SUMMARY
BACKGROUND
In high tuberculosis (TB) burden, resource-poor countries, sputum smear microscopy remains the mainstay of diagnosis. The low sensitivity of this test means that patients with smear-negative but culture-positive TB pass undetected through the health care system. Such clinical episodes are missed opportunities for diagnosis and interruption of transmission, which might be averted through the application of more sensitive diagnostic tests.
OBJECTIVES
To estimate the proportion of incident TB cases that might have been detected earlier than the actual date of diagnosis if a test more sensitive than smear microscopy had been used at an earlier presentation episode.
METHOD
Retrospective cohort study in urban Peru, investigating health care facility interactions for symptoms suggestive of TB prior to TB diagnosis through patient interviews and a review of clinical records.
RESULTS
Of 212 participants enrolled, 58% had one or more clinical interactions prior to their diagnostic episode. Of those with a prior episode, the median number of episodes was three. The median delay to diagnosis from first presentation was 26 days.
CONCLUSION
There are clear missed opportunities for earlier TB diagnosis, delaying treatment initiation and continued spread of Mycobacterium tuberculosis to the community. The implementation of sensitive diagnostic tests appropriate to resource-poor settings should be given high priority.
Keywords: tuberculosis, diagnosis, smear microscopy
FOR THE MAJORITY of tuberculosis (TB) suspects around the world, the only diagnostic test available is sputum smear microscopy.1 Although inexpensive and relatively straightforward, this 100-year-old method fails to detect at least half of cases identified by sputum culture.2–6 As a result, patients with smear-negative but culture-positive TB pass undetected through health care systems where smear microscopy is the only diagnostic modality available, and are told incorrectly that they do not have TB. Some, but presumably not all, find their way back to the clinic when their disease has advanced sufficiently to be detectable by smear microscopy. In the interim, such individuals will have accrued greater morbidity and will have continued transmitting Mycobacterium tuberculosis to their contacts.7 These initial clinical episodes are therefore missed opportunities for earlier diagnosis, treatment and interruption of transmission, which may be avoidable through the application of more sensitive diagnostic tests.
New diagnostic tests suitable for use in resource-poor settings are available.8,9 Liquid culture was recommended in 2007 by the World Health Organization (WHO) for wider implementation in global TB control, as it is faster and more sensitive than solid media culture and can reliably determine drug susceptibility.10,11 Manual Mycobacteria Growth Indicator Tube (MGIT) and microscopic observation drug susceptibility assay (MODS) are sensitive assays with fewer technical and economic constraints than automated platforms.12,13
However, the extent of the potential impact of even a highly sensitive low-cost test depends on the frequency of the opportunities missed for diagnosis. If, in reality, a high proportion of diagnosed TB patients have positive sputum smear results on first presentation to a health facility, there is little room for improvement regardless of the superiority of a new test. Consideration of the frequency of opportunities missed for earlier diagnosis may therefore be at least as important as the sensitivity of the test.
The present study aimed to determine the proportion of incident cases of microbiologically confirmed (smear-positive or culture-positive) pulmonary TB that might have been detected earlier than the actual date of diagnosis if a test more sensitive than smear microscopy had been used at an earlier presentation. The specific outcomes of interest were the proportion of TB patients with at least one clinical interaction prior to their diagnostic episode (and therefore at least one missed opportunity for diagnosis), and within this group the time delay incurred from the first clinical episode to diagnosis.
METHODS
Setting
This retrospective cohort study was undertaken in the adjacent districts of San Juan de Miraflores and Villa María del Triunfo, a high TB burden region in south Lima, Peru (TB incidence 150 per 100 000 in 2006), with an estimated adult human immunodeficiency virus (HIV) prevalence rate of 0.5% and HIV prevalence in incident TB cases of 6.7%.14,15
Study population
Study subjects were current adult TB patients (aged ≥18 years) who had been diagnosed with microbiologically confirmed (acid-fast bacilli smear or Ogawa culture-positive) pulmonary TB within the previous 6 months and who were receiving treatment under the National TB Programme (NTP).
Study procedures
All NTP clinical facilities in the study districts were visited to identify potential participants from the TB register of current patients. Patients meeting the study criteria were invited to participate and were provided with an information sheet describing the study and their potential involvement. Each participant was required to give written informed consent. Participants were interviewed either in the clinical facilities where they received treatment or, when this was not possible, in their homes. A complete history of clinic visits and sputum sample submission was obtained from a semi-structured interview with each participant; relevant TB control programme and clinical and laboratory workbooks were reviewed to identify confirmatory information for each participating patient. When documentation was available, dates and outcomes of the interactions were abstracted using a standardised data collection form, including information from distant and private health facilities, if relevant.
To calculate the number of prior clinical episodes and the subsequent delay to final diagnosis, it was necessary to consolidate several sources of data. Thus, as data abstracted from written records did not always correspond to the information reported by the patients, the following hierarchical approach was taken. The date of TB diagnosis was taken as the recorded date of the initial positive microbiological result. The information from a written record of an episode was used unless this information was either missing or inaccessible, in which case the participant’s estimate was used. If a participant could only recall a date down to the month and not the day, the 15th day of the month was assigned.
Definitions
Information was obtained on health care episodes in the 12 months prior to TB diagnosis. An episode was defined as any clinical interaction at a public or private health care establishment that included consultation with a physician or TB nurse, or the independent submission of sputum specimens for TB smear microscopy unrelated to a clinic visit. It is not uncommon for patients to submit sputum samples for testing directly to their local health centre (which is free), rather than to first consult with a health professional (which entails a nominal fee). The submission of two sputum samples was considered as one episode, as per local guidelines for sputum collection. When submission of one or two sputum samples occurred in conjunction with a clinic visit, all were defined as one episode. On occasions when patients continued to submit further specimens (usually to avoid paying for additional consultations) each successive two specimens were counted as a separate episode. The rationale for this was twofold: the local guidelines for the diagnosis of TB recommend the submission of two specimens per visit, and subsequent pairs of samples were almost never submitted within the same week, and thus represented temporally discrete additional opportunities for diagnosis. Episodes for reasons other than potential TB, or when TB was not considered by the attending health practitioner (based on clinical record review or patient interview), were not included. Each episode was classified as follows: 1) TB considered but sputum smear result not available; 2) TB considered and sputum smear result available. Reasons for which no smear result was available include: a specimen was not requested, the patient was unable or unwilling to produce sputum, and the specimen or the result was lost.
Data entry and analysis
Data were double-entered and analysed in an Access (MS) database (Microsoft, Redmonds, WA, USA). The frequency distribution of prior clinical episodes over time was determined and routine summary statistics were calculated. Proportions were compared by χ2 using Epi Info 6.4 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Ethics
Ethics approval was granted by the Institutional Review Board of the Universidad Peruana Cayetano Heredia. Permission to conduct the study within the Lima Sur region was granted from the regional health department (Dirección de Salud Lima Sur), and local permission from each health facility visited was obtained on arrival.
RESULTS
Study population
Of 424 TB patients identified, microbiological confirmation was lacking for 96 (22.6%), leaving 328 (77.4%) potential participants, of whom 212 (50.0%) were located and enrolled. It was not possible to locate 113 (26.6%) eligible participants; a further three declined the invitation to participate; 43.4% of those who participated were female. The overall median age was 30 years (range 18–86). The median time since diagnosis was 86.4 days (interquartile range [IQR] 46.8–132.3).
Diagnostic profile
The majority of the patients included in this study (96.2%) were diagnosed by positive sputum smear microscopy; eight (3.8%) had a positive sputum culture and negative smear. Symptom profile at the time of diagnosis was generally similar for patients diagnosed at their first visit and those diagnosed after more than 1 visit. Among the 118 patients with multiple episodes, only three symptoms, cough for at least 2 weeks, sputum production and weight loss, were significantly more frequent at the time of diagnosis than at earlier visits (Table).
Table.
Reported symptom profile at diagnosis and at earlier non-diagnostic clinical episodes
| Diagnosed following repeated episodes |
||||
|---|---|---|---|---|
| Symptom | Diagnosed at first episode (n = 94) % |
At diagnosis (n = 118) % |
At episodes prior to diagnosis (n = 203) % |
P value* |
| Cough ≥2 weeks | 91.5 | 92.4 | 84.7 | 0.05 |
| Sputum production | 84.0 | 77.1 | 65.0 | 0.02 |
| Fever | 64.9 | 55.9 | 48.3 | 0.19 |
| Haemoptysis | 33.0 | 41.5 | 35.5 | 0.28 |
| Weight loss | 84.0 | 81.4 | 70.0 | 0.02 |
| Night sweats | 68.1 | 60.2 | 50.2 | 0.08 |
| Chest pain | 72.3 | 74.6 | 68.5 | 0.25 |
| Breathlessness | 59.6 | 63.6 | 57.1 | 0.26 |
χ2 comparison of ‘at diagnosis’ vs. ‘prior to diagnosis’. There were no statistically significant differences between ‘diagnosed at first episode’ and ‘at diagnosis’ for those with repeated episodes.
Diagnostic delay
The 212 participants had a total of 426 clinical episodes: 89 (42.0%) were diagnosed on first presentation, 60 (28.3%) had two episodes, 41 (19.3%) had three, 18 (8.5%) had four, 2 (0.9%) had five and 2 (0.9%) had six episodes. For the 58.0% of patients with at least one prior clinical episode, the diagnostic visit occurred a median of 26 days after their first episode (IQR 6–61.5 days). All participants with prior episodes had at least one negative smear result from one of the prior non-diagnostic episodes.
Missed opportunities
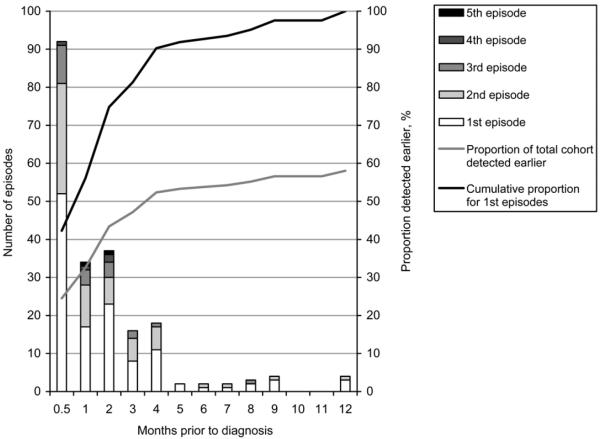
The frequency distribution of episodes failing to result in a diagnosis of TB (missed opportunities) in the 12 months prior to microbiologically confirmed diagnosis is shown in Figure 1. The cumulative proportion of all these episodes, going backward in time, is shown by the curve.
Figure 1.
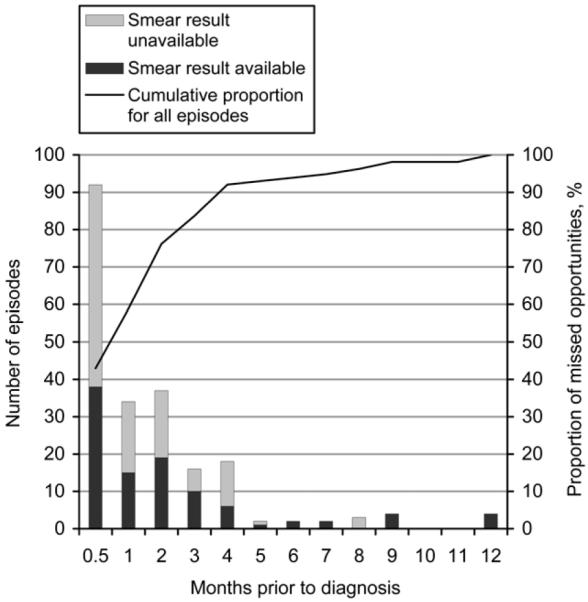
Distribution of missed opportunities in the 12 months preceding diagnosis for participants with at least one prior non-diagnostic episode.
Of the 214 non-diagnostic episodes, 126 occurred during the month prior to diagnosis. The distribution of episodes is shown by available and unavailable smear result. A smear result was not available in 53% of the prior clinical episodes. It can be seen that 76.2% of missed opportunities occurred within the 2-month period prior to diagnosis. Within 3, 4, 6 or 10 months, respectively 83.6%, 92.1%, 93.9% and 98.1% of missed opportunities occurred prior to positive smear-based diagnosis. The temporal distribution of the first and subsequent missed opportunities for each participant is shown in Figure 2.
Figure 2.
Temporal distribution of first and subsequent missed opportunity episodes. The cumulative proportion of first episodes is shown. If all participants had been diagnosed on first presentation, this curve also represents the cumulative proportion of earlier diagnoses at any given time point. Thus, 56.1% of those with a delay in diagnosis would have been diagnosed within the 1 month prior to their diagnosis, and 74.8% within the preceding 2 months. The grey line shows the cumulative proportion for the first episodes of the total cohort, including the 89 who were diagnosed on first presentation. Again assuming TB had been detected on the first presentation of all participants, 32.6% of the total cohort would have been diagnosed up to 1 month earlier. At 2, 6 or 10 months prior to positive smear-based diagnosis, 43.4%, 53.8% or 56.6% of all participants would have been detected earlier.
DISCUSSION
The premise for this study was that patients with TB frequently pass through the health care system more than once on their journey to a TB diagnosis, but the frequency and temporal relation to eventual diagnosis has not been previously described. These data demonstrate that over half of TB patients had prior interactions with clinical services that might represent missed opportunities for diagnosis. Although half of these episodes occurred in the 26 days preceding diagnosis, the remainder occurred over many months. These prolonged periods of undiagnosed disease likely result in increased morbidity and increased household and community transmission.
To what extent are these missed diagnostic opportunities avoidable with the new diagnostic tools that are becoming available? The potential incremental benefit of new diagnostic tool implementation depends upon sensitivity of detection and how frequently that additional sensitivity is needed. At first glance it appears that sputum smear microscopy performs well: 71% of patients were diagnosed either at their first visit (42%) or within the subsequent 26 days (29%); however, this leaves the long tail of 29% of eventually smear-positive cases that are missed.
An important assumption underlying the conclusions of this study is that when TB patients presented to health care services prior to the episode at which the diagnosis was made, they had active TB that would have been detected if they had undergone testing by a more sensitive tool. This requires that 1) TB is considered in the differential and a sample taken for testing; and 2) the patient does indeed have active TB detectable by, for example, culture. As this was a retrospective study, samples from previous non-diagnostic episodes were no longer available. Study results were therefore analysed under two scenarios: those that generated an available smear result and those that did not.
In this study, all patients with at least one episode prior to their diagnostic episode had an interaction that generated a negative sputum smear result, although in just over half of the prior episodes reported, a sputum smear result was unavailable. While it may be argued that episodes without a sputum smear are not missed opportunities—as an improved diagnostic tool is of no use without a specimen—it might also be the case that a more sensitive, rapid diagnostic might influence clinician practice, and thus the proportion of interactions that generate a specimen.
Even if all patients had been tested at every previous clinical interaction, they would not necessarily all have had smear-negative, culture-positive TB at every prior episode. However, the utility of the cumulative frequency percentage curve for missed opportunities in Figure 1 is that the cumulative proportion of missed opportunities that might have resulted in detectable disease at an earlier timepoint can be derived, depending upon the duration selected. Similarly, the cumulative proportion of patients at their first episode that might have been detectable at an earlier timepoint can be derived from Figure 2. The detection of cases at earlier timepoints depends both upon the natural history of smear-positive TB and upon the sensitivity of the test. Although test performance, which affects the sensitivity of smear microscopy, was not directly evaluated in this retrospective study, all laboratories in the NTP network conduct ongoing smear quality control and participate in the National Reference Laboratory external quality assessment programme, and there is no reason to believe that performance was substandard. We thus believe these data to be generalisable.15
Information from the interviews was corroborated with recorded data. In practice, data verification was incomplete, as records were often missing and documentation was patchy, thus a hierarchical approach to consolidate the data was developed. Studies relying on record review will invariably miss the full picture, especially where no single provider oversees the care of an individual. Although participant recall bias is a concern, patients themselves are assumed to provide more comprehensive and complete information than can be found in the records alone.
A possible bias in the results relates to the eligible non-participants who could not be located. This group almost exclusively fell into two categories: those whose time at the clinic or home was minimal due to work and those who had given a false address. Trying to locate patients at their place of work was considered inappropriate on ethical grounds. Enrolled subjects consisted of those employed and unemployed, as well as retired people, students and homemakers. It is not known whether these missing participants would contribute different or similar results.
As only patients with microbiologically confirmed TB were included in this study, the analysis does not take into account the experience of patients with smear-negative disease started on TB treatment based on clinical or radiographic criteria. While they do not contribute to the extent of missed opportunities identified in this study, this subgroup of patients, which formed nearly a quarter of all potential participants, likely represents further opportunities for a more sensitive test to provide earlier diagnosis.
While this is the first study to identify and quantify specific missed opportunities for diagnosis using current diagnostic algorithms in Peru, the urgent need for more accurate, affordable tools to diagnose and control TB is well recognised.16 A recently published systematic review of delay in the diagnosis and treatment of TB found that negative sputum smear was significantly associated with diagnostic delay.17 A cross-sectional study of diagnostic and treatment delay among pulmonary TB patients in Ethiopia found a median delay from the first health provider visit to initiation of treatment of 61 days, although the frequency or timing of visits was not reported.18 The frequency of missed diagnostic opportunities is at least as important as test sensitivity for improving diagnoses. If this study had demonstrated that missed diagnostic opportunities were uncommon, the implementation of even a highly sensitive test would have been unlikely to deliver sufficient incremental benefit to warrant usage. However, these data clearly show that there is a need for wider use of more sensitive diagnostics to bridge this diagnostic gap. Such tests should also deliver rapid results—rapid liquid culture methods satisfy the requirements for increased sensitivity and speed.
CONCLUSION
There are clear opportunities missed for the diagnosis of tuberculosis, resulting in a delay in treatment for patients and the continued spread of M. tuberculosis in the community. The implementation of sensitive diagnostic tests relevant to the resource-poor setting must be a priority.
Acknowledgements
This study was funded by a Clinical Exchange grant from the British Infection Society. SLB is grateful for mentor support from M Newport. DAJM is supported by The Wellcome Trust (078067/Z/05/Z) and is also grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme and mentor support from B Gilman and J Friedland. Thanks also to P Maguina for administrative support. The funders had no input into the contents of this manuscript.
References
- 1.World Health Organization . Global tuberculosis control: surveillance, planning, financing. WHO; Geneva, Switzerland: 2008. WHO/HTM/TB/2008.393. [Google Scholar]
- 2.Frimpong EH, Adukpo R, Owusu-Darko K. Evaluation of two novel Ziehl-Neelsen methods for tuberculosis diagnosis. West Afr J Med. 2005;24:316–320. doi: 10.4314/wajm.v24i4.28224. [DOI] [PubMed] [Google Scholar]
- 3.Githui W, Kitui F, Juma ES, Obwana DO, Mwai J, Kwamanga D. A comparative study on the reliability of the fluorescence microscopy and Ziehl-Neelsen method in the diagnosis of pulmonary tuberculosis. East Afr Med J. 1993;70:263–266. [PubMed] [Google Scholar]
- 4.Perkins MD, Roscigno G, Zumla A. Progress towards improved tuberculosis diagnostics for developing countries. Lancet. 2006;367:942–943. doi: 10.1016/S0140-6736(06)68386-4. [DOI] [PubMed] [Google Scholar]
- 5.Levy H, Feldman C, Sacho H, van der Meulen H, Kallenbach J, Koornhof H. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest. 1989;95:1193–1197. doi: 10.1378/chest.95.6.1193. [DOI] [PubMed] [Google Scholar]
- 6.Urbanczik R. Present position of microscopy and of culture in diagnostic mycobacteriology. Zentralbl Bakteriol Mikrobiol Hyg [A] 1985;260:81–87. doi: 10.1016/s0176-6724(85)80101-2. [DOI] [PubMed] [Google Scholar]
- 7.Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 8.Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part II. Active tuberculosis and drug resistance. Expert Rev Mol Diagn. 2006;6:423–432. doi: 10.1586/14737159.6.3.423. [DOI] [PubMed] [Google Scholar]
- 9.Wattal C. Improving bacteriological diagnosis of tuberculosis. Indian J Pediatr. 2002;69(Suppl 1):S11–S19. [PubMed] [Google Scholar]
- 10.World Health Organization . Strategic TB Advisory Group (STAG-TB): report on conclusions and recommendations. WHO; Geneva, Switzerland: 2007. [Google Scholar]
- 11.Somoskovi A, Kodmon C, Lantos A, et al. Comparison of recoveries of mycobacterium tuberculosis using the automated BACTEC MGIT 960 system, the BACTEC 460 TB system, and Löwenstein-Jensen medium. J Clin Microbiol. 2000;38:2395–2397. doi: 10.1128/jcm.38.6.2395-2397.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adjers-Koskela K, Katila ML. Susceptibility testing with the manual mycobacteria growth indicator tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J Clin Microbiol. 2003;41:1235–1239. doi: 10.1128/JCM.41.3.1235-1239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore DA, Evans CA, Gilman RH, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . UNAIDS/WHO epidemiological fact sheet on HIV and AIDS, Peru, 2008 update. WHO; Geneva, Switzerland: 2009. Joint United Nations Programme on HIV/AIDS, United Nations’ Children’s Fund. [Google Scholar]
- 15.World Health Organization . TB country profile: Peru. WHO; Geneva, Switzerland: 2009. [Google Scholar]
- 16.Millen SJ, Uys PW, Hargrove J, van Helden PD, Williams BG. The effect of diagnostic delays on the drop-out rate and the total delay to diagnosis of tuberculosis. PLoS ONE. 2008;3:e1933. doi: 10.1371/journal.pone.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia: a cross sectional study. BMC Infect Dis. 2005;5:112. doi: 10.1186/1471-2334-5-112. [DOI] [PMC free article] [PubMed] [Google Scholar]