Abstract
Clinical administration of bone marrow-derived stem cells in the setting of acute myocardial infarction (AMI) leads to improved left ventricular ejection fraction. Thymosin beta-4 (TB4) and vascular endothelial growth factor (VEGF) are linked to adult epicardial progenitor cell mobilization and neovascularization and is cardioprotective after myocardial ischemia. This study investigated the time course of TB4 and VEGF during AMI, cardiac arrest, and resuscitation. Fifteen anesthetized and instrumented domestic swine underwent balloon occlusion of the proximal left anterior descending coronary artery. During occlusion, venous blood samples were collected from the right atrium at 5-min intervals until 15 min after the onset of cardiopulmonary resuscitation (CPR). Plasma levels of TB4, VEGF, and matrix metalloproteinase-9 (MMP-9, selected as a marker for remodeling and repair) were measured by ELISA. Generalized linear mixed models were employed to model the time-dependent change in plasma concentration. All variables were natural log transformed, except TB4 values, to normalize distributions. Fifteen animals successfully underwent balloon occlusion of the left anterior descending coronary artery and samples were collected from these subjects. The average onset of spontaneous ventricular fibrillation was 28 min. TB4, VEGF, and MMP-9 demonstrated a statistically significant, time-dependent increase in concentration during ischemia. Following arrest and throughout the first 15 min of resuscitation, MMP-9 had an unchanged rate of rise when compared with the prearrest, ischemic period, with VEGF showing a deceleration in its time-dependent concentration trajectory and TB4 demonstrating an acceleration. Endogenous TB4 and VEGF increase shortly after the onset of AMI and increase through cardiac arrest and resuscitation in parallel to remodeling proteases. These markers continue to rise during successful resuscitation and may represent an endogenous mechanism to recruit undifferentiated stem cells to areas of myocardial injury.
Introduction
The G-actin–sequestering 43-amino acid peptide thymosin beta-4 (TB4), when forming a complex with integrin-linked kinase (ILK), results in the activation of survival kinase Akt, which promotes cardiomyocyte migration, survival, and repair (Bock-Marquette and others 2004). The exogenous administration of TB4 after acute myocardial infarction (AMI) has been shown to upregulate ILK/Akt, enhance early myocyte survival, and improve cardiac function via cardiomyocyte and endothelial cell migration (Srivastava and others 2007). Endogenous TB4 is located in cellular cytoplasm and nuclei and increases in response to myocardial ischemia (Sosne and others 2010). The beneficial effects of TB4 on cardiomyocyte replenishment and regeneration may be secondary to its effects on the Akt pathway, which stimulates production of vascular endothelial growth factor (VEGF) and may provide a pathway in the treatment of AMI (Crockford and others 2010).
VEGF is reported to increase early during acute myocardial ischemia in response to hypoxia-inducible factor in an attempt to stimulate angiogenesis and myocardial-cell survival (Lee and others 2000). Matrix metalloproteinase-9 (MMP-9) is elevated after AMI and is associated with left ventricular remodeling post-MI (Kelly and others 2007). The purpose of this study was to investigate the correlation between TB4 and VEGF during acute myocardial ischemia and their relationship to MMP-9, a marker of myocardial injury.
Materials and Methods
This investigation was approved by our institution's Animal Care and Utilization Review Committee. Yorkshire swine (n = 15) of both sexes were premedicated with ketamine and xylazine. General anesthesia was induced via nasal isoflurane and maintained with isoflurane and oxygen/nitrous oxide after intubation.
Under fluoroscopy and continuous electrocardiographic monitoring, micromanometer-tipped catheters (Millar Instruments, Houston, TX) were positioned in the ascending aorta and right atrium for pressure monitoring. The left anterior descending coronary artery (LAD) was occluded distal to the first septal perforator using a standard PTCA balloon catheter with angiographic confirmation. Animals were observed until ventricular fibrillation (VF) or for 30 min. No further samples were collected after 30 min and the study protocol did not occlude any animals that did not develop spontaneous VF during this period or any animals developing VF due to reperfusion arrhythmias after balloon deflation.
ECG and hemodynamic data were recorded and stored on a laptop computer using PowerLab Chart v. 5.2 (ADInstruments, Castle Hill, Australia). Venous blood was sampled from the right atrium prior to occlusion and at 5-min intervals for 30 min or until VF. Samples were placed in sterile, chilled (0°C) EDTA tubes and centrifuged at 5000 rpm for 10 min. Plasma was immediately separated and stored at −80°C until analysis. TB4, VEGF, and MMP-9 levels were determined using a quantitative sandwich ELISA customized for porcine plasma [TB4 (Alpco Diagnostics, Salem, NH); VEGF and MMP-9 (R&D Systems, Minneapolis, MN)].
Data were entered into an Excel 2003 spreadsheet (v. 11.8; Microsoft Corp., Redmond, WA) and imported into SAS statistical software (9.2; SAS Institute, Cary, NC) for analysis. Spearman rank correlations were employed to measure bivariate associations. Time-dependent changes in the concentrations of TB4 were evaluated using generalized linear mixed models, which account for the auto-correlation inherent in repeated measures designs. Mixed modeling, allowing for fixed and random effects, has the parameter vector for both, can estimate the error covariance matrix for each, and provides the correct standard errors for either the fixed or random effects.
The SAS procedure, PROC MIXED, was invoked for this purpose with ischemia time, measured in minutes, and the intercept (control values) treated as random effects. Model fit was assessed using relative measures such as deviance statistics (for nested models), Akaike's Information Criteria, Bayesian Information Criterion, and percent reductions in random variance components when compared with unconditional means and unconditional growth models. An unstructured variance matrix was used for this analysis. To account for multiple comparisons when contrasting interval measurements to controls, P values were adjusted according to the method of Dunnett-Hsu.
Results
Fifteen animals successfully underwent balloon occlusion of the LAD. Angiographic confirmation was obtained in all animals. Right atrial blood samples were collected from all subjects. The average onset of spontaneous VF was 28 min. TB4, VEGF, and MMP-9 demonstrated a statistically significant, time-dependent increase in concentration during ischemia as demonstrated in Table 1. VEGF values showed a statistically significant increase from control values by 5 min (P = 0.006) as did MMP-9 (P = 0.003). TB4 demonstrated a delayed rise, not reaching a level statistically higher than control values until 15 min postocclusion (P = 0.0003).
Table 1.
Concentrations of Thymosin Beta-4, ET-1, MMP-9, and VEGF During Ischemia and During Resuscitation
| Molecule | Δ Concentration/minute Ischemic period (P value) | Δ Concentration/minute Resuscitative period (P value) | ||
|---|---|---|---|---|
| Thymosin beta-4 | 0.06 pg/mL | (P = 0.0006) | 0.16 pg/mL | (P < 0.0001) |
| ln(ET-1) | 7.0% | (P < 0.0001) | Same | |
| ln(matrix metalloproteinase-9) | 6.2% | (P < 0.0001) | Same | |
| ln(vascular endothelial growth factor) | 8.0% | (P < 0.0001) | 5.1% | (P = 0.02) |
Following arrest and throughout the first 15 min of resuscitation, MMP-9 had an unchanged rate of rise when compared with slope during the prearrest, ischemic period, whereas VEGF showed a statistically significant deceleration in its time-dependent concentration trajectory and TB4 demonstrated a statistically significant acceleration. Prototypical trajectories obtained through mixed modeling are given in Figs. 1–3.
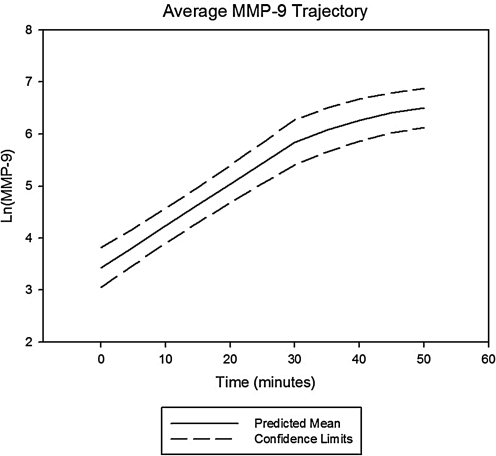
FIG. 1.
Average change trajectory of matrix metalloproteinase-9 (MMP-9) levels after left anterior descending coronary artery (LAD) occlusion. Average change trajectory with 95% confidence bands for serum MMP-9 levels following coronary occlusion and the onset of spontaneous, ischemic ventricular fibrillation (VF). MMP-9 levels were natural log transformed to better normalize its distribution for analysis. Values were statistically higher than control values by 15 min postocclusion (P = 0.0003). The average onset of VF was at the 30 min time point postcoronary occlusion. The rate of rise at this point was not statistically different from that measured prearrest.
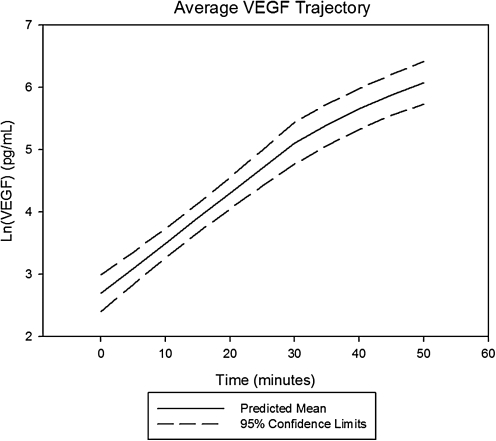
FIG. 3.
Average change trajectory of vascular endothelial growth factor (VEGF) levels post-LAD occlusion. Average change trajectory with 95% confidence bands for serum VEGF levels following coronary occlusion and the onset of spontaneous, ischemic VF. VEGF levels were natural log transformed to better normalize its distribution for analysis. Values were statistically higher than control values by 5 min postocclusion (P = 0.006). The average onset of VF was at the 30 min time point postcoronary occlusion. The rate of rise at this point was statistically slower when compared with the prearrest slope.
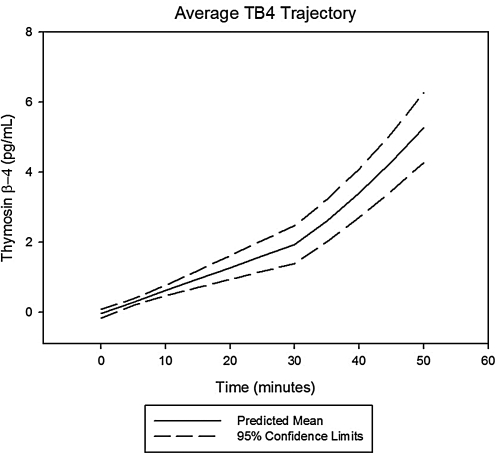
FIG. 2.
Average change trajectory of thymosin beta-4 (TB4) levels post-LAD occlusion. Average change trajectory with 95% confidence bands for serum thymosin β-4 levels following coronary occlusion and the onset of spontaneous, ischemic VF. Values were statistically higher than control values by 5 min postocclusion (P = 0.003). The average onset of VF was at the 30 min time point postcoronary occlusion. The rate of rise at this point was statistically faster when compared with the prearrest slope.
Levels of TB4 were correlated with levels of VEGF (r = 0.80, P < 0.0001) and MMP-9 (r = 0.74, P < 0.0001). VEGF and MMP-9 levels were also correlated (r = 0.63, P < 0.0001) and were not dependent on the sex of the animal or time to VF.
Analysis of samples from animals that underwent instrumentation without balloon occlusion of the LAD showed no rise in either TB4, VEGF, or MMP-9.
Discussion
This study showed that serum levels of VEGF and MMP-9 rise early during myocardial ischemia and are correlated with the rise in TB4. Acute myocardial ischemia may result in a signaling cascade that triggers TB4 to stimulate cardiomyocyte regeneration.
Myocardial ischemia and its resultant damage to the myocardium were thought to be irreversible given the prevailing notion that the heart was a terminally differentiated organ. However, recent studies have reported that the heart is, in fact, capable of regeneration via repopulation by endogenous or circulating progenitor cells, which home to the myocardium and differentiate into functioning myocytes (Lipinski and others 2007). Cell therapy for AMI has focused on the isolation and administration of adult progenitor cells, pretreatment of cells with activators to augment cell homing, improving the delivery of cells to increase the number of active cells, and finally, the identification of endogenous molecules that guide the homing, engrafting, and differentiation of pluripotent cells (Chavakis and others 2010). One of the most important agents involved in recruiting progenitor cells to damaged myocardium is TB4, which also signals migrating progenitor cells to differentiate into endothelial and myocardial cells. VEGF is also responsible for the differentiation of progenitor cells into endothelial cells. This study shows that early myocardial ischemia results in elevated levels of TB4 and VEGF and may explain the cardioprotective effects of TB4 in the setting of myocardial injury.
Left ventricular dilatation after AMI is an important predictor of prognosis and development of congestive heart failure (White and others 1987). Degradation of the extracellular matrix is central to left ventricular remodeling after AMI. MMPs are a family of endopeptidases that act as physiologic regulators of the extracellular matrix. MMP-9 exists within human myocardium during nonpathologic states (Guedez and others 1998).
MMP-9 has been implicated in the structural changes occurring after experimental AMI (Ducharme and others 2000) as well as can predict cardiovascular mortality in patients with coronary artery disease (Blankenberg and others 2003). Squire and others reported that the levels of MMP-9 correlate directly with LV function and presumably infarct size after AMI in man (Squire and others 2004). Other studies have reported that the levels of MMP-9 are associated with postischemic myocardial tissue and also correlates with infarct size (Gonzalez and others 2009). Both myocardial and progenitor cells produce MMP-9 in response to ischemia.
MMP-9 may contribute to weakening and rupture of atherosclerotic plaque and is elevated during AMI (Fang and others 2009). Inhibition of MMP-9 has been associated with improved postinfarct myocardial remodeling and improvement in cardiac function (Huang and others 2009).
TB4 is a highly conserved, 43-amino acid acidic peptide (pI 4.6) initially isolated from bovine thymus. It is present in most tissues and cell lines and is found in high concentrations in blood platelets, neutrophils, macrophages, and other lymphoid tissues. One important function is the regulation of actin polymerization in mammalian nucleated cells, with subsequent effects on actin cytoskeletal organization, necessary for cell motility, organogenesis, and other important cellular events (Crockford 2007). Specifically, TB4 stimulates the capillary-like tube formation of adult coronary endothelial cells and increases embryonic endothelial cell migration and proliferation in vivo, thus leading to repopulation of damaged cells with cells capable of regeneration (Bock-Marquette and others 2009). TB4 has been shown to be a potent stimulator of coronary vasculogenesis and angiogenesis via the proangiogenic cleavage product N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP) (Smart and others 2007; Rossdeutch and others 2008). Other studies have reported that the survival of embryonic and postnatal cardiomyocytes in culture was also enhanced by TB4, which formed a functional complex with PINCH and ILK, resulting in activation of the survival kinase Akt (also known as protein kinase B). After coronary artery ligation in mice, TB4 treatment resulted in upregulation of ILK and Akt activity in the heart, enhanced early myocyte survival, and improved cardiac function. The effects of TB4 on recruitment of progenitor cells are not limited to the heart as recent studies have shown its recruitment effects on the kidney, skin, and liver (Varneaeva and others 2007). Recently, studies have demonstrated that TB4 is a key factor for inducing therapeutic neovascularization (Hinkel and others 2010). Studies investigating the use of exogenous TB4 to patients with AMI are ongoing, with a preliminary study reporting that exogenous synthetic administration of TB4 was well tolerated (Ruff and others 2010).
Stromal-derived factor-1 and its receptor CXCR4 have been widely reported to be essential for hematopoietic progenitor cell recruitment and angiogenesis (Walter and others 2005). VEGF has been shown to induce recruitment of bone marrow-derived circulating myeloid cells via induction of stromal-derived factor-1 (Grunewald and others 2006) via tyrosine kinase receptors, which are potent inducers of vascular growth. In addition to enhancing blood flow, both VEGF-A and VEGF-B may also protect hibernating myocardium after myocardial infarction by altering myocardial metabolism and apoptosis inhibition (Lahteenvuo and others 2009). VEGF-B administration has been reported to induce angiogenesis in mouse hindlimb ischemia (Silvestre and others 2003). Further, overexpression of MMP-9, in the setting of myocardial injury, has been associated with increased VEGF and placenta-derived growth factor (Gargioli and others 2008).
There were several limitations with this study. The first is that we did not obtain tissue specimens to identify progenitor cells or endothelial cells in the area of infarction. Second, we did not objectively assess the size of the infarct, which may independently predict the levels of TB4 and VEGF via staining or magnetic resonance imaging. Subsequent protocols will assess stem cell mobilization based on the data reported in this experiment. MMP-9, a surrogate for myocardial infarct size, rose in accordance with the rise in TB4 and VEGF, suggesting diffuse myocardial injury. Further long-term assessments are needed to justify the hypothesis that the cardioprotective mechanisms of TB4 and VEGF may blunt myocardial injury and MMP-9. VEGF (Eskens and Verweij 2006) and MMP-9 (Neto-Neves and others 2010) inhibitors may trigger or blunt TB4 and warrant further investigation. And, third, the long-term effects of the rise in TB4 and VEGF were not studied as the subjects in this study were sacrificed.
Mesenchymal stem cells have been shown to increase circulating VEGF via a paracrine effect (Tang and others 2005), and the cardiac function postmyocardial infarction is dependent on this effect (Li and others 2009).
The mechanism of this paracrine action has been reported to be due to modulation of the Akt pathway. Lim and others have reported that transplantation of mesenchymal stem cells in a porcine myocardial infarction model resulted in increased signal-regulated protein kinase (ERK) and VEGF (Lim and others 2006).
In conclusion, this study shows that levels of signaling molecules involved in the recruitment and differentiation of pluripotent cells into functioning myocardium rise early during the setting of acute myocardial injury. The levels of TB4 and VEGF are correlated with MMP-9, a marker of myocardial injury, and these levels may reveal an endogenous method of cardioprotection in a porcine infarct model.
Acknowledgment
This research was supported, in part, by a grant from the National Institutes of Health (NHLBI R01 HL076671).
Author Disclosure Statement
The authors have no commercial associations that might create a conflict of interest with the data presented in this manuscript.
References
- Blankenberg S. Rupprecht HJ. Poirier O. Bickel C. Smieja M. Hafner G. Plasma concentrations and genetic variations of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- Bock-Marquette I. Saxena A. White MD. Dimaio JM. Srivastava D. Thymosin beta4 activates integrin linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- Bock-Marquette I. Shrivastava S. Pipes GC. Thatcher JE. Blystone A. Shelton JM. Galindo CL. Melegh B. Srivastava D. Olson EN. Di Maio JM. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol. 2009;46(5):728–738. doi: 10.1016/j.yjmcc.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis E. Koyanagi M. Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: Progress from bench to bedside and back. Circulation. 2010;121:325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Ann NY Acad Sci. 2007;1112:385–395. doi: 10.1196/annals.1415.051. [DOI] [PubMed] [Google Scholar]
- Crockford D. Turjman N. Allan C. Angel J. Thymosin beta4: Structure, function, and biological properties supporting current and future clinical applications. Ann NY Acad Sci. 2010;1194:179–189. doi: 10.1111/j.1749-6632.2010.05492.x. [DOI] [PubMed] [Google Scholar]
- Ducharme A. Frantz S. Aikawa M. Rabkin E. Lindsey M. Rohde LE. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collage accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskens FA. Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: A review. Eur J Cancer. 2006;42(18):3127–3139. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Fang L. Du XJ. Gao XM. Dart A. Acute myocardial infarction enhances production of MMP-9 by peripheral blood mononuclear cells. Heart Lung Circ. 2009;18S:S299–S318. [Google Scholar]
- Gargioli C. Coletta M. De Grandis F. Cannata SM. Cossu G. PIGF, MMP-9 expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14:973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- Gonzalez GE. Rabald S. Briest W. Gelpi RJ. Seropian I. Zimmer HG. Deten A. Ribose treatment reduced the infarct size and improved heart function after myocardial infarction in rats. Cell Physiol Biochem. 2009;24:211–218. doi: 10.1159/000233247. [DOI] [PubMed] [Google Scholar]
- Grunewald M. Avraham I. Dor Y. Bachar-Lustig E. Itin A. Yung S. Chimenti S. Landsman L. Abramovitch R. Keshet E. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Guedez L. Stetler-Stevenson WG. Wolff L. Wang J. Fukushima P. Mansoor A. Stetler-Stevenson M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest. 1998;102:2002–2010. doi: 10.1172/JCI2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkel R. Bock-Marquette I. Hazopoulos AK. Kupatt C. Thymosin beta4: A key factor for protective effects of eEPCs in acute and chronic ischemia. Ann NY Acad Sci. 2010;1194:105–111. doi: 10.1111/j.1749-6632.2010.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CX. Yuan MJ. Huang H. Wu G. Liu Y. Yu SB. Li HT. Wang T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides. 2009;30(12):2286–2291. doi: 10.1016/j.peptides.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kelly D. Cockerill G. Ng LL. Thompson M. Khan S. Samani NJ. Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodeling after acute myocardial infarction in man: A prospective cohort study. Eur Heart J. 2007;3:1–8. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahteenvuo JE. Lahteenvuo MK. Kivela A. Rosenlew C. Falkevall A. Klar J. Heikura T. Rissanen TT. Vahakangas E. Korpisalo P. Enholm B. Carmeliet P. Alitalo K. Eriksson U. Yla-Hertuala S. Vascular endothelial growth factor-b induces myocardium specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1 and neuropilin receptor 1 dependent mechanisms. Circulation. 2009;119:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- Lee SH. Wolf PL. Escudero R. Deutsch R. Jamieson SW. Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- Li Z. Guo J. Chang Q. Zhang A. Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull. 2009;32(8):1343–1346. doi: 10.1248/bpb.32.1343. [DOI] [PubMed] [Google Scholar]
- Lim SY. Kim YS. Ahn Y. Jeong MH. Hong MH. Joo SY. Nam KI. Cho JG. Kang PM. Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70(3):530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Lipinski MJ. Biondi-Zoccai GG. Abbate A. Khianey R. Sheiban I. Bartunek J. Vanderheyden M. Kim HS. Kang HJ. Strauer BE. Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Neto-Neves EM. Dias-Junior CA. Rizzi E. Castro MM. Sonego F. Gerlach RF. Tanus-Santus JE. Metalloproteinase inhibition protects against cardiomyocyte injury during experimental acute pulmonary thromboembolism. Crit Car Med. 2010;39:349–356. doi: 10.1097/CCM.0b013e3181fa3dfe. [DOI] [PubMed] [Google Scholar]
- Rossdeutch A. Smart N. Riley PR. Thymosin beta4 and Ac-SDKP: Tools to mend a broken heart. J Mol Med. 2008;86(1):29–35. doi: 10.1007/s00109-007-0243-9. [DOI] [PubMed] [Google Scholar]
- Ruff D. Crockford D. Girardi G. Zhang Y. A randomized, placebo-controlled, single and multiple dose study of intravenous thymosin beta4 in healthy volunteers. Ann NY Acad Sci. 2010;1194:223–229. doi: 10.1111/j.1749-6632.2010.05474.x. [DOI] [PubMed] [Google Scholar]
- Silvestre JS. Tmarat R. Ebrahimian TG. Le-Roux A. Clergue M. Emmanuel F. Duriez M. Schwartz B. Branellee D. Levy BL. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res. 2003;93:114–123. doi: 10.1161/01.RES.0000081594.21764.44. [DOI] [PubMed] [Google Scholar]
- Smart N. Risebro CA. Melville AA. Moses K. Schwartz RJ. Chien KR. Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445(7124):77–82. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Sosne G. Qiu P. Goldstein AL. Wheater M. Biological activities of thymosin beta 4 defined by active sites in short peptide sequences. FASEB. 2010;24:1–8. doi: 10.1096/fj.09-142307. [DOI] [PubMed] [Google Scholar]
- Squire IB. Evans J. Ng LL. Loftus IM. Thompson MM. Plasma MMP-9 and MMP-2 following acute myocardial infarction in Man: Correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004;10:328–333. doi: 10.1016/j.cardfail.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Srivastava D. Saxena A. Dimaio JM. Bock-Marquette I. Thymosin beta4 is cardioprotective after myocardial infarction. Ann NY Acad Sci. 2007;1112:161–170. doi: 10.1196/annals.1415.048. [DOI] [PubMed] [Google Scholar]
- Tang YL. Zhao Q. Qin Z. Shen L. Cheng L. Ge J. Phillips MA. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80(1):229–237. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- Varneaeva E. Naderzhda A. Hannappel E. Sjogren MH. Rojkind M. Thymosin beta4 upregulates the expression of hepatocyte growth factor and downregulates the expression of PDGF-beta receptor in hepatic stellate cells. Ann NY Acad Sci. 2007;1112:154–160. doi: 10.1196/annals.1415.035. [DOI] [PubMed] [Google Scholar]
- Walter DH. Haendeler J. Reinhold J. Rochwalsky U. Seeger F. Honold J. Hoffmann J. Urbich C. Lehmann R. Arenzana-Seisdesdos F. Aicher A. Heeschen C. Fichtlscherer S. Zeiher AM. Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- White HD. Norris RM. Brown MA. Brandt PW. Whitlock RM. Wild CJ. Left ventricular end systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]