time: 800ms; field of view: 200 mm; flip angle: 8°; matrix: 256×256).
Task Performance
Task performance data were analyzed using one-way ANOVAs to examine the main effect of group upon task performance accuracy: numbers of correct Go and NoGo responses, omissions (misses for Go stimuli) and commissions (incorrect button press for NoGo stimuli) in SPSSv.17(Statistical Analysis Software, Inc.).
Imaging analyses
Data were pre-processed and analyzed using Statistical Parametric Mapping software (SPM5, London, UK). Data for each participant were first corrected for differences in acquisition time between slices; realigned using the first slice as a reference; and unwarped to correct static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. Movement cut off was 2 mm or less. They were co-registered with the participant’s anatomical image, segmented, normalized to standard MNI template, resampled to 3×3×3 mm3 voxels, and spatially smoothed with a Gaussian kernel of 6 mm full-width at half-maximum (FWHM).
A first-level fixed-effect model was constructed for the two blocks (Go and GoNoGo), entered as separate conditions in a block design in the design matrix. Movement parameters from the realignment stage were entered as covariates of no interest to control for participant movement. Trials were modeled using the Canonical Hemodynamic Response Function36. The two conditions were entered as separate t-contrasts into second-level analyses.
A second level random effects model was used for between group comparison. As the main focus of our study was to examine the extent to which the three groups were distinguished by patterns of neural activity during the response inhibition (GoNoGo) and motor control (Go) blocks, we performed a 3-group (ATT, NAT, HC) by 2-condition (GoNoGo versus Go blocks) ANOVA covarying for age to examine the group by condition interaction upon whole-brain activity during task performance. Here, we first used a voxel-wise threshold of p<0.05 for whole brain analyses. We then maintained a cluster-level false positive detection rate of p<0.05 for whole brain activity surviving the above voxel-wise threshold of p<0.05, using two methods of small volume correction, one using FWE within SPM 5 and one with a regional anatomical mask from the WFU Pickatlas37 for each whole brain activity cluster, and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim. AlphaSim is a method of correction for multiple comparisons that accounts for spatial correlations between BOLD signal changes in neighboring voxels. It has been used in previous neuroimaging studies of pediatic populations.38–40
Peak BOLD signal changes were extracted from regions showing a significant group by condition interaction in the 3×2 analysis for each group for each condition. We then performed post-hoc tests on these extracted BOLD signal values to examine the extent to which pair-wise between group differences in activity contributed to the significant group by condition interactions in the above analyses, using independent t-tests and appropriate statistical thresholds to control for multiple tests. In these post-hoc tests for regions showing a significant group by condition interaction in the 3×2 ANOVA, a significance threshold of p<0.05/6=p<0.008 was employed to control for the three independent between group pair-wise tests for each of the two conditions in each region.
In exploratory analyses, we then examined potential relationships between extracted BOLD signal from neural regions showing between group differences in activity during each of the two block types of the task and depression severity, anxiety, suicidal ideation, pubertal status, age, gender, and medication at the time of scanning: medication status (taking versus not taking) and medication load, to account for total number and dose of different psychotropic medications taken.41 We conducted Pearson correlational and independent t-tests as appropriate in SPSSv.17.
Results
Of the 51 eligible participants, 44 completed the study: two were excluded due to claustrophobia; one due to an abnormality detected on the scan; one due to history of marijuana dependence; two due to younger age (10 years old) than the majority of other participants; and one due to excessive movement (>cut off of 2 mm). Median time since last attempt was 25.7 months, and mean lethality of attempt from the SHF was 2.27(injury requiring medical attention). 31 7 ATT attempted suicide impulsively (with no planning), and 3 attempted suicide with less than 3 hours of planning. 10 ATT and 7 NAT were treated with medication for depression. Medications primarily included SSRI antidepressant medication, but also augmentation for treatment of depression with other medications (Table 1). Medication load was computed using a scoring system to account for dose and number of medications.41 ATT had significantly higher BDI, SCARED, and SIQ scores than NAT(Table 1). 7 ATT had BDI scores 10 or greater, and 8 were euthymic. 3 NAT BDI scores 10 or greater, and 12 were euthymic (see Table S1, available online, for further characterization of the suicide attempts).
Table 1.
Demographic information and clinical variables
| ATT(n = 15) | NAT(n = 15) | HC(n = 14) | ||
|---|---|---|---|---|
| Gendera | Male | 4 | 7 | 8 |
| Female | 11 | 8 | 6 | |
| Mean ageb (13–17 years) | 16.20(S.D.: 0.78) | 15.87(S.D.: 1.55) | 15.21(S.D.: 1.42) | |
| Petersenc | 3.13(S.D.: 0.35) | 3.00(S.D.: 0.38) | 3.07(S.D.: 0.62) | |
| BDId | 15.47(S.D.: 15.08) | 4.40(S.D.: 5.58) | 2.07(S.D.: 3.47) | |
| SIQe | 39.73(S.D.: 21.08) | 23.40(S.D.: 11.43) | 16.07(S.D.: 1.49) | |
| SCARED(c)f | 26.93(S.D.: 13.99) | 10.00(S.D.: 8.32) | 8.00(S.D.: 8.01) | |
| SCARED(p)g | 22.73(S.D.: 13.12) | 13.67(S.D.: 8.20) | 6.29(S.D.: 7.37) | |
| Medication: 10 ATT and 7 NAT were taking medication
| ||||
| ATT(n = 10) | NAT(n = 7) | |||
|
| ||||
| Mood stabilizers(n = 2) | Lamotrigine(n = 1) | |||
| Topiramate(n = 1) | ||||
| Antidepressants(n = 17) | Escitalopram(n = 1) | Escitalopram(n=1) | ||
| Citalopram(n=1) | Citalopram(n=1) | |||
| Bupropion(n = 1) | Buproprion(n=1) | |||
| Fluoxetine(n = 4) | Fluoxetine(n = 3) | |||
| Trazodone(n = 2) | ||||
| Duloxetine(n = 1) | ||||
| Sertraline(n = 2) | Sertraline(n= 1) | |||
| Antipsychotics(n = 1) | Aripiprazole(n = 1) | |||
| Other(n = 3) | Levothyroxine sodium(n = 1) | Levothyroxine sodium(n = 2) | ||
Note: Numbers are means; standard deviations in parentheses. Adolescent suicide attempters (ATT), non-attempters with history of depression (NAT), and healthy controls (HC) did not differ significantly in:
gender ratio (χ2= 2.789, p= 0.248).
age (F2, 41=2.166, p= 0.128).
Petersen Pubertal questionnaire (χ2 = 0.646, p= 0.724).
ATT had significantly greater:
Beck depression inventory (BDI) scores than NAT and HC (χ2 = 14.33, p= 0.001).
suicidal ideation questionnaire (SIQ) scores than NAT and HC (χ2 = 21.13, p= 0.0001).
screen for childhood anxiety related emotional disorders, child version (SCARED(c)) than NAT and HC (χ2 = 15.98, p= 0.0001).
screen for childhood anxiety related emotional disorders, parent version (SCARED(p)) scores than NAT and HC (χ2 = 15.73, p= 0.0001).
Task performance data
There was no significant effect of group upon task performance accuracy: percentage of accurate Go responses (ATT 89.98±5.5, NAT 91.56±7.57, HC 90.24±5.22; F2, 43=0.11, p=0.896); percentage of accurate NoGo responses (ATT 84.2±6.4, NAT 85.67 ±11.44, HC 86.9 ± 8.27; F2, 43=0.151, p=0.86); number of omissions (ATT 4.87±3.31, NAT 4.13±4.36, HC 3.35±1.95; F2, 43=0.722, p=0.492); or number of commissions (ATT 4.6±3.16, NAT 4.47±3.48, HC 4.5±3.2; F2, 43=0.007, p=0.993). There was no significant effect of group upon reaction time in milliseconds for Go responses (ATT 371.99±33.19, NAT 356.89±37.56, HC 372.90±20.74; F2, 43=1.203, p=0.311) or for NoGo responses (ATT 385.00±25.40, NAT 380.51±23.63, HC 386.66±23.52; F2, 43=0.253, p=0.778).
Neuroimaging data
All groups activated the right anterior cingulate, left insula, and left dorsolateral prefrontal cortex in response to GoNoGo versus Go blocks (see Table S2, available online.) Movement parameters did not differ between groups: non-attempters versus attempters, r=0.018, p=0.924; controls versus attempters, r=0.218, p=0.732; controls versus non-attempters r=0.282, p=0.684.
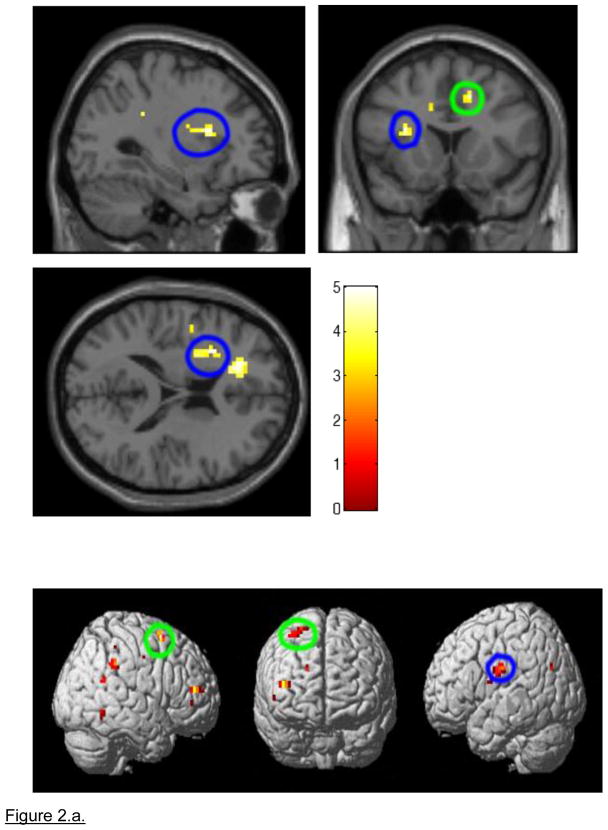
A 3×2 ANOVA, covarying for age, revealed a group by block type interaction in bilateral anterior cingulate gyri (right BA32; p=0.015 uncorrected, p<0.05 corrected; and left BA 32; p=0.01 uncorrected, p<0.05 corrected) and the left insula (BA13; p=0.01 corrected, p<0.05 corrected; Figure 2). 3×2 ANOVA also completed covarying for BDI, SIQ, SCARED-C, SCARED-P, age, and medication load resulted in the same areas of activation. The analyses did not survive SVC with FWE in SPM5, but did survive SVC with alphasim correction at p<0.05(Table 2.a.). Clinical scores did not change the pattern of neural activity (see Table S3, available online).
Figure 2.
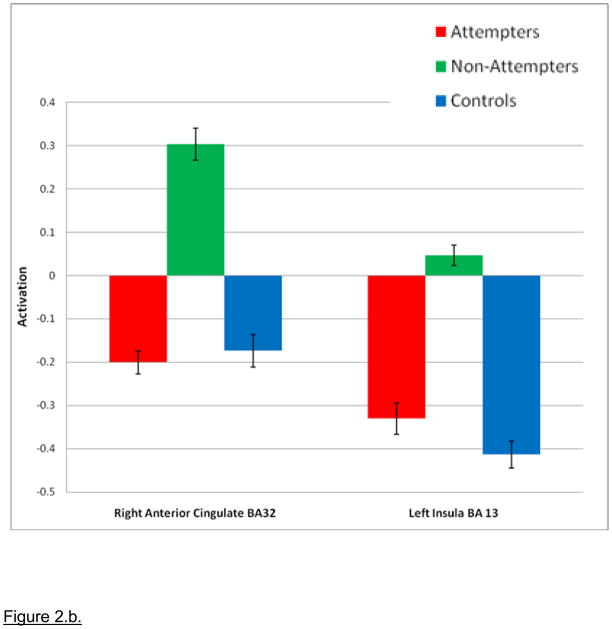
a. Whole brain 3×2 Analysis of Variance (ANOVA) block-design NoGo response inhibition block analyses examining differences in neural activity in the GoNoGo response blocks covarying for age. Note: During GoNoGo blocks, Non-Attempters showed significantly greater activity than Attempters in the right anterior cingulate gyrus (p=0.008, encircled in green) and significantly greater activity than Healthy Controls in the left insula (p=0.004, encircled in blue). Green = Right Anterior Cingulate Gyrus; Blue = Left Insula. b. Non-Attempters > Attempters and Controls during the GoNoGo block. Note: 3×2 Analysis of Variance (ANOVA) Go No Go: Non-Attempters > Attempters and Controls. Magnitude of blood oxygen level dependent (BOLD) signal is depicted in the bar charts. Right Anterior Cingulate Gyrus: p= 0.008 Non-Attempters> Attempters; Left Insula: p= 0.004 Non-attempters>Healthy Controls.
Table 2.
Whole brain activation: 3×2 Analysis of Variance (ANOVA) Group (Attempters (ATT), Non-Attempters (NAT), Healthy Controls (HC)) X Condition (Go, GoNoGo) covarying for age
| a. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | ||||||||||||
| Region | Side | k | SVC k | Alpha Sim K | x | y | z | F | z | Voxelwise | Voxelwise | Voxelwise |
| P, Unc | P, FWE Corr | P, AlphaSim Corr | ||||||||||
| Anterior Cingulate Gyrus (BA32) | R | 39 | 12 | 18 | 15 | 15 | 42 | 4.41 | 2.17 | 0.015 | 1.000 | 0.039 |
| L | 50 | 44 | 17 | −18 | 30 | 18 | 4.92 | 2.34 | 0.01 | 0.997 | 0.048 | |
| Insula (BA13) | L | 24 | 21 | 24 | −30 | 12 | 18 | 4.86 | 2.32 | 0.01 | 0.998 | 0.049 |
| b. | |||||||
|---|---|---|---|---|---|---|---|
| Region | Side | Post Hoc test | Condition | t | df | P | F |
| Anterior Cingulate Gyrus (BA32) | R | NAT>ATT | GO | 2.06 | 28 | 0.049 | 4.22 |
| NAT>ATT | NOGO | 2.84 | 28 | 0.008 | 7.99 | ||
| NAT>HC | NOGO | 2.36 | 27 | 0.026 | 5.53 | ||
| L | NAT>HC | NOGO | 2.36 | 27 | 0.026 | 5.52 | |
| Insula (BA13) | L | NAT>ATT | NO GO | 2.22 | 28 | 0.035 | 4.91 |
| NAT>HC | NOGO | 3.13 | 27 | 0.004 | 9.72 | ||
Note: Values in bold indicate significant between-group differences in neural activity for the post-hoc pair-wise comparisons. FWE= family-wise error, SVC= small volume correction, UNC= uncorrected.
Post-hoc analyses revealed that NAT showed significantly greater activity during GoNoGo response inhibition blocks than HC in left insula (p=0.004), and significantly greater activity during these blocks relative to ATT in right anterior cingulate gyrus (p=0.008). By contrast, ATT did not show this pattern of differential activity relative to HC during GoNoGo blocks. There was no significant between-group difference in neural activity to Go blocks for any of the post-hoc pair-wise comparisons (Table 2.b.).
Exploratory Analyses
Exploratory analyses were performed to examine relationships between development (age, pubertal status), clinical variables (BDI, SCARED child and parent rating, SIQ, medication status and load), gender, and activity in NAT in the regions showing significant differences in activity relative to ATT and HC, that emerged from the 3×2 interaction. A statistical threshold of p=0.05/9(p<0.006) was employed to control for the nine multiple tests (one for each of the demographic and clinical variables above) in each region. These analyses revealed no significant relationships between anxiety, depression, medication status, medication load, gender, age, or pubertal status and activation in these regions. In addition, there was no significant correlation between lethality rating of most lethal attempt or time since attempt and activation in these regions in ATT(see Table S4, available online).
Discussion
Our study is the first to examine neural circuitry supporting response inhibition in adolescents with a history of suicide attempt. Our specific aim was to determine the extent to which adolescent suicide attempters (ATT) showed abnormal activity in this neural circuitry during performance of a GoNoGo response inhibition task relative to adolescent non-attempters (NAT) and healthy controls (HC). Contrary to our hypothesis, ATT did not show a significantly different pattern of neural activity from HC during the GoNoGo response inhibition or the Go motor control blocks.
Our main significant finding was that NAT showed significantly greater activity in right anterior cingulate gyrus to GoNoGo response inhibition blocks relative to ATT, and greater activity in left insula, to these blocks relative to HC. The increase in activity in left insula during GoNoGo blocks shown by NAT relative to HC may reflect abnormally elevated internal state monitoring during the response inhibition blocks, as the insula has been associated with internal state and autonomic function monitoring. 42 The increase in activity in right anterior cingulate gyrus to GoNoGo response inhibition blocks shown by NAT relative to ATT may reflect a compensatory increase in recruitment of right-sided anterior cingulate gyrus to facilitate accurate task performance during response inhibition in NAT relative to ATT. This finding parallels a similar finding of elevated bilateral anterior cingulate gyral activity in depressed, non-suicide attempters relative to healthy adults during attentional control tasks.43 Together, these findings suggest that NAT, but not ATT, may have shown abnormally elevated internal state monitoring, and may have needed to recruit additional attentional control neural circuitry resources relative to ATT, during response inhibition. These findings do not therefore support the hypothesis that ATT have dysfunctional response inhibition circuitry; rather, our findings suggest that ATT were able to perform the task well without the need for recruitment of additional neural circuitry.
The absence of abnormal activity shown by ATT during the GoNoGo task is intriguing for the following reasons. First, previous findings indicate poorer performance on Stroop attentional control tasks in high lethality adult suicide attempters relative to depressed non-attempters. 44 Second, abnormally reduced activity in regions implicated in response inhibition have been shown in other psychiatric illnesses in childhood, including ADHD45 and familial vulnerability to impulse control disorder46, that suggest an association between poor impulse control and impulsive behaviors and dysfunction in response inhibition neural circuitry, that in turn have been proposed to underlie vulnerability to suicide attempt. 47 Given that ATT in the present study performed as well as HC on the GoNoGo task, it is therefore possible that the absence of abnormal activity in response inhibition circuitry during the task in ATT in the present study reflected the normal level of task performance in ATT. Furthermore, our present findings do not support an association between dysfunctional response inhibition neural circuitry and suicide attempt in adolescence, although we did not specifically measure impulsivity in study participants. The majority of ATT in the study did, however, attempt suicide with less than three hours of planning, consistent with impulsive behavior. It is also possible that Type II error may have contributed to the lack of significant difference in activity between ATT and HC, although we were able to demonstrate significant differences in neural activity between NAT and HC. Future studies employing more difficult response inhibition or other attentional control tasks in larger numbers of ATT, NAT and HC in adolescence may help to determine the extent to which poor performance on these tasks is associated with significantly more abnormal neural activity in ATT than either HC or NAT, and the relationship between abnormal neural activity and impulsivity in ATT.
Our exploratory analyses did not reveal any significant relationship between self-reported symptoms of depression and anxiety and patterns of abnormal activity in NAT. Given the continued development of cortical regions throughout childhood and adolescence,14, 48–49 however, future longitudinal studies should examine the extent to which elevated depression and anxiety symptoms are associated with abnormal developmental trajectories of key regions in response inhibition neural circuitry. These abnormal neurodevelopmental trajectories in turn may lead to vulnerability to suicidal behavior in younger children at future risk of suicide, for example, children with a familial history of suicidal behavior.
There were limitations to the current study, which may have contributed to our inability to differentiate ATT from HC. Participants with suicide attempt history had higher depression, anxiety, and suicidal ideation scores than the depression group. These variables were not associated with the outcomes of interest. The block design of our paradigm did not allow for differentiation of correct and incorrect responses. It is possible that susceptibility artifacts may have prevented us from detecting between-group differences in activity in ventral prefrontal cortex. The analyses did not survive SVC with FWE in SPM5, but did survive SVC with AlphaSim correction at p<0.05. SVC with FWE is a far more stringent correction strategy, in which it appears that only clusters greater than 500 voxels would have survived. AlphaSim is a method of correction for multiple comparisons employed in other software packages (e.g., AFNI), and has been used in previous neuroimaging studies of pediatic populations.38–40 In addition, it was necessary to recruit ATT and NAT who were currently taking psychotropic medication, and three of these adolescents had augmentation treatment for depression that included a mood stabilizer or antipsychotic. Our exploratory analyses did not, however, show any significant relationships in NAT between medication status or medication load and activity during response inhibition in those regions showing elevated activity in NAT versus HC and reduced activity in ATT relative to NAT. ATT were studied after attempt, so extrapolation to risk for attempt is difficult. Future studies in larger numbers of unmedicated participants could aim to replicate the findings of the present study.
Our study is the first to examine neural circuitry associated with response inhibition in a GoNoGo task in adolescent ATT relative to NAT and HC. Our findings indicate dissociable patterns of neural activity during response inhibition in ATT and NAT. Our findings suggest that further examination of functional abnormalities in neural regions implicated in attentional and behavioral control processes should be a focus of future studies aiming to identify potential markers of suicide attempt risk throughout childhood, adolescence and adulthood.
Acknowledgments
This research was supported by the American Foundation for Suicide Prevention, the Klingenstein Third Generation Foundation Fellowship for Adolescent Depression, NIMH/NICHD 1K23MH082884-01(LP); and NIH grants MH66775, MH65368, MH56612, MH18951(DB); NIMH MH076971(MP).
We thank the participants who made this research possible.
This manuscript is dedicated to Kathy O’Hern Fowler and her son Lambert, who died unexpectedly from suicide.
Footnotes
All work was completed in the Department of Psychiatry of the University of Pittsburgh, and the Brain Imaging Research Center (University of Pittsburgh & Carnegie Mellon University).
Supplemental material cited in this article is available online.
Disclosure: Drs. Pan, Batezati-Alves, Almeida, Akkal, Hassel, Brent, and Phillips, and Ms. Segreti and Ms. Lakdawala report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Lisa A. Pan, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr. Silvia C. Batezati-Alves, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr. Jorge R.C. Almeida, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Ms. AnnaMaria Segreti, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr. Dalila Akkal, Brain Research Unit, Aalto University, Aalto, Finland.
Dr. Stefanie Hassel, University of Calgary School of Medicine, Calgary, AB, Canada.
Ms. Sara Lakdawala, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr. David A. Brent, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr. Mary L. Phillips, University of Pittsburgh School of Medicine, Pittsburgh, PA. School of Medicine, Cardiff University, Cardiff, UK.
References
- 1.Suicide trends among youths and young adults aged 10–24 years--United States, 1990–2004. MMWR Morb Mortal Wkly Rep. 2007;56(35):905–908. [PubMed] [Google Scholar]
- 2.Fergusson DM, Beautrais AL, Horwood LJ. Vulnerability and resiliency to suicidal behaviours in young people. Psychol Med. 2003;33(1):61–73. doi: 10.1017/s0033291702006748. [DOI] [PubMed] [Google Scholar]
- 3.Molnar BE, Berkman LF, Buka SL. Psychopathology, childhood sexual abuse and other childhood adversities: relative links to subsequent suicidal behaviour in the US. Psychol Med. 2001;31(6):965–977. doi: 10.1017/s0033291701004329. [DOI] [PubMed] [Google Scholar]
- 4.Brent DA, Baugher M, Bridge J, Chen T, Chiappetta L. Age- and sex-related risk factors for adolescent suicide. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1497–1505. doi: 10.1097/00004583-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Mann JJ, Currier D. A review of prospective studies of biologic predictors of suicidal behavior in mood disorders. Arch Suicide Res. 2007;11(1):3–16. doi: 10.1080/13811110600993124. [DOI] [PubMed] [Google Scholar]
- 6.Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Castelnau D, Malafosse A, Courtet P. Impaired decision making in suicide attempters. Am J Psychiatry. 2005;162(2):304–310. doi: 10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- 7.Jollant F, Lawrence NS, Giampietro V, Brammer MJ, Fullana MA, Drapier D, Courtet P, Phillips ML. Orbitofrontal cortex response to angry faces in men with histories of suicide attempts. Am J Psychiatry. 2008;165(6):740–748. doi: 10.1176/appi.ajp.2008.07081239. [DOI] [PubMed] [Google Scholar]
- 8.Bender TW, Gordon KH, Bresin K, Joiner TE. Impulsivity and suicidality: The mediating role of painful and provocative experiences. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Gorlyn M. Impulsivity in the prediction of suicidal behavior in adolescent populations. International J of Adol Med& Health. 2005;17:205–209. doi: 10.1515/ijamh.2005.17.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Maser J, Akiskal H, Schettler P, Scheftner W, Mueller T, Endicott J, Solomon D, Clayton P. Can temperament identify affectively ill patients who engage in lethal or near- lethal suicidal behavior? A 14-year prospective study. Suicide and Life-Threatening Behavior. 2002;32:10–32. doi: 10.1521/suli.32.1.10.22183. [DOI] [PubMed] [Google Scholar]
- 11.Klonsky ED, May A. Rethinking impulsivity in suicide. Suicide Life Threat Behav. 2010;40(6):612–619. doi: 10.1521/suli.2010.40.6.612. [DOI] [PubMed] [Google Scholar]
- 12.Witte TK, Merrill KA, Stellrecht NE, Bernert RA, Hollar DL, Schatschneider C, Joiner TE. “Impulsive” youth suicide attempters are not necessarily all that impulsive. Journal of Affective Disorders. 2008;107:107–116. doi: 10.1016/j.jad.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry. 2001;158(5):735–741. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- 14.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush G, Shin LM. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1(1):308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, McCandliss BD, Fossela J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Oquendo MA, Placidi GP, Malone KM, Campbell C, Keilp J, Brodsky B, Kegeles LS, Cooper TB, Parsey RV, van Heertum RL, Mann JJ. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60(1):14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 18.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 21.Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007;13(3):276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, Kim YY, Yoo SY, Kwon JS. Electrophysiological correlates of behavioral response inhibition in patients with obsessive-compulsive disorder. Depress Anxiety. 2007;24(1):22–31. doi: 10.1002/da.20195. [DOI] [PubMed] [Google Scholar]
- 23.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, Scheres A, Xavier Castellanos F, van Engeland H, Casey BJ. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry. 2007;48(9):881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 26.Raust A, Slama F, Mathieu F, Roy I, Chenu A, Koncke D, Fouques D, Jollant F, Jouvent E, Courtet P, Leboyer M, Bellivier F. Prefrontal cortex dysfunction in patients with suicidal behavior. Psychol Med. 2007;37(3):411–419. doi: 10.1017/S0033291706009111. [DOI] [PubMed] [Google Scholar]
- 27.Halari R, Simic M, Pariante CM, Papadopoulos A, Cleare A, Brammer M, Fombonne E, Rubia K. Reduced activation in lateral prefrontal cortex and anterior cingulate during attention and cognitive control functions in medication-naïve adolescents with depression compared to controls. J Child Psychol and Psychiatry. 2009;50(3):307–316. doi: 10.1111/j.1469-7610.2008.01972.x. [DOI] [PubMed] [Google Scholar]
- 28.Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): Classification of Suicidal Events in the FDA’s Pediatric Suicidal Risk Analysis of Antidepressants. Am J Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 30.Beck AT, Schuyler D, Herman I. Development of Suicidal Intent Scales. In: Beck AT, Resnick HLP, Lettieri DJ, editors. The Prediction of Suicide. Bowie, Maryland: Charles Press; 1974. pp. 45–56. [Google Scholar]
- 31.Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. Standardized Evaluation in Clinical Practice. 2003:103–130. [Google Scholar]
- 32.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 33.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, McKenzie Neer S. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds WM. Suicidal Ideation Questionnaire: Professional Manual. Psychological Assessment Resources Inc; 1987. [Google Scholar]
- 35.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J of Youth Adol. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 36. [Accessed March 2011];Statistical Parametric Mapping Manual. 2009 :66. http://www.fil.ion.ucl.ac.uk/spm.
- 37.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. (WFU Pickatlas, version 3.03) [DOI] [PubMed] [Google Scholar]
- 38.Gilbert AR, Akkal D, Almeida JRC, Mataix-Cols D, Kalas C, Devlin B, Birmaher B, Phillips ML. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2005;48(9):936–944. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 39.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707–19. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disord. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy, and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Harvey PO, Fossati P, Pochon JB, Levy R, LeBastard G, Lehericy S, Allilaire JF, Dubois B. Cognitive control and brain resources in major depression: An fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 44.Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Res. 2008;159(1–2):7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickstein SG, Bannon K, Castellanos FX, Milham M. The neural correlates of attention deficit hyperactivity disorder: and ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 46.Mulder MJ, Baeyens D, Davidson MC, Casey BJ, van den Ban E, van Engeland H, Durston S. Familial vulnerability to ADHD affects activity in the cerebellum in addition to the prefrontal systems. J Am Acad Child Adolesc Psychiatry. 2008;47(1):68–75. doi: 10.1097/chi.0b013e31815a56dc. [DOI] [PubMed] [Google Scholar]
- 47.Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, Currier D, Dougherty DM, Haghighi F, Hodge SE, Kleinman J, Lehner T, McMahon F, Mościcki EK, Oquendo MA, Pandey GN, Pearson J, Stanley B, Terwilliger J, Wenzel A. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psych. 2009;65(7):556–63. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 49.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]