Abstract
Objective: Two chronic, traumatic brain injury (TBI) cases, where cognition improved following treatment with red and near-infrared light-emitting diodes (LEDs), applied transcranially to forehead and scalp areas, are presented. Background: Significant benefits have been reported following application of transcranial, low-level laser therapy (LLLT) to humans with acute stroke and mice with acute TBI. These are the first case reports documenting improved cognitive function in chronic, TBI patients treated with transcranial LED. Methods: Treatments were applied bilaterally and to midline sagittal areas using LED cluster heads [2.1″ diameter, 61 diodes (9 × 633 nm, 52 × 870 nm); 12–15 mW per diode; total power: 500 mW; 22.2 mW/cm2; 13.3 J/cm2 at scalp (estimated 0.4 J/cm2 to cortex)]. Results: Seven years after closed-head TBI from a motor vehicle accident, Patient 1 began transcranial LED treatments. Pre-LED, her ability for sustained attention (computer work) lasted 20 min. After eight weekly LED treatments, her sustained attention time increased to 3 h. The patient performs nightly home treatments (5 years); if she stops treating for more than 2 weeks, she regresses. Patient 2 had a history of closed-head trauma (sports/military, and recent fall), and magnetic resonance imaging showed frontoparietal atrophy. Pre-LED, she was on medical disability for 5 months. After 4 months of nightly LED treatments at home, medical disability discontinued; she returned to working full-time as an executive consultant with an international technology consulting firm. Neuropsychological testing after 9 months of transcranial LED indicated significant improvement (+1, +2SD) in executive function (inhibition, inhibition accuracy) and memory, as well as reduction in post-traumatic stress disorder. If she stops treating for more than 1 week, she regresses. At the time of this report, both patients are continuing treatment. Conclusions: Transcranial LED may improve cognition, reduce costs in TBI treatment, and be applied at home. Controlled studies are warranted.
Introduction
Traumatic brain injury (TBI) is a significant socio-economic burden;1 1.4 million individuals are affected by TBI annually in the U.S.2 Closed-head, mild TBI does not show focal lesions on computed tomography or magnetic resonance imaging scans.3 Instead, diffuse axonal injury4 in the anterior corona radiata and frontotemporal regions is often seen.5 Brain positron emission tomography scans have shown low levels of regional glucose metabolism in bilateral frontal and temporal lobes in chronic TBI cases.6 Other studies have also shown abnormal frontal lobe activation.7–9 Two regions within the frontal lobes susceptible to damage during TBI are the prefrontal cortex and the anterior cingulate gyrus. The prefrontal cortex is involved in maintaining, monitoring, and manipulating information in working memory,10 and particularly in sustained attention.11,12 The anterior cingulate gyrus has been implicated in divided attention, novelty detection, working memory, memory retrieval, Stroop tasks (inhibition), evaluative judgment, motivation, and performance monitoring.11–16 The anterior cingulate gyrus is considered an initiating or inhibiting region, in that it initiates appropriate, and suppresses inappropriate, behavior.13,15,16
Chronic post-traumatic stress disorder (PTSD) cases share some of the same abnormality in neurocircuitry with chronic TBI cases, including medial prefrontal cortex dysfunction. This includes inadequate top-down governance of the amygdala by the medial prefrontal cortex.17
Low-level laser (light) therapy (LLLT, 500mW or less) using coherent lasers, or non-coherent light-emitting diodes (LEDs) has been shown to produce beneficial cellular and physiological effects in controlled trials (reviewed in Desmet et al., 2006).18 During LLLT, absorption of red or near infra-red (NIR) photons by cytochrome c oxidase in the mitochondrial respiratory chain19 causes an increase in cellular respiration that continues for much longer than the duration of exposure to the light, when light treatment is delivered at appropriate fluence and exposure durations. Primary cellular effects include changes in intracellular calcium,20 increases in ATP synthesis,20–22 increases in nerve cell proliferation and migration,23 and production of low levels of reactive oxygen species (ROS) in mitochondria, causing NF-kB activation via the redox sensitive sensor enzyme protein kinase D1.24 One of the most upregulated genes after NF-kB activation is the antioxidant, mitochondrial superoxide dismutase (MnSOD).25
Transcranial NIR light that penetrates the scalp and skull can significantly reduce damage from experimentally induced stroke in rats23 and rabbits,26 improve memory in middle-aged mice,27 reduce the amount of brain damage and sequelae stemming from acute TBI in mice,28 and significantly improve the outcome in human cases of acute stroke.29,30 A single transcranial, NIR LED application to the left (L) and right (R) lateral forehead areas (F3, F4 in the 10/20 EEG system) has been observed to significantly reduce depression and anxiety for 2 weeks, in patients diagnosed with chronic, major depression.31
Neurogenesis in the TBI-damaged brain is not the rare event it was once thought to be.32 LLLT has stimulated neuron repair in the spinal cord in an animal model,33 and could increase neurogenesis in TBI-damaged brain tissue. LLLT increases expression (and activation) of growth factors such as transforming growth factor-β (TGF-β) and vascular endothelial growth factor (VEGF), which may contribute to positive brain remodeling after TBI. In a rat stroke model, transcranial LLLT triggered the expression of TGF-β1 (in addition to reducing NO levels).34 In a rat heart infarcts model, LLLT significantly increased VEGF expression levels, which are correlated with increased angiogenesis.35
Materials and Methods
LED Devices
Two similar red/NIR LED devices were used. The first had three, square-shaped LED cluster heads, each with a dimension of 4.4 × 4.4 cm (approximately 1.75 × 1.75 in). The treatment area was 19.39 cm2; each cluster head contained 49 diodes (40 NIR 870 nm diodes, 12.25 mW each, and 9 red 633 nm diodes, 1 mW each). Total power was 500 mW (±20%), continuous wave (CW); power density, 25.8 mW/cm2 (+20%); 1 J = 2 s, 1 J/cm2 = 38.8 s.
The second device had one (or three), circular-shaped cluster heads, each with a diameter of 5.35 cm (2.1 in). Treatment area was 22.48 cm2; each cluster head contained 61 diodes (52 NIR 870 nm diodes, and 9 red 633 nm diodes, 12–15 mW each). Total optical output power was 500 mW (±20%), CW; power density, 22.2 mW/cm2 (±20%); 1 J = 2 s, 1 J/cm2 = 45 s. The devices were FDA-approved for treatment of musculoskeletal pain. They were used off-label; informed consent was obtained.
Results
Case Report, P1
P1 (59F, R-handed) sustained closed-head TBI in a motor vehicle accident (MVA) (April 1997). Her head snapped back, hitting a rigid head-rest. She was wearing a seatbelt and did not lose consciousness. Head X-rays and structural magnetic resonance imaging (MRI) scan were normal.
She had two Master's degrees, knew three languages, had written three books, and was a member of Mensa. She had been director of marketing for an internet marketing company, and taught web-design at a major university. At 5 months post-MVA, she was diagnosed by a neurologist as having post-concussive syndrome, was told she might never recover (even for 5 years), and resigned from all professional work due to cognitive dysfunction.
At 2 years post-MVA, her cognitive abilities were re-evaluated. Her divergent reasoning abilities were “significantly impaired across all verbal tasks,” and “executive functioning ability was severely impaired.” During years 2 and 4 post-MVA, she completed three cognitive training programs (20–40 days, each). She could then work on her computer for 20 min at a time, but was still unable to perform professional work. She suffered from depression, with a suicidal gesture.
At 7 years post-MVA, she received her first transcranial LED treatment (May 2004). The first LED device (described above) was used on the L and R forehead areas, 8 J/cm2 to each area; treatment time, 5 min, 10 s per area. She drove herself home (30 min), then slept through dinner and most of the next day. On day 3 after the first LED treatment, she had “improved concentration and focus” and she was able to work at her computer for 40 min (compared with 20 min, previously).
Note, when 8 J/cm2 is applied to the head, it is estimated that only 2–3% of the NIR photons reach brain cortex at depths of 1 cm from the skin or scalp surface (on the basis of post-mortem studies).36 Only 0.2–0.3% are estimated to reach depths of 2 cm (into white matter). Thus, the transcranial LED treatment with 8 J/cm2 delivered to skin on the L and R forehead areas is estimated to deliver only 0.24 J/cm2 to brain cortex (3%); and 0.024 J/cm2 to white matter (0.3%).
One week later, the same LED treatment was repeated. She had no return of excessive sleepiness, and was able to work at her computer for 40 min at a time during the week. During weeks 3 to 8, she continued with weekly treatments, with the following areas undergoing treatment: bilateral forehead (targeting the frontal poles/prefrontal cortex); midline at hairline (targeting the anterior cingulate gyrus); and bilateral temples (targeting the anterior temporal lobes). The hair was carefully parted on the scalp under each LED cluster head to help reduce blockage of the red/NIR photons into the scalp from hair.
The treatment parameters were gradually increased from 8 J/cm2 (5 min, 10 s) per area to 20 J/cm2 (12 min, 54 s) per area. After 8 weeks, P1 was able to work at her computer for 3 h at a time.
After 7 months of weekly LED treatments, in January 2005 (7 years, 9 months post-MVA) she obtained a home treatment unit, the second LED device (single LED cluster head) (Fig. 1). She treated 6 forehead/scalp areas per night (10 min per area; 13.3 J/cm2 per area; 0.4 J/cm2 to cortex). The areas included were: bilateral forehead, temples, posterior-superior to the ears (likely angular gyrus areas, which she reports helped her to “remember what she read”), base of occiput (which “removed the extreme sensitivity of the L scalp area that had bothered her when her hair was being cut”), midline at front hairline, and the center, vertex of her head (which “improved her ability to do math”). She has not been formally tested.
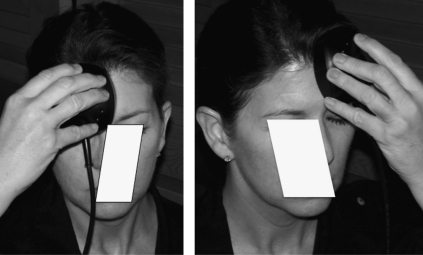
FIG. 1.
Location of right and left forehead placement areas for transcranial LED treatments performed by the patient at home, using a single, circular-shaped cluster head. The usual treatment time is 10 min per area (13.3 J/cm2). See text for further description of this second LED device.
She prefers to treat at bedtime because this LED protocol “improves her sleep.” The 6 areas treated vary from night to night. After 3.5 years, she also added an acupuncture point on the sole of the foot, Kidney 1, a point used historically to reverse coma and improve mentation,37 or top, base of toes - Ba Feng. At the time of this report (March 2010), she has continued to treat herself with this basic home treatment protocol for 5 years. She sometimes treats for 3 weeks in a row, then stops for 1 week.
If she stops the transcranial LED treatments for more than 2 weeks, she slowly regresses; her focus and attention become compromised. As is common with chronic TBI patients, she has fallen occasionally since the MVA. This includes two falls at home since acquiring the home treatment unit. She feels that using the LED cluster head, transcranially, as soon as possible after a fall, helps her to recover faster. (Note: because transcranial LED may increase cerebral blood flow, it would be contra-indicated if acute cerebral hemorrhage was present; see Discussion.)
After 6 years of transcranial LED treatments, she continues to perform computer work for 3 h at a time. She reports “improved self-awareness of both limitations and successes, as well as improved inhibition of inappropriate behavior and angry outbursts.” She also uses the LED unit to help manage her osteoarthritis knee pain. She notes there are some remaining cognitive problems; she cannot multi-task as well as she would like. She still needs to make notes, to be sure all things are accomplished; her overall quality of life, however, is much improved (now age 72, and 13 years post-MVA). She continues to take the drug, Concerta, which she had begun several years before starting the transcranial LED treatments.
Case Report, P2
P2 (52F, R-handed) referred herself for neuropsychological evaluation (March 2009) due to changes in her cognitive functioning over the preceding 2 years, particularly the last 4–5 months. She holds a Bachelor's degree and had a distinguished military career as a high-ranking officer. After 20 years of service, she retired and was working full-time as an executive consultant for an international, technology consulting firm.
She had a history of multiple concussions without loss of consciousness (LOC) during civilian traumas (rugby, sky diving), and military deployment. In 2007, she fell backwards from a swing, hitting the back of her head on concrete (with LOC, likely several minutes). Soon after, she noticed changes in cognition (could not concentrate, stay on task, or multi-task). In October 2008, she stopped working and required medical disability due to cognitive dysfunction.
In December 2008, an EEG showed right temporal lobe slowing (monorhythmic) without epileptiform activity. Structural MRI showed a slightly larger left frontal horn compared with the right frontal horn; and bilateral, deep prominent sulci were present throughout, especially in high frontal and parietal cortical areas (Fig. 2). No enhancing lesions were demonstrated with contrast. A toxicology screen was carried out, and a high mercury level of 1.2 μg/g (reference <0.80) was observed. The nickel level was 0.40 μg/g (reference <0.30).
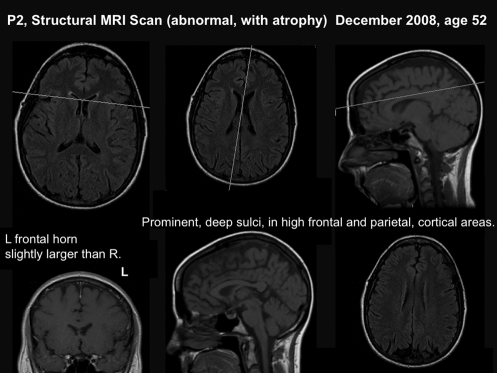
FIG. 2.
Structural, T-1 weighted MRI scan for P2 (age 52), obtained in December 2008, 1 month after starting medical disability, for cognitive dysfunction. This MRI scan shows a left frontal horn that is slightly larger than the right one. Cortical atrophy is also present. Deep prominent sulci are present, especially in the high frontal and high parietal cortical areas. The images in the top row show the plane of section (white lines) for the coronal, mid-sagittal and axial views in the bottom row.
Neuropsychological testing was performed in March 2009, prior to any LED treatments. This evaluation showed deficits in executive functioning, including working memory, processing speed, and cognitive flexibility, all of which are consistent with frontal lobe dysfunction. The scores from March 2009 are described later, in the context of comparison with those following 9 months of transcranial LED treatments (December 2009).
In March 2009, P2 began transcranial LED treatments (second LED device, three LED cluster heads). Each night, the cluster heads were placed on the skin/scalp over the bilateral forehead, high-frontal, high-parietal, and temporoparietal areas (areas with prominent cortical atrophy on her MRI).
The duration of treatment for each area was gradually increased over a 4-week period. During the first week, the LED cluster heads were applied daily for 7 min/area (9.3 J/cm2 at scalp, 0.28 J/cm2 to cortex). Following the first treatment (performed in the afternoon) she was sleepy for the next several hours. During the second week, the LED cluster heads were applied nightly (at bedtime) for 8 min/area (10.6 J/cm2, 0.32 J/cm2); during the third week, for 9 min/area (12 J/cm2, 0.36 J/cm2); and thereafter, for 10 min/area (13.3 J/cm2, 0.4 J/cm2). In addition to the transcranial LED areas, one additional area was treated on the sole of the foot, acupuncture point Kidney 1.37 See Fig. 3.
FIG. 3.
Second LED device (console model with three LED cluster heads) used by P2 during home treatments. These were used in three different areas at the same time. Here, two LED cluster heads are held in place, on left and right high-frontal areas on the scalp, with a loose-fitting elastic cap. The third LED cluster head has been placed on the sole of the foot (acupuncture point, Kidney 1); it is held in place with a soft, flexible, elastic band, secured with a Velcro strap. The usual treatment time is 10 min per area (13.3 J/cm2). See text for further description of this second LED device.
In July 2009, after 4 months of transcranial LED treatments, her medical disability was discontinued; she returned to work full-time, as executive consultant with the international, technology consulting firm (employer prior to October 2008). She continues with this full-time employment as of March 2010.
Neuropsychological test results, pre- and post- 9 months transcranial LED treatments, P2
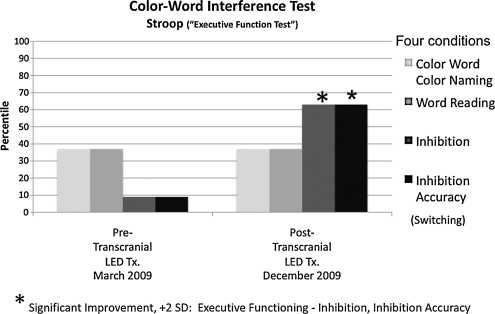
Post-LED neuropsychological testing showed significant improvement (+1, +2 SD) on tests of executive function and inhibition, and on memory. On the Stroop test, a test for executive function (color-word interference test),38 there was significant improvement in condition 3, showing improved inhibition. This task requires naming the ink color in which discrepant color words are printed. There was a change of +2 SD from a score of −1.5 SD below the mean for this test (9th percentile, March 2009) to a score of +0.5 SD above the mean (63rd percentile, December 2009). There was also a significant improvement in condition 4, showing improved inhibition accuracy. This task requires switching back and forth between naming the ink colors and reading the words. There was a change of +2 SD from a score of −1.5 SD below the mean for this test (9th percentile, March 2009) to a score of +0.5 SD above the mean (63rd percentile, December 2009). These scores reflect significant improvement (+2 SD) in executive functioning, inhibition, and inhibition accuracy (Fig. 4).
FIG. 4.
Neuropsychological test results for P2, pre- and post-transcranial LED Treatments. Post-testing was obtained after 9 months of nightly, transcranial LED treatments performed at home. After the LED treatments, significant improvement (+2 SD) in executive function, in terms of both inhibition and inhibition accuracy, was observed.
On the Wechsler Memory Scale - Revised39 there was significant improvement in logical memory passages, where the examinee repeats information in two paragraphs (one at a time) read aloud by the examiner, both immediately, and after a 30-min delay. In addition, inquiry regarding recognition of facts within each story follows the delayed recall. In March 2009, immediate recall was in the 83rd percentile, and in December 2009, in the 95th percentile, reflecting a +1 SD improvement. In March 2009, delayed recall was in the 83rd percentile, and in December 2009, in the 99th percentile, reflecting a +2 SD improvement. These scores show a +1, +2 SD improvement in memory.
PTSD, P2
In December 2009 (9 months post-LED treatments), P2 participated in an interview with a co-author (JK), a clinical neuropsychologist specializing in PTSD. As is true for many people who suffer a physical injury with TBI, the index event also qualifies diagnostically for PTSD as a psychological trauma. Clinical effects can manifest in difficulties modulating memories, emotions, and physiological reactivity that develop from personal internal as well as from environmental cues. Managing these reactions can be further impaired by an overlapping TBI, or multiple overlapping TBIs.40
Prior to the LED treatments, P2 was having noticeable difficulties employing appropriate levels of social behavior, with appropriate levels of emotional intensity in social and work milieus. Her sleep was disrupted and less regular than before the injury. After a few months of transcranial LED treatments, she noticed improved levels of self-awareness, self-monitoring, and self-regulation in social and work settings. Her sleep was improved and impulsivity to react with irritation and anger were reduced. Her medications include Lexapro (since 2002, but discontinued in June, 2009), Provigil, Ritalin (30 mg per day, initiated in June, 2009), Armour thyroid replacement, liquid glutathione, and twice weekly vitamin B injections.
Discussion
The mechanisms of action underlying the observed improved cognition through the use of transcranial, red/NIR LEDs in these two mild TBI cases are unknown. Five possible mechanisms are reviewed briefly, below.
Mitochondria display a significant amount of dysfunction after TBI.41,42,43 The primary mechanism for improvement posited by Lampl et al. (2007),29 in their study of acute, human stroke patients, was an increase in ATP.19–22,44,45 This mechanism would involve the utilization of photons by cytochrome c oxidase in the mitochondria to increase ATP (likely to a greater degree in cortical/subcortical cells that are hypoxic). An increase in ATP following red/NIR LED treatments in chronic TBI cases would have beneficial effects, including an increase in cellular respiration and oxygenation.
Oxidative stress plays a role in the damage seen after TBI.46 One hypothesis is that LLLT produces low levels of ROS in mitochondria of illuminated cells, and these ROS cause NF-kB activation via the redox-sensitive sensor enzyme protein kinase D1,24 resulting in upregulation of mitochondrial superoxide dismutase (MnSOD).25 A single exposure of LLLT/LED in vitro with fibroblasts has been observed to increase NF-kB in the short term.47 In stimulated dendritic cells in the longer term, however, NF-kB and pro-inflammatory cytokines were reduced.48 Thus, in the long-term, repeated LED treatments are hypothesized to decrease inflammation (less NF-kB), and up-regulate gene products that are cytoprotective, such as superoxide dismutase, glutathione peroxidase, and heat shock protein 70.24,25,49,50 It is hypothesized that an overall protective response occurs with repeated LED treatments, with a reduction in major ROS-mediated damage and chronic inflammation known to occur in the brain after TBI.
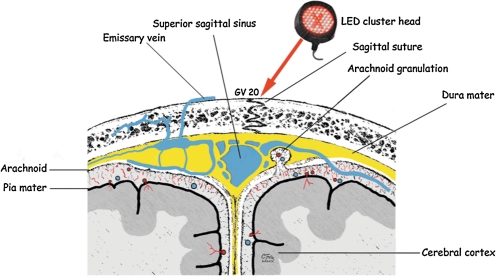
Acupuncture points located on the scalp were treated with the red/NIR LEDs. This includes points along the Governing Vessel (GV) acupuncture meridian, located on the midline of the skull (including, in part, the mid-sagittal suture line). Some acupuncture points located on the GV meridian have been used historically to help treat patients with coma37 and stroke51 - e.g., GV 16 (inferior to occipital protuberance), GV 20 (vertex) (Fig. 5), and GV 24 (near center-front hairline); these points were treated in this study. Stimulation of point GV 26, located on this same meridian (on the midline, philtrum area near the upper lip), has been observed to increase oxygenation to the frontal cortex in rats.52 PO2 microelectrodes were inserted into the cortex of the rats through small burr holes. When a needle was inserted into GV26, there was an immediate increase in PO2 in the frontal cortex; the effect was reproducible and comparable to that obtained with increased inspiratory CO2, known to induce arterial vasodilation and capillary perfusion pressure. Thus, stimulation of acupuncture points on the GV meridian with red/NIR LED may have promoted vasodilation in the frontal cortex. Additional acupuncture points located on the forehead/scalp were also treated, including some along the Bladder (Bl), Gall Bladder, and Stomach meridians. During functional MRI, increased activation (blood flow) in the ipsilateral visual association (occipital) cortex was observed when a distal acupuncture point (Bl 67, on the 5th toe) was stimulated with red-beam laser (10 mW, 670 nm), likely affecting Bl 1 (located at the medial canthus of the eye).53 Sham laser had no effect. Bl 1 and Bl 67 are acupuncture points used historically to treat visual disorders.51 Thus, stimulation of acupuncture points even distal to the brain can likely cause increased blood flow and activation within specific brain regions, including brain regions separate from the somatotopic sensory cortex representation for the body point stimulated with laser or needle. Additional functional imaging studies are needed.
Transcranial red/NIR LED may have irradiated the blood via the valveless, emissary veins located on the scalp surface, but interconnecting with veins in the superior sagittal sinus (Fig. 5) (Mary Dyson, personal communication). If red/NIR photons penetrate deeply enough to reach cortex, it is also possible they are entering small vessels located between the arachnoid and the pia mater, including those that supply arterial blood to superficial areas of the cortex (Fig. 5). Direct, in vitro irradiation of blood with red-beam laser has been observed to improve erythrocyte deformability (flexibility) and rheology.54 A beneficial effect from direct laser blood irradiation in vivo has been observed during stenting procedures in which low-level, red-beam laser (10 mW, 650 nm) was used to irradiate directly into a coronary artery.55 The re-stenosis rate was reduced and there were no negative side effects or complications. Thus, blood irradiation at the scalp may have affected local, intracerebral blood and circulation; however, this is unknown and would require further study.
-
There may have been an increase in regional cerebral blood flow (rCBF) specific to the frontal lobes. P2 showed significant improvement on neuropsychological testing for executive function (inhibition and inhibition accuracy) and memory after LED treatment. These results suggested improved function in prefrontal cortex and anterior cingulate gyrus regions. Significant improvement for P2, on “inhibition and inhibition accuracy” on the Stroop test particularly, suggests improved function of the medial prefrontal cortex, anterior cingulate gyrus area.11–16 It is possible that this medial prefrontal cortex area could have been treated with NIR photons, especially when the LED cluster head was placed over the midline, front hairline area. The dorsolateral prefrontal cortex was also likely irradiated when the LEDs were placed on the L and R high-frontal areas of the scalp (Fig. 3).
There could also have been increased rCBF to frontal pole areas, as observed in a recent transcranial LED study to treat major depression.31 An increase in mean rCBF was observed at the frontal pole areas (measured with NIR spectroscopy, INVOS system) during the real LED condition (810 nm, 225 mW/cm2, 60 J/cm2) as compared with the sham LED condition, although the mean L and R change did not reach statistical significance (p = 0.16). The LEDs were placed over the L and R lateral forehead areas (F3 and F4 in the 10/20 EEG system); areas also treated in this study. Those results are relevant to the present study, because three out of ten cases in that study had PTSD, and P2 in the present study also had PTSD. P2 reported that after a few months of using the transcranial LED treatments, her “impulsivity to react with irritation and anger” was reduced. Also, although P1 in the present study did not have PTSD, she reported “improved inhibition of inappropriate behavior and angry outbursts,” following transcranial LED. Both cases reported improved sleep. No negative side effects were reported.
FIG. 5.
Diagram of structures present on coronal view at vertex of the skull, where one of the LED cluster heads was placed. When placed here, an acupuncture point located on the Governing Vessel (GV) meridian was treated (GV 20). See text for historical use of this point.51 Several other structures were also likely irradiated with the red/NIR LED cluster heads, including the valveless emissary veins that interconnect with veins in the superior sagittal sinus. Only 3% of NIR photons delivered at the surface of the skull are estimated to reach 1 cm deep (to cortex).36 Suture lines, and the superior sagittal sinus, may be areas where red/NIR photons have better potential for penetration.
Conclusions
Results from the two chronic TBI cases described here, along with those from previous transcranial LLLT/LED studies with acute stroke patients and chronic, major depression cases, suggest that further, controlled research with this methodology is warranted. Further study is required to determine the optimal treatment parameters. Transcranial red/NIR LED may be an inexpensive, noninvasive treatment, suitable for home treatments, to improve cognitive function in TBI patients, as well as to reduce symptom severity in PTSD patients. It is recommended that future studies include pre- and post-neuropsychological testing, as well as functional brain imaging and diffusion tensor imaging scans, to better understand the possible physiological changes that may take place with transcranial LLLT/LED treatments.
A preliminary version of this paper was published as a proceedings paper.56
Acknowledgments
The authors thank Charles Foltz for the graphics art in Figure 5; Carole Palumbo, Ph.D. for assistance with MRI analysis; Paula Martin, Anna Kharaz, Michael Ho, Ph.D., and Ethan Treglia, M.S., for manuscript assistance; and Michael and Winter Robinson for photography. M. Naeser was supported by Merit Review Funding, Department of Veterans Affairs. M. Hamblin was supported by the NIH (grant RO1AI050875), US Air Force MFEL program (contract FA9550-04-1-0079), Center for Integration of Medicine and Innovative Technology (DAMD17-02-2-0006), and Congressionally Directed Medical Research Program (W81XWH-09-1-0514).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sosin D.M. Sniezek J.E. Thurman D.J. Incidence of mild and moderate brain injury in the United States. Brain Inj. 1996;10:47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 2.Thurman D.J. Alverson C. Dunn K.A. Guerrero J. Sniezek J.E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Mittl R.L. Grossman R.I. Hiehle J.F., et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. Am. J. Neuroradiol. 1994;15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- 4.Taber K.H. Warden D.L. Hurley R.A. Blast-related traumatic brain injury: what is known? J. Neuropsychiatry Clin. Neurosci. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- 5.Niogi S.N. Mukherjee P. Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato T. Nakayama N. Yasokawa Y. Okumura A. Shinoda J. Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J. Neurotrauma. 2007;24:919–926. doi: 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Carrion R. Gomez P.V. Junque C., et al. Frontal hypoactivation on functional magnetic resonance imaging in working memory after severe diffuse traumatic brain injury. J. Neurotrauma. 2008;25:479–494. doi: 10.1089/neu.2007.0417. [DOI] [PubMed] [Google Scholar]
- 8.Strangman G.E. O'Neil-Pirozzi T.M. Goldstein R., et al. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch. Phys. Med. Rehabil. 2008;89:974–981. doi: 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 9.McAllister T.W. Flashman L.A. McDonald B.C. Saykin A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J. Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 10.Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann. N.Y. Acad. Sci. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- 11.Lewin J.S. Friedman L. Wu D., et al. Cortical localization of human sustained attention: detection with functional MR using a visual vigilance paradigm. J. Comput. Assist. Tomogr. 1996;20:695–701. doi: 10.1097/00004728-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pardo J.V. Fox P.T. Raichle M.E. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- 13.Cabeza R. Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 14.Dehaene S. Kerszberg M. Changeux J.P. A neuronal model of a global workspace in effortful cognitive tasks. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swick D. Jovanovic J. Anterior cingulate cortex and the Stroop task: neuropsychological evidence for topographic specificity. Neuropsychologia. 2002;40:1240–1253. doi: 10.1016/s0028-3932(01)00226-3. [DOI] [PubMed] [Google Scholar]
- 16.Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- 17.Shin L.M. Rauch S.L. Pitman R.K. Structural and functional anatomy of PTSD. New York (NY): Guilford Press; 2005. [Google Scholar]
- 18.Desmet K.D. Paz D.A. Corry J.J., et al. Clinical and experimental applications of NIR-LED photobiomodulation. Photomed. Laser Surg. 2006;24:121–128. doi: 10.1089/pho.2006.24.121. [DOI] [PubMed] [Google Scholar]
- 19.Lane N. Cell biology: power games. Nature. 2006;443:901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 20.Yu W. Naim J.O. McGowan M. Ippolito K. Lanzafame R.J. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem. Photobiol. 1997;66:866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 21.Mochizuki-Oda N. Kataoka Y. Cui Y. Yamada H. Heya M. Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002;323:207–210. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 22.Oron U. Ilic S. De Taboada L. Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed. Laser Surg. 2007;25:180–182. doi: 10.1089/pho.2007.2064. [DOI] [PubMed] [Google Scholar]
- 23.Oron A. Oron U. Chen J., et al. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 24.Storz P. Mitochondrial ROS–radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Sompol P. Xu Y. Ittarat W. Daosukho C. St. Clair D. NF-kappaB-associated MnSOD induction protects against beta-amyloid-induced neuronal apoptosis. J. Mol. Neurosci. 2006;29:279–288. [PubMed] [Google Scholar]
- 26.Lapchak P.A. Salgado K.F. Chao C.H. Zivin J.A. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Michalikova S. Ennaceur A. van Rensburg R. Chazot P.L. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol. Learn. Mem. 2008;89:480–488. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Oron A. Oron U. Streeter J., et al. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 29.Lampl Y. Zivin J.A. Fisher M., et al. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 30.Zivin J.A. Albers G.W. Bornstein N., et al. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 31.Schiffer F. Johnston A.L. Ravichandran C., et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. www.behavioralandbrainfunctions.com/content/5/1/46. Behav. Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson R.M. Sun D. Bullock M.R. Neurogenesis after traumatic brain injury. Neurosurg. Clin. N. Am. 2007;18:169–181. doi: 10.1016/j.nec.2006.10.007. xi. [DOI] [PubMed] [Google Scholar]
- 33.Byrnes K.R. Waynant R.W. Ilev I.K., et al. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 34.Leung M.C. Lo S.C. Siu F.K. So K.F. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg. Med. 2002;31:283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 35.Tuby H. Maltz L. Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg. Med. 2006;38:682–688. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 36.Wan S. Parrish J.A. Anderson R.R. Madden M. Transmittance of nonionizing radiation in human tissues. Photochem Photobiol. 1981;34:679–681. doi: 10.1111/j.1751-1097.1981.tb09063.x. [DOI] [PubMed] [Google Scholar]
- 37.Frost E. Acupuncture for the Comatose Patient. Am. J. Acupunct. 1976;4:45–48. [Google Scholar]
- 38.Delis D.C. Kaplan E. Kramer J.H. Delis-Kaplan Executive Function System (D-KEFS): Examiner's manual. San Antonio (TX): The Psychological Corporation; 2001. [Google Scholar]
- 39.Wechsler D. Wechsler Memory Scale-Revised. San Antonio (TX): Psychological Corporation; 1987. [Google Scholar]
- 40.Knight J. Taft C. Assessing neuropsychological concomitants of trauma and PTSD, in: Assessing psychological trauma and PTSD. In: Wilson J., editor; Keane T.M., editor. New York: The Guilford Press; 2004. pp. 344–388. [Google Scholar]
- 41.Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- 42.Gilmer L.K. Roberts K.N. Sullivan P.G. Miller K. Scheff S. Early mitochondrial dysfunction following cortical contusion injury. J. Neurotrauma. 2009;26:1271–1280. doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lifshitz J. Sullivan P.G. Hovda D.A. Wieloch T. McIntosh T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Eells J.T. Henry M.M. Summerfelt P., et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. U.S.A. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karu T. Mechanisms of low-power laser light action on cellular level. Proc. of S.P.I.E. 2000;4159:1–17. [Google Scholar]
- 46.Ansari M.A. Roberts K.N. Scheff S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen A.C.H. Arany P.R. Huang Y.Y., et al. Low level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. SPIE. 2009;7165:7165081–10. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen A.C.H. Huang Y.Y. Sharma S.K. Hamblin M.R. Effects of 810-nm laser on murine bone marrow derived dendritic cells. Photomed. Laser Surg. 2010. in press. [DOI] [PMC free article] [PubMed]
- 49.Avni D. Levkovitz S. Maltz L. Oron U. Protection of skeletal muscles from ischemic injury: low-level laser therapy increases antioxidant activity. Photomed. Laser Surg. 2005;23:273–277. doi: 10.1089/pho.2005.23.273. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y.H. Takahashi K. Jiang G.Z., et al. In vivo production of heat shock protein in mouse peritoneal macrophages by administration of lipopolysaccharide. Infect. Immun. 1994;62:4140–4144. doi: 10.1128/iai.62.10.4140-4144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deadman P. Al-Khafaji M. A Manual of Acupuncture. Hove, East Essex, England: Journal of Chinese Medicine Publications; 1998. pp. 256–325. 548–560. [Google Scholar]
- 52.Chen G.S. Erdmann W. Effects of acupuncture on tissue-oxygenation of the rat brain. Comp. Med. East West. 1977;5:147–154. doi: 10.1142/s0147291777000210. [DOI] [PubMed] [Google Scholar]
- 53.Siedentopf C.M. Golaszewski S.M. Mottaghy F.M. Ruff C.C. Felber S. Schlage A. Functional magnetic resonance imaging detects activation of the visual association cortex during laser acupuncture of the foot in humans. Neurosci. Lett. 2002;327:53–56. doi: 10.1016/s0304-3940(02)00383-x. [DOI] [PubMed] [Google Scholar]
- 54.Mi X.Q. Chen J.Y. Liang Z.J. Zhou L.W. In vitro effects of helium-neon laser irradiation on human blood: Blood viscosity and deformability of erythrocytes. Photomed. Laser Surg. 2004;22:477–482. doi: 10.1089/pho.2004.22.477. [DOI] [PubMed] [Google Scholar]
- 55.De Scheerder I.K. Wang K. Kaul U., et al. Intravascular low-power laser irradiation after coronary stenting: Long-term follow-up. Lasers Surg. Med. 2001;28:212–215. doi: 10.1002/lsm.1040. [DOI] [PubMed] [Google Scholar]
- 56.Naeser M.A. Saltmarche A. Krengel M.H. Hamblin M.R. Knight J.A. Transcranial LED therapy for cognitive dysfunction in chronic, mild traumatic brain injury: Two case report. Proc. of SPIE. 2010;7552:75520L1–12. [Google Scholar]