Abstract
Within the past decade, it has been recognized that a majority of patients with essential mixed cryoglobulinemia (MC) are chronically infected with hepatitis C virus (HCV). Although the underlying mechanisms have not been fully elucidated, cryoglobulin formation is clearly linked to the attempt of the host to clear the significant quantities of virions generated daily by the chronic infection. This review summarizes the current understanding of the relationship between chronic HCV infection and the development of MC, and discusses the interaction between the immune system and HCV and how this interaction can lead to the development of lymphoproliferative disorders.
Keywords: hepatitis C virus, B lymphocytes, cryoglobulinemia
Hepatitis C virus (HCV) is a small single-stranded RNA virus with a prevalence of approximately 170 million people worldwide.1 It affects approximately 3.2 million Americans.2 Chronic infection with HCV causes the progression of liver disease from chronic hepatitis to cirrhosis, and liver failure, as well as possible development of hepatocellular carcinoma. Chronic HCV infection is now the leading indication for liver transplantation in the United States. Although much of the focus in chronic HCV infection has been centered on hepatic sequelae, it is now apparent that clinically significant extrahepatic manifestations are important in some patients.3
Within the past decade, it has been recognized that a majority of patients with essential mixed cryoglobulinemia (MC) are chronically infected with HCV.4,5 Although the underlying mechanisms have not been not fully elucidated, cryoglobulin formation is clearly linked to the attempt of the host to clear the significant quantities of virions generated daily by the chronic infection.6 Thus, management of these patients has focused on either the reduction of virus production, the elimination of immune complexes, the function of B cells, or the suppression of the inflammatory response of the host to the cryoglobulin immune complex.
This review summarizes the current understanding of the relationship between chronic HCV infection and the development of MC. We discuss the interaction between the immune system and HCV and how this interaction can lead to the development of lymphoproliferative disorders. As more is learned about the key players in the immune response to chronic HCV infection, we are better able to target specific participants in this pathway to treat the clinical manifestations of this disease.
Cryoglobulins and Cryoglobulinemia
Cryoglobulins are serum proteins that precipitate at low temperatures and then redissolve during incubation at 37°C.7,8 The temperature at which the proteins precipitate is determined by the thermal amplitude.9 This in-vitro phenomenon can be observed in a spectrum of hematologic, infectious, and rheumatologic disorders.8
Different categories of cryoglobulins have been described that refer to their different immunologic compositions, and different clinical syndromes (cryoglobulinemias) have been attributed to their occurrence. Type I cryoglobulins are complexes of a single monoclonal immunoglobulin (Ig), usually IgM. They account for 10–15% of cryoglobulinemia syndromes.7,8 Type I cryoglobulinemia is mainly found in patients with hematologic malignancies such as Waldenstrom macroglobulinemia and multiple myeloma. The clinical findings are those of increased serum viscosity, due to the fact that IgM is a relatively large molecule and the formation of large IgM complexes may lead to peripheral vessel occlusion, manifesting as stroke, Raynaud phenomenon, or ischemic limb ulcers.7
Type II and type III cryoglobulins constitute MC. Type II is composed of polyclonal IgG and monoclonal IgM. Type III is composed of polyclonal IgG and/or polyclonal IgM. Type II accounts for 50–60% of cryoglobulinemias, whereas type III accounts for 30–40%. The IgG component is always polyclonal with both kappa and lambda light chains. The IgM fraction in type II mounts kappa chains and usually possesses rheumatoid factor (RF) activity. The RF activity allows the IgM component to bind intact IgG at both the Fc and Fab fragments, conferring stability of circulating IgG-IgM immune complexes.7,10 The ability to bind the Fc fragment of autologous IgG confers rheumatoid activity.
MC is marked by a systemic vasculitis that damages small- and medium-sized arteries and veins of many different organs. The immune complexes, composed of different cryoglobulins, deposit in the vessel walls. Through the complement system, they mediate intense activation of the inflammatory response.11 Clinically, this systemic vasculitis can manifest as myriad symptoms, ranging from mild to severe, including purpuric skin eruptions that may become ischemic ulcers, polyneuropathy, glomerulonephritis, Raynaud phenomenon, and arthralgias. Despite the occasional association of this clinical spectrum with autoimmune, hematologic, or infectious (eg, hepatitis B virus [HBV]) diseases, it has historically arisen without any predisposing disorder and was thus termed “essential MC.” With the discovery of HCV in 1989, it quickly became evident that chronic infection with HCV accounted for at least 90% of cases of MC.4,5 Supporting the etiologic role of HCV, studies have demonstrated the presence of HCV antibody and HCV RNA in higher concentrations within the immune complexes than in the sera of MC patients.4
Pathogenesis
Hepatitis C Virus, Essential Mixed Cryoglobulinemia, and Cryoglobulinemic Vasculitis
Although overt symptoms of cryoglobulinemic vasculitis develop in only approximately 5% of chronic HCV infection cases,12 circulating mixed cryoglobulin complexes are much more common and are detected with a prevalence of40–50% in chronic HCV-infected patients. Circulating immune complexes comprise HCV virions with low- or very low-density lipoproteins,13 IgG-IgM-RF antibody complexes, and complement. Significant geographic diversity appears among patients with HCV-related cryoglobulinemia, with greater prevalence in southern Europe compared to northern Europe and North America. It is not clear whether this variability is related to differences in host or virus, or whether it reflects differences in the ability of local laboratories to detect cryoglobulins.
Hepatitis C Virus-specific Effects on B Cells
A common hypothesis for HCV-related cryoglobulinemia is chronic antigenic stimulation of the humoral immune system, which facilitates clonal B-lymphocyte expansion.10 However, other chronic viral infections, including HBV, are not associated with the same high prevalence.10 Furthermore, the B lymphocytes that accumulate in peripheral blood in patients with HCV-related cryoglobulinemia consist of a naive, resting phenotype without evidence of activation.14 Nevertheless, global B-cell stimulation may still prove to be fundamental to HCV-related cryoglobulinemia.
A number of other possibilities have been proposed to explain the effects of chronic HCV infection on B cells. Peripheral blood mononuclear cells in patients chronically infected with HCV, and especially in those with cryoglobulinemic vasculitis, show a t(14:18) translocation that is responsible for Bcl-2 activation.15 This proto-oncogene increases B-cell survival by inhibiting apoptosis and could lead to increased B-cell quantities. The consequent expansion of B lymphocytes could explain the increased production of autoantibodies and cryoglobulins.
The HCV E2 envelope protein binds to the cell surface glycoprotein CD81 that is present on B cells as well as on hepatocytes. Interaction with HCV E2 reduces the threshold for B-cell activation and might increase the frequency of VDJ rearrangement in antigen-reactive B cells.
HCV-specific proteins also demonstrate molecular mimicry. NS5A and NS core proteins can simulate host autoantigens, possibility resulting in B-lymphocyte activation and autoantibody production.
Effector signals that enhance survival of immunocompetent cells are the subject of current major research. A newly described cytokine, B-cell activating factor of the tumor necrosis factor family (BAFF)—also known as B-lymphocyte stimulator,16 THANK, TALL-1, and zTNF4—may be important in HCV-related cryoglobulinemia, as well as several systemic autoimmune diseases, including rheumatoid arthritis (RA), primary Sjogren's syndrome (pSS), and systemic lupus erythematosus (SLE). BAFF mRNA is present mainly in lymphoid tissue and is expressed by monocytes, macrophages, dendritic cells, and growth factor-stimulated neutrophils on exposure to interferons or CD40 ligand.17 BAFF mRNA, however, is absent from B cells.18,19 BAFF binds to three receptors selectively expressed by B cells: B-cell maturation antigen; transmembrane activator, calcium modulator, and cyclophilin ligand interactor; and BAFF receptor. BAFF has several effects on B cells: it plays a critical role in B-cell maturation,20 in long-lived bone marrow plasma cell survival,21 in the promotion of humoral immune response,22 and in CD40-independent immunoglobulin class-switch recombination.17
Autoreactive B cells are more dependent on BAFF for survival than alloreactive B cells.23–25 Because autoreactive B cells are associated with the development of autoimmune disorders such as SLE, RA, and pSS, as well as cryoglobulinemia, BAFF levels may be significant in the pathogenesis of these disorders. The recent demonstration of increased serum BAFF levels in SLE, RA, and pSS,26–29 and, in at least two recent studies, elevated serum levels in chronic HCV patients compared to controls and patients with chronic HBV infection,30,31 strongly suggests that BAFF plays a role in HCV-related cryoglobulinemia. Interestingly, serum BAFF levels were even higher in those patients with chronic HCV infection and symptoms indicative of systemic vasculitis or mixed cryoglobulinemia, HCV-MC.30–32 Levels were also higher in type II than type III HCV-MC.30
Source of Hepatitis C Virus—related Mixed Cryoglobulins
Expansion of RF-synthesizing B cells appears to be a hallmark of HCV-MC. RFs are tolerance-resistant autoantibodies directed against the Fc regions of IgG molecules. They appear to have a physiologic immunoregulatory function in healthy individuals and transiently increase in chronic inflammatory diseases, implying a normal response to immune complexes. Continued production in disease states such as RA suggests a pathogenic significance.33
Analysis of the Ig variable (IgV) gene in RF B cells in HCV-MC suggests antigen-driven expansion. IgV heavy and light chains are heavily mutated, suggesting germinal-center derivation. Most B-cell expansions display a complementarity determining region-3 (CDR-3) homologous to RF-CDR-3, suggesting derivation from a precursor strongly selected for auto-IgG specificity.
The B-cell receptor (BCR) ligand in HCV-MC could potentially develop in one of at least two ways. First, molecular mimicry due to shared epitopes between HCV and IgG-Fc domains may allow cross-reaction between a virus-associated immunodominant epitope and IgG autoantigen. Evidence for this has been demonstrated in several MC patients between HCV-NS3 and IgG-Fc.34 Immunization with immune complexes can also result in generation of T-cell-dependent RF with high affinity.35 Repetitive stimulation of RF-BCR by continuous production of IgG-HCV complexes could, thus, induce secretion of RF-IgM.36
In either event, the interaction between HCV and B and T cells is essential for the clearance of the more than 1 trillion copies of virus produced daily6 and is likely to generate significant amounts of immune complexes that could lead to stimulation of high-affinity RF, as previously described. Polyclonal activation of CD5-positive cells are regarded as the major source of the IgM-RF molecule in type III MC.37 Emergence of a single dominant clone could explain type II.
Association with Non-Hodgkin Lymphoma
The above discussion highlights the profound impact that chronic HCV infection has on B-cell stimulation and survival. Chronic HCV infection and HCV-associated MC have been closely associated with the development of non-Hodgkin lymphoma (NHL).36,38–41 A recent study demonstrated a prevalence of 10% in NHL after a 10-year follow-up of a cohort of MC patients.42 NHL that arises in cryoglobulinemic patients is often a low-grade lymphoma with bone marrow involvement, but can develop into a high-grade lymphoma phenotype. Extranodal involvement is common in the liver, salivary glands, and spleen. Regression following successful antiviral therapy of HCV with interferon has served to further strengthen the association.43 Although the driving force behind lymphoproliferation in chronic HCV infection is unclear, increased levels of BAFF may play a role. Mice transgenic for BAFF develop lymphocytic disorders, including lymphomas.44
Clinical and Laboratory Manifestations of Cryoglobulinemia
HCV-associated cryoglobulinemic vasculitis manifests as a multitude of clinical symptoms affecting many different organs. Serum cryoglobulin or complement levels do not tend to correlate with disease severity. Typically, complement levels are low. It is generally assumed that this reflects systemic activation of the complement system with consumption of its components CH50, C4, C1q, and a smaller decrease of C3.8 Tests for antinuclear, smooth-muscle, mitochondrial, and antineutrophil cytoplasmic antibodies are generally negative, whereas tests for RF are typically positive.
Target Organ Involvement in Hepatitis C Virus-Mixed Cryoglobulinemia
Cutaneous and Joint Involvement
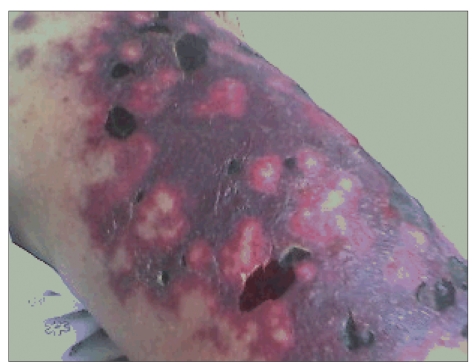
Purpura is the most common clinical manifestation of MC, occurring in at least 90% of cases. Skin lesions often begin in the legs and may progress to deep ulcers in approximately 10% of patients.8 Purpuric lesions can also be found on the buttocks, trunk, and upper extremities, but they rarely involve the face.7 Itching is rare. Skin eruptions can be intermittent, and repeated episodes can lead to hyperpigmentation of the involved skin, whereas ulcers usually appear above the malleoli. Purpura is often preceded by paresthesiae or a prickling sensation in the involved limb.7 Progression of skin lesions to frank skin necrosis and gangrene can occur, but is less common (Figure 1).
Figure 1.
Cutaneous necrotizing vasculitis consistent with cryoglobulinemic vasculitis on histologic examination in a chronic hepatitis C virus patient with a cryocrit measuring 42%.
Raynaud phenomenon occurs in up to one third of cases and involves hands, feet, lips, ears, and the tip of the nose. Arthralgia without arthritis is commonly experienced, typically affecting the proximal interphalangeal joints of the hands, metacarpophalangeal joints, knees, and hips.7 Sicca syndrome can be experienced clinically without the presence of autoantibodies often seen in Sjogren's syndrome.
Neurologic Involvement
The neurologic manifestation of MC is most frequently peripheral neuropathy, commonly polyneuropathy, reportedly occurring in 36–86% of patients.7,45 The pathogenic mechanism of peripheral nerve damage has been postulated to be vasculitis of the vasa nervorum, as well as autoimmune nerve damage, with cases of demonstrated demyelination.46 Abnormalities on electrophysiologic studies can be readily detected and can often be progressive with involvement of new areas over time, even if overall cryoglobulin levels decline with therapy.45 There is no clear central nervous system involvement. However, cognitive function has been shown to be decreased in MC patients relative to nonaffected HCV patients.47 Abnormalities in periventricular white matter were more prevalent on brain magnetic resonance imaging of MC patients than unaffected HCV patients and healthy controls.
Renal Involvement
Renal impairment in MC is often due to membranoproliferative glomerulonephritis (MPGN) and is clinically evident in 20–30% of MC patients.7,8,10 Renal biopsy often demonstrates glomerular subendothelial deposits characteristic of type I MPGN; by immunofluorescence, these deposits are typically found to be composed of IgM, IgG, and C3.48 These subendothelial deposits are identical to the circulating complexes found in patients with type II MC.49 Other patterns on biopsy that have been described include subepithelial deposits typical of type III MPGN, membranous nephropathy, renal artery vasculitis, and tubulointerstitial inflammation and scarring.48,49
The clinical symptoms and signs of MC can precede the onset of renal involvement for years. The most common clinical findings from renal disease in MC are proteinuria with microscopic hematuria, moderate renal insufficiency, and hypertension. However, acute onset, marked by a sudden rise in creatinine with severe proteinuria, is the first renal manifestation in approximately 25% of patients.49 Oliguria and dialysis requirement occur in a small minority of patients. HCV-associated MPGN can develop following liver and renal transplantation.48 A recent report from a large Italian center showed age, serum creatinine, and proteinuria as independent risk factors for the development of progressive renal failure at follow-up.50 Renal involvement is commonly the greatest cause of morbidity in MC and often justifies more aggressive treatment of the disease.
Endocrine Dysfunction
A variety of endocrine gland dysfunctions have been described to be more common in patients with HCV-related MC than in age- and sex-matched controls.11 The most well-described disorders are type II diabetes, hypothyroidism with positive antithyroid antibodies, and gonadal dysfunction.
Correlation With Liver Disease Severity
MC tends to correlate with duration of HCV infection and older age. However, cryoglobulinemia and/or the presence of detectable cryoglobulins in the serum of HCV patients has been associated with increased risk of advanced fibrosis and cirrhosis in patients with chronic HCV infection, irrespective of age or disease duration.51–53 The presence of serum cryoglobulins has been shown to correlate with the severity of hepatic steatosis on liver biopsy.53
Complement, Symptomatic Hepatitis C Virus—Mixed Cryoglobulinemia, and the Liver
Complement proteins are produced constitutively by macrophages and hepatocytes, and are differentially expressed in patients with chronic HCV and chronic HCV-related hepatocellular cancer.54 Hypocomplementemia has been described in association with hepatitis C viremia in healthy blood donors.55 Thus, complement may be the link between liver disease severity and HCV-MC.
The role of complement in the solubilization of immune complexes was first described by Miller and Nussenzweig.56 Complement solubilization is primarily mediated via the alternative pathway, but factors in the classic pathway accelerate the process. Components of the membrane attack complex are not involved. Through the alternative pathway, C3b is deposited over antigen-antibody complexes, breaking down peptide bonds that are associated with precipitation. As a result, small immune complexes are formed that are rich in C3b, which then degrades to inactive, soluble C3bi. Thus, the resulting soluble complexes are less able to bind to C3b receptors or set up inflammatory reactions. The complement-solubilized immune complexes that lose their binding affinity for C3b receptors and Fc receptors are able to bind to macrophages via C3bi receptors. These complement-solubilized immune complexes are removed from circulation by Kupffer cells in the liver.57–59 There is evidence to suggest that IgG-antigen complexes that are not solubilized by complement are deposited outside the reticulo-endothelial system.60 This may be especially relevant in patients with compromised or deficient complement function58 and has been demonstrated in patients with chronic renal failure on hemodialysis.61
Complement solubilization of immune complexes could provide an explanation for the observation that symptomatic HCV-MC correlates with advanced liver disease when synthesis by hepatocytes might be expected to be low. Furthermore, reduced HCV clearance might increase de novo infection and the infected hepatocyte mass.
Mannan-binding lectin (MBL) is a pattern recognition molecule that binds micro-organisms via interactions with glycans on the target surface. Bound MBL subsequently activates MBL-associated serine protease proenzymes (MASPs) and can activate complement via a specific MBL pathway.62 A role for MBL in HCV infection has been indicated by studies examining MBL levels and polymorphisms in relation to disease progression and treatment response. HCV encodes two highly glycosylated envelope proteins, E1 and E2, which are potential targets for interaction with MBL. Mutant MBL2 haplotypes have been linked to disease progression and response to therapy in HCV infection. Median values of MBL/MASP-1 complex activity were higher in sera from patients with liver disease than in healthy controls. MBL/MASP-1 complex activity levels correlated with severity of fibrosis after adjusting for confounding factors (P=.003). MBL/MASP-1 complex activity was associated more significantly with fibrosis than MBL concentration. The potential role of MBL/MASP-1 complex activity in HCV disease progression may reflect the importance of this pathway in the clearance of HCV and Ig-HCV immune complexes.63,64
We recently analyzed clinical data from 43 patients with HCV-related cryoglobulinemia who presented to our large hepatitis C referral center.65 These patients were identified from one or more of the standard clinical triad: purpura, neuropathy, or polyarthralgias. In several patients, cryoglobulinemia was detected only after repeat testing at a number of different laboratories, reflecting the high frequency of errors in serum collection and preparation in the detection of cryoglobulins in clinical samples. A separate study found the most common laboratory errors in the detection of cryoglobulins to be loss of cryoglobulins due to failure of proper separation of serum from whole blood, loss of cryoprecipitate due to refrigeration before centrifugation, and an inadequate volume of serum for testing cryoglobulins at low levels.9 Our study confirmed the presence of advanced cirrhosis or fibrosis, which have been frequently reported in these patients.12,51,66 Our study also underscored the very low complement levels associated with symptomatic HCV-MC.
Treatment Options
Therapy should be initiated for patients with symptomatic MC, and is directed both at the virus and at the immunemediated inflammation. Patients with mild to moderate cryoglobulinemic symptoms (purpura, arthralgias, peripheral sensory neuropathy) are typically managed with low doses of steroids. More severe manifestations, including skin ulcers, motor neuropathy, glomerulonephritis, and disseminated vasculitis, warrant rapid and aggressive therapy to control the widespread immune devastation. Initial treatment with high-dose steroids is often initiated along with plasma exchange as a more attractive option to immunosuppression with agents such as cyclophosphamide.67 Plasma exchange works rapidly and effectively at halting the progression of several of the severe manifestations of cryoglobulinemic vasculitis.11 Plasma exchange is often used repeatedly in the first few weeks of therapy but is not a good long-term option, given the need for continued intravascular access and its inherent complications.
Antiviral Therapy
Although steroids and plasma exchange are effective at gaining initial control of systemic inflammation, therapy directed against HCV is the mainstay of long-term control of both the hepatic and extrahepatic manifestations of chronic infection with HCV. Antiviral therapy should be started after systemic vasculitis has come under initial control with immunosuppression and/or plasma exchange, as the disease can be exacerbated with the initiation of interferon (IFN) therapy in some patients.68,69 Increased BAFF levels due to IFN provides a ready explanation for these observations.17
IFN alfa monotherapy can reduce viral load and induce clinical improvement in MC. However, relapse within a few months of therapy withdrawal is common.70 Therapy with pegylated IFN (PEG-IFN) and ribavirin has significantly increased sustained virologic response (SVR) to therapy.71,72 In general, patients with HCV-MC who achieve SVR also achieve prolonged clinical remission. However, at the end of a 6-month follow-up study, several patients developed asymptomatic relapse in their cryocrit. This cohort of patients had a mix of HCV genotypes, leading to an overall SVR rate that was lower than the rate found in non-MC HCV patients.73 A second author found both SVR and subsequent clinical remission rates that appeared superior to conventional SVR rates for HCV patients without HCV-MC.74 These patients were treated with a higher dose of PEG-IFN alfa-2b for approximately 6 weeks longer, which may have contributed to the higher SVR rate. One patient who relapsed was controlled again with re-initiation of therapy. Several patients who achieved SVR and clinical remission still had cryoglobulins detected at the end of the study. It is not clear at this time whether PEG-IFN and RBV therapy can produce long-term remission of HCV-MC and whether achieving SVR means that patients are free from the risk of relapse of their HCV-MC symptoms.
Rituximab
Rituximab (Rituxan, Genentech) is a humanized murine monoclonal antibody that binds to CD20, a transmembrane protein expressed on the surface of pre-B lymphocytes and mature lymphocytes. Potential mechanisms that govern rituximab elimination of B cells include antibody-dependent cellular cytotoxicity, complementdependent cytotoxicity, and apoptosis. Treatment with rituximab at a dose of 375 mg/m2 weekly for 4 consecutive weeks results in B-cell reduction to less than 1% of peripheral mononuclear blood cells, with recovery of B-cell counts approximately 6–9 months after therapy.75 Rituximab has become part of the standard treatment regimens used in a variety of B-cell NHL and chronic lymphocytic leukemia therapies.76 The B cells targeted by rituximab are in late pre-B-cell development and do not include plasma cells not expressing CD20.77 Elimination of these cell lines proves particularly effective in reducing IgM production.78 Thus, rituximab has been used as an agent to target autoimmune diseases in which antibodies, and especially RF activity, play a pivotal role in pathogenesis.78–80
The safe and effective use of rituximab in MC associated with chronic HCV infection has been demonstrated in a number of studies (Table 1).75,81–85 In one study, rituximab induced complete or partial response in most symptom categories evaluated, with the majority of patients experiencing clinical benefit.82 Therapy with rituximab also allowed most patients to discontinue maintenance therapy with corticosteroids. In another study, 80% of patients achieved complete response within 4 months of rituximab therapy, with their skin, joint, and neuromuscular symptoms showing strong response to treatment. The only patient with renal involvement in this study did not respond.75 A high response rate was observed in a third study that involved a small number of HCV-MC patients with glomerulonephritis who received rituximab.83 Most of the patients in the above studies experienced durable clinical remission. The average time to relapse was approximately 7 months.83
Table 1.
Studies Using Rituximab As Therapy for Mixed Cryoglobulinemia
| Study | Number of patients (n) | Outcomes |
|---|---|---|
| Sansonno et al.75 | 20 |
|
| Lamprecht et al.81 | 1 |
|
| Zaja et al.82 | 15 |
|
| Roccatello et al.83 | 6 |
|
| Basse et al.84 | 7 |
|
| Quartuccio et al.85 | 5 |
|
Complete response (CR) defined as reduction of cryocrit to less than 75% of initial value and at least two of the following: disappearance of purpura or arthralgia, or weakness or improvement of neuropathy.
- IFN
interferon
- RBV
ribavirin
- NHL
non-Hodgkin's lymphoma
- PEG
pegylated
- HCV
hepatitis C virus
- MPGN
membranous proliferative glomerulonephritis
- BM
basement membrane
- PR
partial response.
Significant reductions in serum levels of IgM, cryoglobulins, and RF were demonstrated with a rise in C4 levels, indicating reduced formation of immune complexes. No significant changes in IgG or IgA levels were noted. These results are consistent with rituximab's lack of effect on plasma cells, the main source of IgG and IgA in serum. This pattern of immunologic response has also been described in other series of HCV-MC patients treated with rituximab.76,83
Several studies have demonstrated increased HCV viral levels with rituximab therapy, with some patients experiencing small rises in serum aminotransferases.75,82,86 However, a brief report failed to demonstrate a significant increase.83 Complications of NHL therapy with rituximab, manifested by increased levels of HCV RNA in blood have been recently reported.87 Nevertheless, the authors concluded that rituximab was safe in HCV-MC patients. One patient developed retinal artery thrombosis,82 leading the other investigators to treat the patients on rituximab with aspirin.83 Two cases of disseminated opportunistic infections (which resulted in 1 death) in immunosuppressed renal transplant patients who received rituximab as rescue therapy for MC have been reported.84 Although effective at controlling the systemic phenomena of MC, rituximab should be used with caution in patients already taking significant immunosuppressive therapy, such as in the posttransplant setting.
Several recent reports regarding HBV virus reactivation during rituximab therapy suggest that caution may be warranted in patients with markers of current or past HBV infection, including antibody to hepatitis B surface antigen.88–98 Concomitant antiviral therapy for HBV in at-risk patients may prevent reactivation.89 The use of rituximab in 2 patients with SLE has been recently linked to the development of progressive multifocal leukoencephalopathy.99 These reports also highlight a potential role for B cells in the control of other chronic viral infections.
References
- 1.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(suppl 1):62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 4.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- 5.Misiani R, Bellavita P, Fenili D, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 6.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-a therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 7.Dammacco F, Sansonno D, Piccoli C, et al. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628–638. doi: 10.1046/j.1365-2362.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 8.Morra E. Cryoglobulinemia. Hematology Am Soc Hematol Educ Program. 2005:368–372. doi: 10.1182/asheducation-2005.1.368. [DOI] [PubMed] [Google Scholar]
- 9.Kallemuchikkal U, Gorevic PD. Evaluation of cryoglobulins. Arch Pathol Lab Med. 1999;123:119–125. doi: 10.5858/1999-123-0119-EOC. [DOI] [PubMed] [Google Scholar]
- 10.Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4–13. doi: 10.1136/jcp.55.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferri C, Mascia MT. Cryoglobulinemic vasculitis. Curr Opin Rheumatol. 2006;18:54–63. doi: 10.1097/01.bor.0000198002.42826.c2. [DOI] [PubMed] [Google Scholar]
- 12.Lunel F, Musset L, Cacoub P, et al. Cryoglobulinemia in chronic liver diseases: role of hepatitis C virus and liver damage. Gastroenterology. 1994;106:1291–1300. doi: 10.1016/0016-5085(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang AC, Wells JV, Fudenberg HH. Chemical analyses of cryoglobulins. Immunochemistry. 1974;11:341–345. doi: 10.1016/0019-2791(74)90186-4. [DOI] [PubMed] [Google Scholar]
- 14.Ni J, Hembrador E, Di Bisceglie AM, et al. Accumulation of B lymphocytes with a naive, resting phenotype in a subset of hepatitis C patients. J Immunol. 2003;170:3429–3439. doi: 10.4049/jimmunol.170.6.3429. [DOI] [PubMed] [Google Scholar]
- 15.Zignego AL, Ferri C, Giannelli F, et al. Prevalence of bcl-2 rearrangement in patients with hepatitis C virus-related mixed cryoglobulinemia with or without B-cell lymphomas. Ann Intern Med. 2002;137:571–580. doi: 10.7326/0003-4819-137-7-200210010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 17.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–265. doi: 10.1159/000082106. [DOI] [PubMed] [Google Scholar]
- 19.Mackay F, Mackay CR. The role of BAFF in B-cell maturation, T-cell activation and autoimmunity. Trends Immunol. 2002;23:113–115. doi: 10.1016/s1471-4906(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 20.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor BP, Raman VS, Erickson LD, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Do RK, Hatada E, Lee H, et al. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigenengaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 25.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Ittah M, Miceli-Richard C, Eric Gottenberg J, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjogren's syndrome. Arthritis Res Ther. 2006;8:R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stohl W, Metyas S, Tan SM, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 29.Tan SM, Xu D, Roschke V, et al. Local production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritis. Arthritis Rheum. 2003;48:982–992. doi: 10.1002/art.10860. [DOI] [PubMed] [Google Scholar]
- 30.Sene D, Ghillani-Dalbin P, Thibault V, et al. Long-term course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31:2199–2206. [PubMed] [Google Scholar]
- 31.Toubi E, Gordon S, Kessel A, et al. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27:134–139. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Fabris M, Quartuccio L, Sacco S, et al. B-lymphocyte stimulator (BLyS) upregulation in mixed cryoglobulinaemia syndrome and hepatitis-C virus infection. Rheumatology (Oxford) 2007;46:37–43. doi: 10.1093/rheumatology/kel174. [DOI] [PubMed] [Google Scholar]
- 33.Milosevic-Jovcic N, Ciric D, Hajdukovic-Dragojlovic L, Mircetic V. Differences in the relationship of specificity to titre and functional affinity between circulating Ga- and pan-reactive IgM rheumatoid factors in rheumatoid arthritis. Rheumatology (Oxford) 2004;43:1190–1193. doi: 10.1093/rheumatology/keh287. [DOI] [PubMed] [Google Scholar]
- 34.De Re V, Sansonno D, Simula MP, et al. HCV-NS3 and IgG-Fc crossreactive IgM in patients with type II mixed cryoglobulinemia and B-cell clonal proliferations. Leukemia. 2006;20:1145–1154. doi: 10.1038/sj.leu.2404201. [DOI] [PubMed] [Google Scholar]
- 35.Fehr T, Bachmann MF, Bucher E, et al. Role of repetitive antigen patterns for induction of antibodies against antibodies. J Exp Med. 1997;185:1785–1792. doi: 10.1084/jem.185.10.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dammacco F, Sansonno D, Piccoli C, et al. The lymphoid system in hepatitis C virus infection: autoimmunity, mixed cryoglobulinemia, and Overt B-cell malignancy. Semin Liver Dis. 2000;20:143–157. doi: 10.1055/s-2000-9613. [DOI] [PubMed] [Google Scholar]
- 37.Curry MP, Golden-Mason L, Doherty DG, et al. Expansion of innate CD5pos B cells expressing high levels of CD81 in hepatitis C virus infected liver. J Hepatol. 2003;38:642–650. doi: 10.1016/s0168-8278(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 38.De Rosa G, Gobbo ML, De Renzo A, et al. High prevalence of hepatitis C virus infection in patients with B-cell lymphoproliferative disorders in Italy. Am J Hematol. 1997;55:77–82. doi: 10.1002/(sici)1096-8652(199706)55:2<77::aid-ajh5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri F, Sperotto A, Fanin R. Hepatitis c and lymphoma. Curr Oncol Rep. 2000;2:172–175. doi: 10.1007/s11912-000-0090-0. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri F, Barillari G, Fanin R, et al. Hepatitis C virus infection among cryoglobulinemic and non-cryoglobulinemic B-cell non-Hodgkin's lymphomas. Haematologica. 1997;82:314–317. [PubMed] [Google Scholar]
- 41.Mazzaro C, Zagonel V, Monfardini S, et al. Hepatitis C virus and non-Hodgkin's lymphomas. Br J Haematol. 1996;94:544–550. doi: 10.1046/j.1365-2141.1996.6912313.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferri C, Sebastiani M, Giuggioli D, et al. Mixed cryoglobulinemia: demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum. 2004;33:355–374. doi: 10.1016/j.semarthrit.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Hermine O, Lefrère F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 44.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammendola A, Sampaolo S, Ambrosone L, et al. Peripheral neuropathy in hepatitis-related mixed cryoglobulinemia: electrophysiologic follow-up study. Muscle Nerve. 2005;31:382–385. doi: 10.1002/mus.20184. [DOI] [PubMed] [Google Scholar]
- 46.Boukhris S, Magy L, Senga-mokono U, et al. Polyneuropathy with demyelinating features in mixed cryoglobulinemia with hepatitis C virus infection. Eur J Neurol. 2006;13:937–941. doi: 10.1111/j.1468-1331.2006.01416.x. [DOI] [PubMed] [Google Scholar]
- 47.Carbonari M, Caprini E, Tedesco T, et al. Hepatitis C virus drives the unconstrained monoclonal expansion of VH1-69-expressing memory B cells in type II cryoglobulinemia: a model of infection-driven lymphomagenesis. J Immunol. 2005;174:6532–6539. doi: 10.4049/jimmunol.174.10.6532. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RJ, Willson R, Yamabe H, et al. Renal manifestations of hepatitis C virus infection. Kidney Int. 1994;46:1255–1263. doi: 10.1038/ki.1994.393. [DOI] [PubMed] [Google Scholar]
- 49.D'Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 50.Roccatello D, Fornasieri A, Giachino O, et al. Multicenter study on hepatitis C virus-related cryoglobulinemic glomerulonephritis. Am J Kidney Dis. 2007;49:69–82. doi: 10.1053/j.ajkd.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36((4 pt 1)):978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- 52.Siagris D, Christofidou M, Tsamandas A, et al. Cryoglobulinemia and progression of fibrosis in chronic HCV infection: cause or effect? J Infect. 2004;49:236–241. doi: 10.1016/j.jinf.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Saadoun D, Asselah T, Resche-Rigon M, et al. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology. 2006;43:1337–1345. doi: 10.1002/hep.21190. [DOI] [PubMed] [Google Scholar]
- 54.Lee IN, Chen CH, Sheu JC, et al. Identification of complement C3a as a candidate biomarker in human chronic hepatitis C and HCV-related hepatocellular carcinoma using a proteomics approach. Proteomics. 2006;6:2865–2873. doi: 10.1002/pmic.200500488. [DOI] [PubMed] [Google Scholar]
- 55.Itoh K, Tanaka H, Shiga J, et al. Hypocomplementemia associated with hepatitis C viremia in sera from voluntary blood donors. Am J Gastroenterol. 1994;89:2019–2024. [PubMed] [Google Scholar]
- 56.Miller GW, Nussenzweig V. A new complement function: solubilization of antigen-antibody aggregates. Proc Natl Acad Sci U S A. 1975;72:418–422. doi: 10.1073/pnas.72.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi N, Fujita T, Takata Y, Tamura N. Interaction of complement solubilized complexes with mouse peritoneal macrophages and their clearance and tissue uptake. Clin Exp Immunol. 1985;61:176–182. [PMC free article] [PubMed] [Google Scholar]
- 58.Petersen I, Baatrup G, Jepsen HH, Svehag SE. Complement-mediated solubilization of immune complexes and their interaction with complement C3 receptors. Complement. 1985;2:97–110. doi: 10.1159/000467850. [DOI] [PubMed] [Google Scholar]
- 59.Sanchez-Cuenca JM. Complement mediated solubilization: role of the complement in the clearance of circulating immune-complexes. Allergol Immunopathol (Madr) 1994;22:197–203. [PubMed] [Google Scholar]
- 60.Skogh T, Stendahl O. Complement-mediated delay in immune complex clearance from the blood owing to reduced deposition outside the reticuloendothelial system. Immunology. 1983;49:53–59. [PMC free article] [PubMed] [Google Scholar]
- 61.Inagi R, Miyata T, Hong K, et al. Decreased activity of complement-mediated immune complex clearance in hemodialysis patients. Clin Immunol Immunopathol. 1993;68:333–339. doi: 10.1006/clin.1993.1135. [DOI] [PubMed] [Google Scholar]
- 62.Jensenius JC. The mannan-binding lectin (MBL) pathway of complement activation: biochemistry, biology and clinical implications. Adv Exp Med Biol. 2005;564:21–22. doi: 10.1007/0-387-25515-X_6. [DOI] [PubMed] [Google Scholar]
- 63.Brown KS, Keogh MJ, Tagiuri N, et al. Severe fibrosis in hepatitis C virus-infected patients is associated with increased activity of the mannan-binding lectin (MBL)/MBL-associated serine protease 1 (MASP-1) complex. Clin Exp Immunol. 2007;147:90–98. doi: 10.1111/j.1365-2249.2006.03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown KS, Ryder SD, Irving WL, et al. Mannan binding lectin and viral hepatitis. Immunol Lett. 2007;108:34–44. doi: 10.1016/j.imlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Al Sibae M, Jacobson IM, Lake-Bakaar G. Clinical spectrum of chronic hepatitis C virus-related cryoglobulinemia in the US. Presented at Digestive Disease Week, May 19–24, 2007, Washington, DC. Abstract M1838.
- 66.Schott P, Hartmann H, Ramadori G. Hepatitis C virus-associated mixed cryoglobulinemia. Clinical manifestations, histopathological changes, mechanisms of cryoprecipitation and options of treatment. Histol Histopathol. 2001;16:1275–1285. doi: 10.14670/HH-16.1275. [DOI] [PubMed] [Google Scholar]
- 67.Tavoni A, Mosca M, Ferri C, et al. Guidelines for the management of essential mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13(suppl 13):S191–S195. [PubMed] [Google Scholar]
- 68.Gordon AC, Edgar JD, Finch RG. Acute exacerbation of vasculitis during interferon-alpha therapy for hepatitis C-associated cryoglobulinaemia. J Infect. 1998;36:229–230. doi: 10.1016/s0163-4453(98)80022-4. [DOI] [PubMed] [Google Scholar]
- 69.Boonyapisit K, Katirji B. Severe exacerbation of hepatitis C-associated vasculitic neuropathy following treatment with interferon alpha: a case report and literature review. Muscle Nerve. 2002;25:909–913. doi: 10.1002/mus.10118. [DOI] [PubMed] [Google Scholar]
- 70.Misiani R, Bellavita P, Baio P, et al. Successful treatment of HCV-associated cryoglobulinaemic glomerulonephritis with a combination of interferon-alpha and ribavirin. Nephrol Dial Transplant. 1999;14:1558–1560. doi: 10.1093/ndt/14.6.1558. [DOI] [PubMed] [Google Scholar]
- 71.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 72.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 73.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 74.Cacoub P, Saadoun D, Limal N, et al. PEGylated interferon alfa-2b and ribavirin treatment in patients with hepatitis C virus-related systemic vasculitis. Arthritis Rheum. 2005;52:911–915. doi: 10.1002/art.20958. [DOI] [PubMed] [Google Scholar]
- 75.Sansonno D, De Re V, Lauletta G, et al. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818–3826. doi: 10.1182/blood-2002-10-3162. [DOI] [PubMed] [Google Scholar]
- 76.Cheung MC, Haynes AE, Meyer RM, et al. Rituximab in lymphoma: a systematic review and consensus practice guideline from Cancer Care Ontario. Cancer Treat Rev. 2007;33:161–176. doi: 10.1016/j.ctrv.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 78.De Vita S, Quartuccio L. Treatment of rheumatoid arthritis with rituximab: an update and possible indications. Autoimmun Rev. 2006;5:443–448. doi: 10.1016/j.autrev.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Grillo-Lopez AJ, Hedrick E, Rashford M, Benyunes M. Rituximab: ongoing and future clinical development. Semin Oncol. 2002;29(1) suppl 2:105–112. [PubMed] [Google Scholar]
- 80.Patel DD. B cell-ablative therapy for the treatment of autoimmune diseases. Arthritis Rheum. 2002;46:1984–1985. doi: 10.1002/art.10476. [DOI] [PubMed] [Google Scholar]
- 81.Lamprecht P, Lerin-Lozano C, Merz H, et al. Rituximab induces remission in refractory HCV associated cryoglobulinaemic vasculitis. Ann Rheum Dis. 2003;62:1230–1233. doi: 10.1136/ard.2002.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaja F, De Vita S, Mazzaro C, et al. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827–3834. doi: 10.1182/blood-2002-09-2856. [DOI] [PubMed] [Google Scholar]
- 83.Roccatello D, Baldovino S, Rossi D, et al. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2004;19:3054–3061. doi: 10.1093/ndt/gfh469. [DOI] [PubMed] [Google Scholar]
- 84.Basse G, Ribes D, Kamar N, et al. Rituximab therapy for de novo mixed cryoglobulinemia in renal transplant patients. Transplantation. 2005;80:1560–1564. doi: 10.1097/01.tp.0000183749.79424.b4. [DOI] [PubMed] [Google Scholar]
- 85.Quartuccio L, Soardo G, Romano G, et al. Rituximab treatment for glomerulonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford) 2006;45:842–846. doi: 10.1093/rheumatology/kel004. [DOI] [PubMed] [Google Scholar]
- 86.Lake-Bakaar G, Dustin L, McKeating J, et al. Hepatitis C virus and alanine aminotransferase kinetics following B-lymphocyte depletion with rituximab: evidence for a significant role of humoral immunity in the control of viremia in chronic HCV liver disease. Blood. 2007;109:845–846. doi: 10.1182/blood-2006-08-041525. [DOI] [PubMed] [Google Scholar]
- 87.Aksoy S, Abali H, Kilickap S, et al. Accelerated hepatitis C virus replication with rituximab treatment in a non-Hodgkin's lymphoma patient. Clin Lab Haematol. 2006;28:211–214. doi: 10.1111/j.1365-2257.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- 88.Dai MS, Chao TY, Kao WY, et al. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769–774. doi: 10.1007/s00277-004-0899-y. [DOI] [PubMed] [Google Scholar]
- 89.Tsutsumi Y, Tanaka J, Kawamura T, et al. Possible efficacy of lamivudine treatment to prevent hepatitis B virus reactivation due to rituximab therapy in a patient with non-Hodgkin's lymphoma. Ann Hematool. 2004;83:58–60. doi: 10.1007/s00277-003-0748-4. [DOI] [PubMed] [Google Scholar]
- 90.Ng HJ, Lim LC. Fulminant hepatitis B virus reactivation with concomitant listeriosis after fludarabine and rituximab therapy: case report. Ann Hematol. 2001;80:549–552. doi: 10.1007/s002770100346. [DOI] [PubMed] [Google Scholar]
- 91.Zell JA, Yoon EJ, Ignatius Ou SH, et al. Precore mutant hepatitis B reactivation after treatment with CHOP-rituximab. Anticancer Drugs. 2005;16:83–85. doi: 10.1097/00001813-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 92.Soong YL, Lee KM, Lui HF, et al. Hepatitis B reactivation in a patient receiving radiolabeled rituximab. Ann Hematol. 2005;84:61–62. doi: 10.1007/s00277-004-0948-6. [DOI] [PubMed] [Google Scholar]
- 93.Tsutsumi Y, Kawamura T, Saitoh S, et al. Hepatitis B virus reactivation in a case of non-Hodgkin's lymphoma treated with chemotherapy and rituximab: necessity of prophylaxis for hepatitis B virus reactivation in rituximab therapy. Leuk Lymphoma. 2004;45:627–629. doi: 10.1080/1042819031000151923. [DOI] [PubMed] [Google Scholar]
- 94.Hernandez JA, Diloy R, Salat D, et al. Fulminant hepatitis subsequent to reactivation of precore mutant hepatitis B virus in a patient with lymphoma treated with chemotherapy and rituximab. Haematologica. 2003;88:ECR22. [PubMed] [Google Scholar]
- 95.Law JK, Ho JK, Hoskins PJ, et al. Fatal reactivation of hepatitis B post-chemotherapy for lymphoma in a hepatitis B surface antigen-negative, hepatitis B core antibody-positive patient: potential implications for future prophylaxis recommendations. Leuk Lymphoma. 2005;46:1085–1089. doi: 10.1080/10428190500062932. [DOI] [PubMed] [Google Scholar]
- 96.Sarrecchia C, Cappelli A, Aiello P. HBV reactivation with fatal fulminating hepatitis during rituximab treatment in a subject negative for HBsAg and positive for HBsAb and HBcAb. J Infect Chemother. 2005;11:189–191. doi: 10.1007/s10156-005-0385-z. [DOI] [PubMed] [Google Scholar]
- 97.Westhoff TH, Jochimsen F, Schmittel A, et al. Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood. 2003;102:1930. doi: 10.1182/blood-2003-05-1403. [DOI] [PubMed] [Google Scholar]
- 98.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology. 2006;43:209–220. doi: 10.1002/hep.21051. [DOI] [PubMed] [Google Scholar]
- 99.US Food and Drug Administration. FDA Warns of Safety Concern Regarding Rituxan in New Patient Population. [August 8, 2007]. Available at http://www.fda.gov/bbs/topics/NEWS/2006/NEW01532.html.