Abstract
Exosomes are small membrane vesicles secreted from various types of cells. Tumor-derived exosomes contain MHC class I molecules and tumor-specific antigens, receiving attention as a potential cancer vaccine. For induction of efficient anti-tumor immunity, CD4+ helper T cells are required, which recognize appropriate MHC class II-peptide complexes. In this study, we have established an MHC class II molecule-expressing B16F1 murine melanoma cell line (B16F1-CIITA) by transduction of the CIITA (Class II transactivator) gene. Exosomes from B16-CII cells (CIITA-Exo) contained a high amount of MHC class II as well as a tumor antigen TRP2. When loaded on dendritic cells (DCs), CIITA-Exo induced the increased expression of MHC class II molecules and CD86 than the exosomes from the parental cells (Exo). In vitro assays using co-culture of immunized splenocytes and exosome-loaded DCs demonstrated that CIITA-Exo enhanced the splenocyte proliferation and IL-2 secretion. Consistently, compared to B16-Exo, CIITA-Exo induced the increased mRNA levels of inflammatory cytokines such as TNF-α, chemokine receptor CCR7 and the production of Th1-polarizing cytokine IL-12. A tumor preventive model showed that CIITA-Exo significantly inhibited tumor growth in a dose-dependent manner. Ex vivo assays using immunized mice demonstrated that CIITA-Exo induced a higher amount of Th1-polarized immune responses such as Th1-type IgG2a antibodies and IFN-γ cytokine as well as TRP2-specific CD8+ T cells. A tumor therapeutic model delayed effects of tumor growth by CIITA-Exo. These findings indicate that CIITA-Exo are more efficient as compared to parental Exo to induce anti-tumor immune responses, suggesting a potential role of MHC class II-containing tumor exosomes as an efficient cancer vaccine.
Keywords: cancer vaccine, CIITA, exosomes, immunotherapy, MHC class II molecules
Introduction
Exosomes have be small membrane vesicles (40-100 nm in diameter) that originate from multivesicular bodies that release internal vesicles into the extracellular space by fusion with plasma membranes (Denzer et al., 2000). These vesicles are produced by various cell types including reticulocytes, intestinal epithelial cells, hematopoietic cells as well as tumor cells (Johnstone et al., 1987; Raposo et al., 1996; Zitvogel et al., 1998; Heijnen et al., 1999; van Niel et al., 2001; Blanchard et al., 2002; Skokos et al., 2002). Proteomic analyses of exosomes were showed that exosomes contain a selective enrichment of a discrete set of cellular proteins associated with antigen presentation, signal transduction, migration/adhesion including major histocompatibility complex (MHC) molecules, heat shock proteins (Hsp70), MFG-E8, and tetraspanins (Escola et al., 1998; Thery et al., 2001; Wubbolts et al., 2003).
Recent studies have suggested that exosomes can serve as a new cell-free vaccine with therapeutic effect in cacner immunotherapy (Hao et al., 2007). Exosomes derived from tumor peptide-pulsed DCs have shown to bear peptide-loaded MHC molecules and costimulatory molecules and inhibited tumor growth by tumor specific T cell responses (Zitvogel et al., 1998).
A variety of tumor cells also constitutively release exosomes (Wolfers et al., 2001; Andre et al., 2002). Tumor-derived exosomes contain tumor-specific antigens and tumor peptide/MHC class I complexes that can prime tumor-specific CTLs and can elicit a potent tumor-specific immune response (Wolfers et al., 2001). Malignant effusions accumulate exosomes bearing tumor antigens and antigen-presenting molecules that can generate anti-tumor T cell responses (Andre et al., 2002), and exosomes from different tumor cells showed that exosomes prohibited not only syngeneic but also allogeneic tumor growth (Andre et al., 2002; Cho et al., 2005). These studies provide a rationale that tumor-derived exosomes have the potential as an efficient cell-free therapeutic vaccine.
Many strategies in cancer immunotherapy have aimed to trigger anti-tumor immune responses by an MHC class I-restricted tumor-specific CTL response (Takenoyama et al., 2001; Kass et al., 2003). However, to generate efficiently CD8+ T effector and memory cells, CD4+ T helper (Th) cells are essentially required (Baxevanis et al., 2000). Th-derived cytokines, especially Th1 type cytokines and other soluble mediators in the tumor microenvironment are fundamental for maturation of CTL precursors with the cytolytic function leading to tumor regression (Ciavarra et al., 1991). Additionally, the generation of MHC class II-restricted CD4+ Th cells are required for optimal induction of both humoral and cellular effector mechanisms in cancer immunotherapy (Kern et al., 1986).
Most tumor cells express MHC class I molecules, but not MHC class II. As expression of all MHC class II alleles are controlled by a master regulatory gene MHC class II transcriptional activator (CIITA), CIITA-introduced tumor cells can provide antigen processing and presentation by both MHC class I and II molecules (Nagarajan et al., 2002). An interesting study using modified tumor cells to express MHC class II molecules by transfection with the CIITA-encoded AIR-1 locus showed tumor-rejection effects in vivo by the stimulation of tumor-specific CD4+ and CD8+ T cells (Meazza et al., 2003).
In this study, we transduced murine melanoma cell line B16F1 cells with the CIITA gene to express MHC class II molecules and investigated whether exosomes from the tumor cells (CIITA-Exo) possessed the enhanced capability of immune stimulation as compared to exosomes from parental tumor cells (Exo). Our results demonstrated that exosomes from CIITA-transduced tumor cells exhibited greater effects on tumor regression as compared to the Exo, suggesting CIITA-Exo as a potential vaccine for cancer immunotherapy.
Results
MHC class II expression at exosomes from CIITA-transduced tumor cells
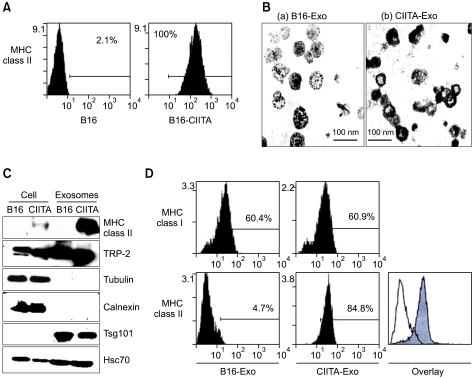
B16F1 melanoma cells transduced with a mock or CIITA-incorporated retrovirus were cloned, and were analyzed for the expression of MHC class II molecules (Figure 1A). When compared with the mock-tranduced cells (B16F1), CIITA-transduced cells (B16F1-CIITA) displayed high levels of MHC class II molecules on the cellular surface.
Figure 1.
Characterization of exosomes from CIITA-transducted tumor cells. (A). MHC class II expression on CIITA-transfected B16F1 tumor cells. The clone cell were analysed by flow cytometry to evaluation expression of MHC class II molecules. (B) exosomes were observed by electron microscopy (Bar=100 nm) (C) cellular proteins and exosomes from mock (B16) or CIITA transduced (CIITA) cells were analyzed by immunoblot analysis with each indicated antibodies against MHC class II, TRP-2, Tsg101, Hsc70, tubulin, and Calnexin. (D) Bead-exosomes complex were incubated with PE-conjugated anti-MHC class I and II antibodies, and analyzed by flow cytometry.
To investigate whether the MHC class II molecules were also present at exosomes from B16F1-CIITA cells, we isolated exosomes from both B16F1 and B16F1-CIITA cells as described in Methods. The morphology of the isolated exsomes was observed using transmission electron microscopy (Figure 1B). We performed Western blotting to compare proteins expression of cell and exosome. As shown in Figure 1C, MHC class II molecules were highly enriched in exosomed from CIITA-Exo as compared to Exo. TRP-2 tumor antigen and heat shock cognate (Hsc70) were increased exosomes compared with cells. Exosomes were contained Tsg101 and not calnexin.
We subsequently examined the presence of MHC class II molecules on the surfaces of CIITA-Exo as on the cells by FACS analysis using a bead-conjugated method (Kass et al., 2003). Figure 1D showed that MHC class II molecules were detected on the surfaces of CIITA-Exo, demonstrating that MHC class II molecules were successfully loaded onto exosomes by CIITA-transduction into cells.
Enhanced immune effects of exosomes from CIITA-transduced tumor cells on dendritic cell maturation
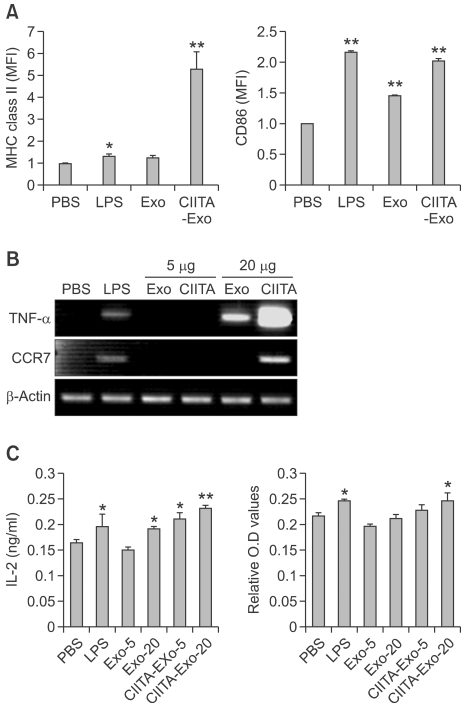
The efficient T cell mediated antitumor immune responses require the uptake, processing and presentation of tumor antigens by DCs (Escudier et al., 2005). Tumor-derived exosomes provide a source of tumor antigen for cross-presentation by DCs, and in vivo, DCs-loaded with tumor derived exosomes induce tumor regection (Wolfers et al., 2001). We examine the immune activity of CIITA-Exo on DCs by pulsing with either with PBS, LPS, positive control, Exo or CIITA-Exo, respectively at the indicated concentration for 24 hours. The pulsed DCs showed an increase in the expression of immunostimulatory molecules such as MHC class II molecules and CD86 (Figure 2A). CIITA-Exo significantly induced higher level of MHC class II molecules than Exo. The mean fluorescence intensity (MFI) of costimulatory molecules, CD86, was slightly enhanced by CIITA-Exo, to the extent similar with that of LPS but greatly higher than that of Exo. Therefore, CIITA-Exo exhibited potent activity on induction of DC maturation phenotypically with Exo.
Figure 2.
Immune stimulating effects of CIITA-Exo on dendtitic cell line. (A) The surface expression of MHC class II and CD86 on the pulsed DCs was detected by flow cytometry. The number above the bars indicates the fold increase of mean fluorescence intensity (MFI) (B) The mRNA level of TNF-a, CCR-7 and β-actin in the pulsed DCs was analyzed by RT-PCR. (C) The culture supernatants were collected to determine IL-2 release by ELISA and CTLL-2D proliferation assay (alarmar blue). Statistical significance, *: P <0.05, ** : P <0.005.
To further analyze the immunostimulatory properties of CIITA-Exo on DC maturation, we harvested Exo-pulsed DCs and performed RT-PCR. The results revealed that mRNA levels of imflammatory cytokine, TNF-α and another maturation marker, CCR-7, were significantly induced at a much higher level by CIITA-Exo than Exo (Figure 2B). These results indicated that CIITA-Exo were more powerful immunogen than Exo, implicating that they might be more capable of eliciting immune response in vivo.
Increased proliferation and activation of immune cells by CIITA-Exo
Since the enhanced immunologic function of CIITA-Exo on DC maturation was revealed, we first examined the effects of CIITA-Exo on naïve immune cells though DCs. DCs were pulsed either with PBS, LPS, Exo, and CIITA-Exo and mytomycin C for 4 hours were treated respectively. The Exoloaded DCs were co-cultured with naïve syngeneic splenocytes for 48 hours, followed by the culture supernatants were collected and analyzed the IL-2 cytokine production and proliferation assay of CTLL-2D, IL-2 dependent T cell line. As show in Figure 2C, CIITA-Exo pulsed DCs were markedly induced the IL-2 production of naïve splenocytes, suggesting the CIITA-Exo might be more capable of activity CD4+ helper T cells and enhancing the production of IL-2 cytokine. Consistent with this data, CTLL-2D, which were incubated with coculture supernatant of CIITA-Exo pulsed DCs, were more proliferated than other groups including Exo. These data showed that CIITA-Exo was able to activate the immune cells than Exo, implicating that they might enhance powerful anti-tumor effects.
Improved preventive anti-tumor immune response of exosomes from CIITA-transduced tumor cells
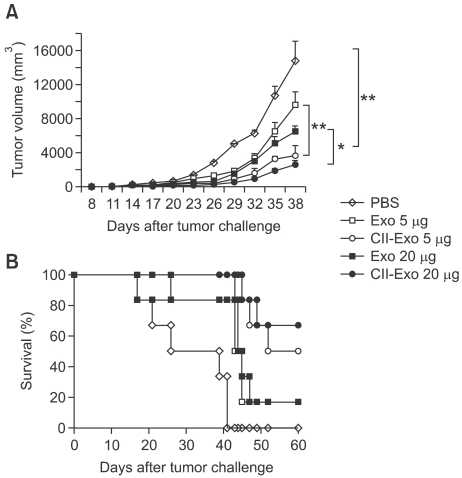
To evaluate anti-tumor effects of the exosomes in vivo, we first observed the using of a preventive tumor model. C57BL/6 mice were vaccinated with either PBS, Exo, or CIITA-Exo and then were challenged with B16F1 tumor cells one week after the last immunization. As the tumor growth and survival rate was monitored (Figure 3), CIITA-Exo significantly inhibited tumor growth in a dose-dependent manner (Figure 3A), and survival rate in mice vaccinated with CIITA-Exo was 50% (5 µg) or 67% (20 µg) as compared to mice vaccinated with Exo where survival rate was 18% (5 and 20 µg) at 60 days (Figure 3B).
Figure 3.
More potent protective antitumor immunity induced by immunization with CIITA-Exo in preventive tumor models. C57BL/6 mice (n=7 per group) were intradermally immunized with PBS (◊), Exo 5 µg (□), Exo 20 µg (■), CIITA-Exo 5 µg (○) or CIITA-Exo 20 µg (●). One week after the last immunization, the immunized mice were challenged with B16F1 tumor cells. (A) Tumor volume was measured every three days from one week after tumor challenge, and was calculated using the formula longer length × shorter length2/2. (B) Survival of each group of mice was monitored until two months after tumor challenge. Statistical significance, *: P <0.05, ** : P <0.005.
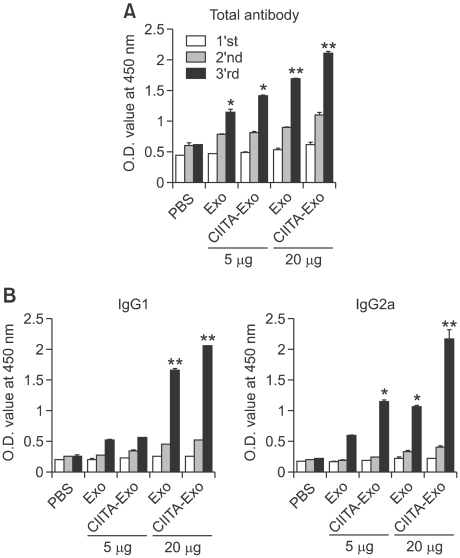
To analyze the immune mechanisms induced by the exosomes, we examined indicators for humoral and cellular immune responses in the immunized mice. For analysis of the tumor-specific humoral immune responses, sera from immunized mice were subjected to an ELISA. The findings showed that the amount of tumor-specific antibody was considerably higher in mice immunized with CIITA-Exo as compared to control mice immunized treated with PBS or mice immunized with Exo (Figure 4A). When antibody isotyping was performed to determine which antibody isotype was specifically and dominantly produced, we found that CIITA-Exo markedly increased the production of Th-1 type antibody IgG2a while the level of Th-2 type antibody IgG1 was comparable with Exo (Figure 4B), indicating that Th-1 type immune responses were successfully triggered by CIITA-Exo.
Figure 4.
Humoral immune responses by exosomes were analyzed. Mice were immunized three times with either PBS, Exo 5 µg or Exo 20 µg or CIITA-Exo 5 µg or CIITA-Exo 20 µg. The experiments were repeated twice and displayed similar results. Sera from the immunized mice were obtained and were pooled one week after each immunization (1st, 2nd and 3rd week). (A) Anti-mouse IgG and IgM antibody was used to detect whole antibodies. (B) Anti-IgG1- and anti-IgG2a-speicific antibodies were used to detect antibody isotypes. Statistical significance; ns: not significant, *: P <0.05, **: P <0.01.
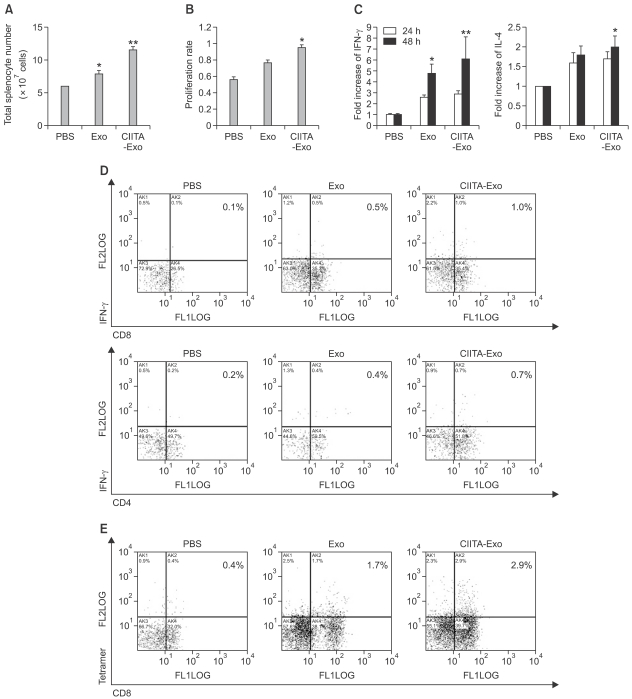
To identify the characteristics of cellular immune responses induced by CIITA-Exo, splenocytes from immunized mice (n=10) were extracted 10 days after the last immunization. Splenomegaly was observed from the mice immunized with both types of exosomes but CIITA-Exo induced more dramatic increase of splenic size and total splenocyte number (Figure 5A). Respective splenocyte samples were stimulated with irradiated B16F1 cells in vitro. After a 3 days in vitro stimulation, the splenocytes were collected to investigate whether CIITA-Exo could effectively trigger tumor-specific Th1 type cellular immune responses. Equal numbers of splenocyte from respective immunizaed mouse groups were stimulated with mitomycin C-treated B16F1 cells. After 72 h stimulation, the stimulated splenocytes were assayed by alarma blue assay for cell proliferation rate. Proliferation rate of splenocyte was increased in group of CIITA-Exo (Figure 5B). The producing rate of IFN-γ was significantly increased, but the producing rate of IL-4 was slightly increased in CIITA-Exo as compared to Exo (Figure 5C). These results indicated that CIITA-Exo could effectively trigger tumor-specific Th-1 type cellular immune responses.
Figure 5.
Cellular immune responses by exosomes were analyzed. Mice were immunized three times with either PBS, Exo 5 µg or Exo 20 µg or CIITA-Exo 5 µg or CIITA-Exo 20 µg. Spleens from the respective groups were extracted 10 days after the last immunization. (A) The absolute cell numbers of splenocytes were counted by trypan blue exclusion assay. (B) One week after the last immunization, splenocytes from the respective groups were harvested and pooled together. Equal numbers of splenocytes from respective immunized mouse groups were stimulated with mitomycin C-treated B16 cells. After 72 hr stimulation, the stimulated splenocytes were assayed by alarma blue assay for cell proliferation. The experiments were performed twice and demonstrated similar result. Statical significance; *P <0.05. (C) The co-culture supernatnats were collected after 24 or 48 h incubation and utilized for ELISA to measure the quantity of IFN-γ or IL-4 secreted from the stimulated splenocytes. *P <0.05. (D) Intrcellular cytocine assay. The stimulated splenocytes were surface-stained with FITC anti CD-4 or anti CD-8 antibodies and intracelluarly labeled with PE anti-IFN-γ or anti-IL-4 antibodies. (E) Tetramer assay. The stimulated splenocytes were stained with FITC-tagged anti-CD8 antibodies and were reacted with PE-conjugated H-2b/TRP2 tetramer. The percentage of the double-positive cell population is displayed on each panel.
When analyzed the T cell subset of immunized splenocyte, CIITA-Exo vaccinated splenocyte possessed slightly higher number of CD4+ or CD8+ T cells (data not shown). We performed flow cytometry to evaluate whether tumor-specific CD8+ T cells were activated by the exosomes. Figure 5D shows that the IFN-γ-producing CD8+ T cell population was increased approximately 2 folds and IFN-γ-producing CD4+ T cell population was also increased 1.8 folds in splenocytes immunized with CIITA-Exo as compared to splenocytes immunized with Exo. Further investigation using a tetramer assay demonstrated that the tumor antigen (TRP2)-specific CD8+ T cell population was approximately 1.7 folds higher with CIITA-Exo injection than Exo injection (Figure 5E). Collectively, the results demonstrate that the CIITA-Exo could induce the increased Th-1 type humoral and cellular immune responses required for efficient anti-tumor effects, suggesting CIITA-Exo as stronger immunologic stimulants than parental Exo.
In vivo therapeutic anti-tumor effects of exosomes from CIITA-transduced tumor cells
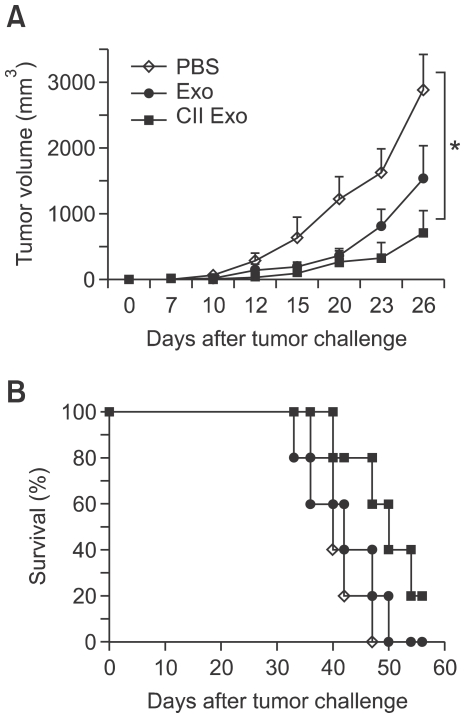
The anti-tumor effects of respective exosomes were tested in a therapeutic model. Tumor-bearing C57BL/6 mice were intradermally injected with either PBS, Exo, or CIITA-Exo, and tumor growth was monitored. Figure 3 showed that CIITA-Exo exerted a greater capability of tumor regression as well as an increased survival rate. As shown in Figure 6A, CIITA-Exo delayed the tumor growth than the Exo, which was consistent with the result of the preventive model as shown in Figure 3. The survival rate of the tumor-bearing mice was also increased up to 20% by CIITA-Exo as compared to the Exo with 0% at 60 days post last exosome injection (Figure 6B). This finding demonstrates that CIITA-Exo certainly exerted enhanced antitumor effects in vivo as compared to Exo.
Figure 6.
In vivo anti-tumor effects of Exo in tumor-bearing mouse were evaluated. C57BL/6 mice (n = 7 per group) were injected with B16F1 tumor cells. When the tumor size reached 1 cm2, either PBS (◊), Exo 20 µg (●) or CIITA-Exo 20 µg (■) was intradermally injected three times with a two-week interval in therapeutic tumor models. (A) Tumor size was measured one or two times a week. (B) The survival rate of the mice was determined until 60 days after the last injection. Representative data are shown from two repeated independent experiments. Statistical significance; *: P <0.05.
Taken together, CIITA-enrichment in tumor-derived exosomes by CIITA gene transduction into tumor cells could be an innovative way to improve imuune response of tumor-derived exosomes as a potent therapeutic cancer vaccine.
Discussion
Exosomes are a candidate with a great potential to function as a cell-free cancer vaccine. Previously, exosome-based immunotherapeutic trials have primarily focused on dendritic cells (DCs) (Escudier et al., 2005; Morse et al., 2005; Shortman et al., 2009). These studies have indicated that DC-derived exosomes can be used as a cell-free cancer vaccine. Interestingly, tumor cell-derived exosomes, which are enriched in MHC-I molecules, costimulatory molecules, heat shock protein, intracellular adhesion molecules, and tumor associated antigens, were found to carry tumor antigens that were capable of triggering an effective immune response (Hao et al., 2007). Accordingly, tumor-derived exosomes have be a cell-free cancer vaccine potency for clinical application.
Nevertheless, tumor-derived exosomes were shown to promote tumor growth rather than inhibit, possibly due to negative effects of tumor-derived exosomes on IL-2 induced CTL responses via TGF-β which enhances regulatory T cell induction (Clayton et al., 2007). To alleviate the negative effects as well as to increase the anti-tumor effects, it is necessary to develop an innovative method for tumor-derived exosomes. Previously, we have reported improved anti-tumor effects of manipulated tumor-derived exosomes by enrichment with human tumor antigen MUC-1 and Hsp70 that efficiently suppressed tumor growth (Cho et al., 2005, 2009). Therefore, tumor-derived exosomes-based immunotherapy needs to be modified to increase therapeutic efficacy and enhance immunogenicity.
In this study, we provide a novel strategy using exosomes from CIITA-transducted tumor cells, CIITA is a major transcriptional activator of MHC class II molecule. We expected that CIITA introduction into tumor cells could cause the expression of MHC class II molecules in a complex form with diverse endogenous tumor antigenic peptides, and produce exosomes with those MHC class II/antigenic peptides complexes. The data in this study identified their morphology and protein composition, indicating that exosomes expressed protein such as Hsc70, TRP-2, MHC class I and gp100, and MHC class II successfully induced the high expression of MHC class II molecules at cells and exosomes. Furthermore, we expected that the augmented immunogenicity in such exosomes could extend or evoke CD4+ helper T cell (CD4+ Th1) and subsequent CD8+ cytolytic T cell responses (CD8+ T) against tumor cells with diverse tumor antigenic peptides. Preveously, the CIITA has been used in other vaccine systems, particularly tumor cell-based vaccines, to successfully improve vaccine potency (Armstrong et al., 1997; Thompson et al., 2006, 2008). Furthermore, tumor cells transfected with CIITA/or CD80 have been shown to activate tumor-specific CD4+ T cells (Thompson et al., 2006, 2008). Moreover, the amplitude of protective immune response directly correlated with the amount of CIITA-mediated MHC-II expression, and CIITA-transfected cells efficiently processed and presented nominal antigens to antigen-specific CD4+ T cells (Mortara et al., 2006). Therefore, the preparation of MHC class II-containing exosomes from fusion gene-modified cancer cells might be practical for developing a vaccine potency and were certainly stronger to elicit tumor-specific immune responses and tumor regression effects in vivo as compared to the Exo.
The exact mechanism of immunostimulation by CIITA-Exo is not clear yet. A previous study has shown that immature DC-derived exosomes induced Th-1 and CTL responses in an indirect way via mature DCs by transferring their MHC-peptide complexes to the mature DCs due to the weakness of costimulatory molecule and ICAM-1 expression required for direct stimulation of adaptive immunity (Thery et al., 2002; Andre et al., 2004). Accordingly, exosomes from the CIITA-introduced tumor cells are assumed to exert their activities through antigen-presenting DCs rather than directly activating CD4+ T cells. That is, the TRP2-reactive CD8+ T cells could be generated by help from the tumor-specific CD4+ T cells activated via DCs with an MHC class II/tumor antigenic peptide complexes that might had been provided from the CIITA-Exo. These antigen-specific T-cells are probably involved in the killing of tumor cells, and facilitated immune protection after vaccination. These results indicate that CIITA-dependent MHC class II expression induced a strong protective anti-tumor effects particularly at the level of CD4+Th cell triggering, necessary also for better induction of CD8+ CTLs. The exact molecular mechanism performed by CIITA-Exo will be further studied.
We tried test to know whether exosome have liver toxicity (data not shown). But liver toxicity by exosomes was not detected. Consequently we can use safely as vaccine.
Our study was distinguished from several studies reported by others that used tumor cells modified with CIITA gene as cancer vaccine (Armstrong et al., 1997; Mortara et al., 2009). By using exosomes, we could expect fewer side effects than when tumor cells themselves were used. In addition, we could take advantages of the immunostimulatory components in exosomes, which are enriched as compared to cells. This study demonstrated that not only melanoma could be treated with exosomes derived from the tumor cells, but also the therapeutic effect could be higher with exosomes derived from the tumor cells with CIITA gene introduced.
In conclusion, this study presented for the first time that exosomes from CIITA-introduced tumor cells have the elevated a strong protective antitumor immunity suggesting that MHC class II-containing tumor-derived exosomes (CIITA-Exo) have a cancer vaccine potency. We expect that this strategy will be a new preventive vaccine and adjuvant in cancer immunotherapy.
Methods
Cell line establishment
A murine melanoma cell line B16F1 (H-2b) were transduced with a mock or CIITA-inserted retrovirus kindly provided by Professor. K.C. Jung (Department of Pathology, Seoul National University College of Medicine, Seoul, Korea).
Exosome isolation and purification
Cells were cultured in antibiotics-containing DMEM supplemented with 10% fetal bovine serum previously centrifuged overnight at 100,000 µg to eliminate bovine-derived exosomes. Exosomes were isolated from cell culture supernatants by successive centrifugation (300 g for 5 min, 1,200 g for 20 min, 10,000 g for 30 min) and a final ultra-centrifugation step at 100,000 g for 1 hour, followed by resuspension in PBS. For further purification, exosomes were resuspended in 2.5 M sucrose in 20 mM Hepes buffer (pH 7.4) and were subsequently loaded on the bottom of a SW41 tube. Hepes buffer (20 mM) with 2 M sucrose followed by Hepes buffer (20 mM) with 0.25 M sucrose was carefully loaded on top of the exosomes to produce a discontinuous 2-0.25 M sucrose gradient. After centrifugation overnight at 100,000 µg in a SW41 swing rotor, 1 ml of each fraction was collected from the top of the tube.
Western blot analysis
Cells or exosomes lysed in lysis buffer (10 mM Triton X-100, pH 7.5, 150 mM NaCl) for 1 h on ice were centrifuged at 4℃, and protein level determination in supernatants was performed by use of the BCA method (Pierce, Rockford, IL). Proteins resolved by SDS-PAGE were transferred to a PVDF membrane (Millipore, Billerica, MA) that was then blocked with 5% skim milk in TTBS (10 mM Tris, 150 mM NaCl, 0.1% Tween 20), followed by incubation with primary antibodies and subsequent HRP-tagged secondary antibodies. The labeled proteins were visualized by use of an ECL detection system (Uppsala, Sweden, Amersham Biosciences).
Antibodies
Antibodies used in this study included purified forms of anti-tsg101, anti-calnexin, anti-TRP2, and anti-MHC class II (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies included HRP-tagged anti-mouse (Pierce), anti-rabbit and anti-goat (Santa Cruz Biotechnology) antibodies.
Antibodies used for flow cytometric analysis were FITC-conjugated anti-CD8, or anti-MHC class I, anti-MHC class II, anti-IFN-γ antibodies (Pharmingen, BD Biosciences, San Diego, CA), and H-2b/TRP2 tetramer (Proimmune, Bradenton, FL).
Isotyping of antibody production
All mice (5-6 weeks old) purchased from Charles River Laboratories (Yokohama, Japan) were housed in animal facilities regulated by the Experimental Animal Committee at Seoul National University. C57BL/6 mice (n = 5) were immunized three times by intradermal injection with PBS, Exo, or CIITA-Exo. Seven days after each immunization, serum samples were obtained from the retroorbital plexus and were pooled together. Ninety-six well plates coated with Exo (1 mg/ml) were blocked with 3% BSA in PBS for 2 h. Diluted serum in blocking buffer was added to each well and was incubated for 1 h. Either an anti-mouse IgG1 or IgG2 antibody (mouse isotyping kits, Sigma, St. Louis, MO) and subsequently, a biotin-tagged anti-mouse immunoglobulin antibody was incubated for 1 h, followed by the addition of HRP-conjugated streptavidin. TMB solution was added to the plates and the reaction was stopped by the addition of H2SO4 followed by the measurement of optical density at 450 nm.
Immune response monitoring (intracellular cytokine staining and tetramer assay)
C57BL/6 mice (n = 5 per group) were intradermally immunized with PBS, Exo, or CIITA-Exo three times in a two-week interval. Ten days after the last immunization, splenocytes were extracted from the respective groups and were pooled together, followed by the stimulation with irradiated B16F1 cells for 48 h. For intracellular cytokine staining, the stimulated splenocytes were treated with brefeldin A for 4 h, and then were surface-stained with FITC-conjugated anti-CD8 antibody. After 1 h-incubation on ice, the stained cells were fixed in 2% PFA-containing PBS followed by permeablization on ice with permeablization buffer (0.1% BSA, 0.01% saponin in PBS) and then were incubated with PE-tagged anti-IFN-γ antibodies. For tetramer assay, the stimulated splenocytes were stained with PE-conjugated H-2b/TRP2 tetramer (Proimmune) and FITC-tagged anti-CD8 antibodies. All the stained cells were analyzed with a Beckman Coulter FACScan (Epics XL; coulter, Marseille, France).
In vivo animal study
For preventive model, C57BL/6 mice (n = 10 per group) were intradermally immunized three times with either PBS, Exo (5 or 20 µg), or CIITA-Exo (5 or 20 µg), and challenged with subcutaneously-injected 2×105 B16F1 cells one week after the last immunization. For therapeutic model, approximately 100 mm2-size tumor-bearing C57BL/6 mice (n = 7 per group) by subcutaneous injection of 1×106 B16F1 cells were intradermally injected three times in a one-week interval with either PBS, Exo (20 µg), or CIITA-Exo (20 µg). Tumor size was measured twice a week and calculated by use of the following formula: (longer length × shorter length2)/2.
Statistical analysis
Statistical analyses were performed primarily with use of the Student's t-test with SigmaPlot 2001 software (Systat software, Chicago, IL). For the analysis of animal experiments, two-way ANOVA with Prism 3.0 software was utilized (GraphPad Software, La Jolla, CA). P-values of less than 0.05 were regarded as statistically significant.
Acknowledgements
This work was supported by The Ministry of Knowledge Economy (10035353) and partly supported by the Korean Science & Engineering Foundation (KOSEF) through the Tumor Immunity Medical Research Center (TIMRC) at Seoul National University College of Medicine.
Abbreviations
- CIITA
MHC class II transcriptional activator
- CTL
cytotoxic T lymphocytes
- Exo
exosomes
- Hsc70
heat shock cognate
- Hsp70
heat shock proteins
- MFI
mean fluorescence intensity
- MHC
major histocompatibility complex
References
- 1.André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 2.André F, Schartz NE, Chaput N, Flament C, Raposo G, Amigorena S, Angevin E, Zitvogel L. Tumor-derived exosomes: a new source of tumor rejection antigens. Vaccine. 2002;(Suppl 4):A28–A31. doi: 10.1016/s0264-410x(02)00384-5. [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong TD, Clements VK, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94:6886–6891. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Gritzapis AD, Sotiriadou R, Papamichail M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J Immunol. 2000;164:3902–3912. doi: 10.4049/jimmunol.164.7.3902. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 7.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW. Exosomes: a new delivery system for tumor antigens in cancer immunotherapy. Int J Cancer. 2005;114:613–622. doi: 10.1002/ijc.20757. [DOI] [PubMed] [Google Scholar]
- 9.Ciavarra RP. T helper cells in cytotoxic T lymphocyte development: analysis of the cellular basis for deficient T helper cell function in the L3T4-independent T helper cell pathway. Cell immunol. 1991;134:427–441. doi: 10.1016/0008-8749(91)90315-3. [DOI] [PubMed] [Google Scholar]
- 10.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 11.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 12.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Dorval T, Chaput N, André F, Caby MP, Novault S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao S, Moyana T, Xiang J. Review: cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm. 2007;22:692–703. doi: 10.1089/cbr.2007.368-R. [DOI] [PubMed] [Google Scholar]
- 15.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 16.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exo somes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 17.Kass R, Agha J, Bellone S, Palmieri M, Cané S, Bignotti E, et al. In vitro induction of tumor-specific HLA class I-restricted CD8+ cytotoxic T lymphocytes from patients with locally advanced breast cancer by tumor antigen-pulsed autologous dendritic cells. J Surg Res. 2003;112:189–197. doi: 10.1016/s0022-4804(03)00147-1. [DOI] [PubMed] [Google Scholar]
- 18.Kern DE, Klarnet JP, Jensen MC, Greenberg PD. Requirement for recognition of class II molecules and processed tumor antigen for optimal generation of syngeneic tumor-specific class I-restricted CTL. J Immunol. 1986;136:4303–4310. [PubMed] [Google Scholar]
- 19.Kleijmeer MJ, Ossevoort MA, van Veen CJ, van Hellemond JJ, Neefjes JJ, Kast WM, et al. MHC class II compartments and the kinetics of antigen presentation in activated mouse spleen dendritic cells. J Immunol. 1995;154:5715–5724. [PubMed] [Google Scholar]
- 20.Meazza R, Comes A, Orengo AM, Ferrini S, Accolla RS. Tumor rejection by gene transfer of the MHC class II transactivator in murine mammary adenocarcinoma cells. Eur J Immunol. 2003;33:1183–1192. doi: 10.1002/eji.200323712. [DOI] [PubMed] [Google Scholar]
- 21.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortara L, Frangione V, Castellani P. Irradiated CIITA-positive mammary adenocarcinoma cells act as a potent anti-tumor-preventive vaccine by inducing tumor-specific CD4+ T cell priming and CD8+ T cell effector functions. Int Immunol. 2009;21:655–665. doi: 10.1093/intimm/dxp034. [DOI] [PubMed] [Google Scholar]
- 23.Mortara L, Castellani P, Meazza R, Tosi G, De Lerma Barbaro A, et al. CIITA-induced MHC class II expression in mammary adenocarcinoma leads to a Th1 polarization of the tumor microenvironment, tumor rejection, and specific antitumor memory. Clin Cancer Res. 2006;12:3435. doi: 10.1158/1078-0432.CCR-06-0165. [DOI] [PubMed] [Google Scholar]
- 24.Nagarajan UM, Bushey A, Boss JM. Modulation of gene expression by the MHC class II transactivator. J Immunol. 2002;169:5078–5088. doi: 10.4049/jimmunol.169.9.5078. [DOI] [PubMed] [Google Scholar]
- 25.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shortman K, Lahoud MH, Caminschi I. Improving vaccines by targeting antigens to dendritic cells. Exp Mol Med. 2009;41:61–66. doi: 10.3858/emm.2009.41.2.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skokos D, Goubran-Botros H, Roa M, Mecheri S. Immunoregulatory properties of mast cell-derived exosomes. Mol Immunol. 2002;38:1359–1362. doi: 10.1016/s0161-5890(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 28.Takenoyama M, Yoshino I, Eifuku R, So T, Imahayashi S, Sugaya M, et al. Successful induction of tumor-specific cytotoxic T lymphocytes from patients with non-small cell lung cancer using CD80-transfected autologous tumor cells. Jpn J Cancer Res. 2001;92:309–315. doi: 10.1111/j.1349-7006.2001.tb01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 30.Théry C, Duban L, Segura E, Véron P, Lantz O, Amigorena S. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 31.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JA, Dissanayake SK, Ksander BR, Knutson KL, Disis ML, Ostrand-Rosenberg S. Tumor cells transduced with the MHC class II transactivator and CD80 activate tumor-specific CD4+ T cells whether or not they are silenced for invariant chain. Cancer Res. 2006;66:1147. doi: 10.1158/0008-5472.CAN-05-2289. [DOI] [PubMed] [Google Scholar]
- 33.Thompson JA, Srivastava MK, Bosch JJ, Clements VK, Ksander BR, Ostrand-Rosenberg S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol Immunother. 2008;57:389–398. doi: 10.1007/s00262-007-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 35.Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 36.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Möbius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 37.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]