Abstract
Rheumatoid arthritis (RA) is characterized by synovial inflammation mediated by T-cells, monocytes and macrophages. The homing of these cells to the inflamed synovium is regulated by chemokine-receptors and their ligands. A 32-basepair deletion (Δ32) in the gene encoding CCR5, a chemokine-receptor, results in a non-functional receptor. A negative association between CCR5-Δ32 and RA has been described, although other studies found no associations. Furthermore, the observation that individuals homozygous for CCR5-Δ32 develop RA has raised questions about the role of CCR5-Δ32. This meta-analysis of all published case-control association studies confirms the negative association between CCR5-Δ32 and RA (Odds Ratio = 0.65; 95 % confidence intervals = 0.55 - 0.77; p < 0.0001), suggesting that CCR5-Δ32 is protective against the development of RA. CCR5 blockade in animal models of RA results in amelioration of arthritis, suggesting that CCR5 blockade could also modify disease in patients with RA.
Keywords: Rheumatoid arthritis, CCR5, association, case-control, meta-analysis, chemokines
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory arthritis with a prevalence of ∼1 % in the United States. While genetic associations between RA and variants of the human leukocyte antigens (HLA) are well established, these only account for part of the genetic susceptibility to RA, suggesting variation in non-HLA genes might play important roles in the susceptibility to RA1. Association and linkage studies have successfully identified several non-HLA variants associated with RA. Case-control association studies face several limitations, including poor choice of candidate genes and subjects, population stratification, and lack of power due to inadequate number of subjects. These limitations can result in spurious associations and/or lack of replication2. One way to overcome these challenges is to ensure that studies have adequate sample sizes and by selecting candidate genes with a compelling role in the pathogenesis of the phenotype being examined.
RA is characterized by the expansion of inflamed synovial tissue, which demonstrates prominent infiltrates of T-cells, plasma cells, and macrophages. A complex network of chemokines and adhesion molecules coordinates the recruitment of leukocytes to sites of inflammation. CCR5 is a chemokine receptor involved in the migration of immune cells to the inflamed RA synovium3,4. CCR5 is preferentially expressed on the surfaces of T-cells, monocytes and macrophages and its ligands include the proinflammatory chemokines RANTES, macrophage inflammatory proteins (MIP)-1α and MIP-1β.
Synovial T-cells from patients with active RA express high levels of CCR5 5-7. In addition, RA synovial fluid also contains high levels of the CCR5 ligands MIP-1α and RANTES8,9. These observations suggest that CCR5 is involved in the recruitment of inflammatory cells into the inflamed synovial tissue in RA. This led to the hypothesis that alterations in the expression of CCR5 would influence the susceptibility to, and/or the phenotype of, RA. A 32-basepair deletion in the open reading frame of the gene encoding CCR5 (CCR5-Δ32) results in a truncated protein which cannot be detected at the cell surface10. It is plausible that this loss of function mutation, which has an allele frequency of ∼10 % in some European populations 11, could have a protective effect against RA.
To date, five case-control association studies of RA and CCR5-Δ32 in populations of European ancestry have been published12-16. Two of them demonstrated statistically significant negative associations between CCR5-Δ32 and RA13,14. Three other studies did not demonstrate statistically significant associations but suggested a negative association. It has been reported that individuals heterozygous for CCR5-Δ32 have a milder phenotype of disease12,15. However, others have concluded that the presence of CCR5-Δ32 does not influence the phenotype of RA13,17. Counter to the proposed role of CCR5 in RA are observations of individual RA patients who are homozygous for CCR5-Δ3216-19. In addition, one study showed no difference in the predominance of CCR5-positive cells in the synovial fluid between patients with RA that are homozygous for the wild type CCR5 allele and those heterozygous for CCR5-Δ3219. Together, these observations raise questions about the influence of CCR5-Δ32 on the pathogenesis and the phenotype of RA.
The objective of the present study was to perform a meta-analysis of the published studies in order to: 1) increase statistical power, 2) determine if the associations described in some studies could be replicated, and 3) quantify the genetic risk of CCR5-Δ32. All five published case-control association studies of RA and CCR5-Δ32 in populations of European ancestry were included. An association study of Mexican Amerindian patients with RA and a study that included cases that did not fulfill criteria for a diagnosis of RA were excluded, as detailed in methods (See legend to table 1)17,20.
Table 1. Allele frequencies in patients with RA and healthy controls for the CCR5-Δ32 polymorphism.
| AUTHOR (REF) | COUNTRY | RA | CONTROLS | OR (95 % CI) | ||||
|---|---|---|---|---|---|---|---|---|
| SUBJECTS | CCR5-W N (%) | CCR5-Δ32 N (%) | SUBJECTS | CCR5-W N (%) | CCR5-Δ32 N (%) | |||
| Cooke (16) | W Europe | 278 | 514 (93) | 42 (7) | 266 | 475 (89) | 57 (11) | 0.68 (0.45 – 1.03) |
| Garred (12) | Denmark | 163 | 292 (90) | 34 (10) | 151 | 261 (86) | 41 (14) | 0.74 (0.46 – 1.20) |
| Gomez-Reino (13) | Spain | 673 | 1268 (94) | 78 (6) | 815 | 1515 (93) | 115 (7) | 0.81 (0.60 – 1.09) |
| Zapico (15) | Spain | 160 | 302 (94) | 18 (6) | 500 | 904 (90) | 96 (10) | 0.56 (0.33 – 0.94) |
| Pokorny (14) | New Zealand | 516 | 976 (95) | 56 (5) | 985 | 1777 (90) | 193 (10) | 0.53 (0.39 – 0.72) |
| Pooled | 1790 | 3352 (94) | 228 (6) | 2717 | 4932 (90) | 502 (10) | 0.65 (0.55 – 0.77) | |
Published case-control association studies of CCR5-Δ32 and RA were identified using a PUBMED search. To be included studies had to be in English and report genotype and/or allele frequency information on CCR5-Δ32 among patients with RA and ethnicity matched controls. Only studies performed on individuals of European ancestry were included to minimize population stratification. A PUBMED search using the terms “rheumatoid arthritis” and “CCR5” uncovered 64 articles, of which six were case-control association studies. Of these, five studies were performed on individuals of European ancestry. The references of these studies were also reviewed for other association studies not included in the PUBMED database. One study included individuals of Amerindian ancestry and was excluded.20 Another study had genotype information on cases with inflammatory arthritis but had no controls.17 That study also included individuals with inflammatory arthritis and only 45 % of them met criteria for a diagnosis of RA at diagnosis, and only 75 % met criteria for a diagnosis of RA at 5 years. For these reasons, that study was also excluded. Data extracted included the year of publication, ethnicity of subjects, numbers of cases and controls, allele and genotype frequencies, and information regarding gender distribution and phenotype of cases. Refined analysis by rheumatoid factor status and gender were precluded by the lack of availability of such information from all studies. Of the 5 studies, three studies provided proportion of female patients12,14,15, which ranged from 71 % to 84 %, similar to frequencies of female RA patients in other published studies. Only one study provided the proportion of patients positive for rheumatoid factor.12
Genotype and allele frequency data were obtained from the published studies. Odds ratios (OR) and 95 % confidence intervals (95 % CI) shown were calculated for the presence of the CCR5-Δ32 allele. The pooled OR was calculated under a fixed-effects model. The studies were not weighted by size. Mantel-Haenszel Chi square tests were performed for the individual studies and for the studies combined, stratifying for the different studies using the FREQ procedure in SAS, version 9.1. Meta-analysis was considered to be significant if the 95 % CI around the pooled OR for the combined studies excluded 1.0. The Breslow-Day test was performed to test for heterogeneity among the different studies, with significance for heterogeneity set at p <0.05. The Breslow-day Chi-square was 4.5, (4 df), p > 0.344, suggesting no heterogeneity among the studies.
Results and discussion
Genotype and allele frequency data were available on a total of 4507 individuals (1790 RA cases and 2717 controls). Cases and controls were individuals of European ancestry from Spain, Denmark, New Zealand and England. The frequency of the CCR5-Δ32 allele ranged from 5 % to 10 % among patients with RA, with a mean frequency of 6 % (Table 1). Among the controls, the frequency of CCR5-Δ32 allele was higher, ranging from 7 % to 14 %, with a mean frequency of 10 %. Odds ratios (OR) from these five studies were all less than one, ranging from 0.33 to 0.81, although some of the 95 % confidence intervals (CI) overlapped 1.0 and were not statistically significant (Figure 1). The pooled OR was 0.65, (95 % CI: 0.55 to 0.77; p <0.0001), demonstrating a highly significant negative association between RA and CCR5-Δ32 in populations of European ancestry. The non-significant p value (p > 0.34) for the Breslow-Day test indicated no heterogeneity in the studies, suggesting that data from these studies could be pooled with confidence.
Figure 1. Meta-analysis of 5 studies addressing the association between CCR5-Δ32 and rheumatoid arthritis.
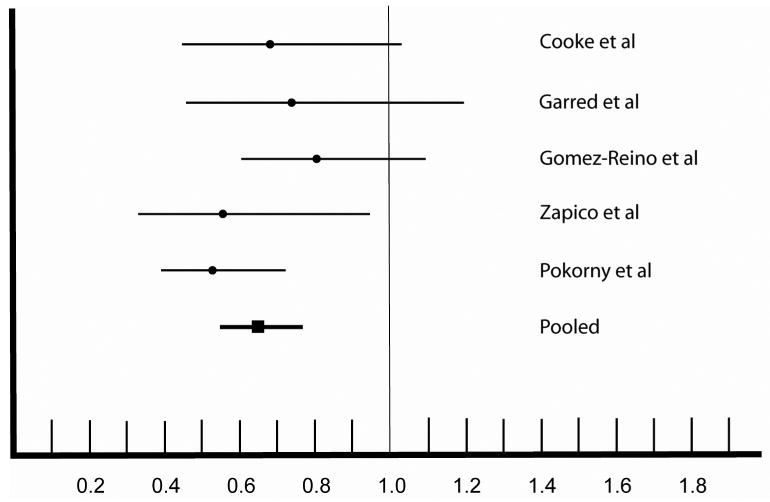
This figure depicts the odds ratio (OR) and 95 % confidence intervals (CI) of the association between CCR5-Δ32 and RA. The data were obtained from the five studies indicated on the right. The estimated OR from each study is shown as a dark circle, with horizontal lines extending to the 95 % CI of the OR. The pooled OR from the five studies is shown as a square, with the thick horizontal line depicting the 95 % CI. Values below 1.0 indicate a negative association with RA indicating protection against RA. Confidence intervals that overlap 1.0 indicate lack of statistical significance.
These five studies were also analyzed for associations of different CCR5 genotypes with RA (Table 2). The pooled OR for the comparison of the frequency of wild-type CCR5 homozygotes to the frequency of CCR5-Δ32 heterozygotes was 0.70 (95 % CI: 0.59 to 0.83; p = 0.0001). The Breslow-Day test was not statistically significant (p = 0.35). When the data were analyzed by comparing the frequency of wild-type CCR5 homozygotes to the frequency of CCR5-Δ32 homozygotes, the pooled OR was much lower, with an OR (95% CI) of 0.16 (0.05 to 0.48; p = 0.0008). The Breslow-Day test was not statistically significant (p = 0.08). It should be noted, however, that there were only 4 individuals with RA that were homozygous for CCR5-Δ32, and the individual OR could not be calculated for three studies. Nevertheless, the proportion of cases with the CCR5-Δ32/CCR5-Δ32 genotype was about a fifth that of controls. The fact that CCR5-Δ32 homozygosity confers a much greater protective effect than does CCR5-Δ32 heterozygosity suggests a gene dosage effect.
Table 2. Genotype frequencies in patients with RA and healthy controls for the CCR5-Δ32 polymorphism.
| REF | RA | Controls | OR (95 % CI) W/W vs. Δ32/W | OR (95 % CI) W/W vs. Δ32/ Δ32 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | W/W | W/ Δ | Δ / Δ | N | W/W | W/ Δ | Δ / Δ | |||
| 16 | 278 | 238 (86) | 38 (14) | 2 (0.7) | 266 | 212 (80) | 51 (19) | 3 (1.4) | 0.66 (0.42 – 1.05) | 0.59 (0.10 – 3.6) |
| 12 | 163 | 131(80) | 30 (18) | 2 (1.2) | 151 | 112 (74) | 37 (25) | 2 (1.3) | 0.69 (0.40 – 1.20) | 0.85 (0.12 – 6.2) |
| 13 | 673 | 595 (88) | 78 (12) | 0 (0) | 815 | 707 (87) | 101 (12) | 7 (0.9) | 0.92 (0.67 – 1.30) | NC |
| 15 | 160 | 142 (89) | 18 (4) | 0 (0) | 500 | 410 (82) | 84 (17) | 6 (1.2) | 0.62 (0.36 – 1.07) | NC |
| 14 | 516 | 460 (89) | 56 (11) | 0 (0) | 985 | 804 (82) | 169 (17) | 12 (1.2) | 0.58 (0.42 – 0.80) | NC |
| 1790 | 1566 (87) | 220 (12) | 4 (0.2) | 2717 | 2245(83) | 442 (17) | 30 (1.1) | 0.70 (0.59 – 0.84) | 0.16 (0.05 –0.48) | |
This table shows the results of comparisons between individuals carrying the CCR5/CCR5 genotype (W/W) versus those with CCR5/CCR5-Δ32 (W/Δ) and those with CCR5-Δ32/CCR5-Δ32 genotypes using methods described in the legend to table 1. NC: Not calculated since there were no cases with the Δ32/ Δ32 homozygous genotype.
In addition to confirming that large sample sizes are required for detecting variants with modest associations with a phenotype, the findings from this meta-analysis have several implications. First, these results strongly demonstrate that the CCR5-Δ32 allele confers protection against RA in individuals of European ancestry. While this could be due to the role played by CCR5 in directing T-cells to the inflamed RA synovium, it is conceivable that CCR5 might play other roles. For instance it has been shown that RANTES-CCR5 signaling serves as an important regulator of cell survival and growth in astrocytes21. The protection conferred by CCR5-Δ32 against the development of RA, however, does not seem to be absolute, as demonstrated by the small numbers of individuals with RA who are homozygous for CCR5-Δ32. The chemokine system is redundant, and it is likely that other receptors and pathways play a role in the development of RA in these individuals. Furthermore, RA is a complex trait, and it is likely that variation in different genes contribute to susceptibility and/or phenotype (genetic heterogeneity). Examining the phenotypes of a large cohort of individuals with RA homozygous for CCR5-Δ32 would enable us to determine more accurately whether they tend to have a milder phenotype of RA.
It is possible that the observed association is due to another variant that is in linkage disequilibrium with CCR5-Δ32, such as the G allele of a single nucleotide polymorphism (A–2459G) in the 5′-cis regulatory region of CCR522,23. The association between CCR5-Δ32 and RA also raises the possibility that other functional variants at the CCR5 locus could affect susceptibility to RA. The 5′ cis-regulatory region of CCR5 has several single nucleotide polymorphisms. Some of these show alterations in binding of transcription factors, and consequently different CCR5 haplotypes are associated with differential transcriptional regulation24. Furthermore, different CCR5-haplotypes have been shown to be associated with different disease-modifying effects among patients with acquired immune deficiency syndrome22. Association studies of these other CCR5 variants in large cohorts of individuals with inflammatory arthritis including RA would also provide insights about genetic variation at the CCR5 locus and arthritis.
This meta-analysis does have some limitations. Ideally, analyses where data from individual subjects are pooled are preferable to a meta-analysis of published studies25, but a pooled analysis of data was beyond the scope of this study. Although very few studies were available for the meta- analysis, combining them did result in substantial numbers of cases and controls. Analysis of the data stratified by gender or rheumatoid factor status would have provided more information, but such information was not available from all of these studies. Another potential limitation is the presence of heterogeneity among studies. Although steps were taken to minimize heterogeneity by limiting analysis to individuals with RA with a European ancestry, it is possible that heterogeneity still existed between these studies. The statistical tests used to detect heterogeneity have low sensitivity, and hence, in spite of the absence of a statistically significant heterogeneity, some heterogeneity might still exist. However, it should be noted that the effects of all five studies included in the analysis were in the same direction, i.e., protective. It is also possible that there were differences in the definition of the phenotype. All studies reported that the cases had definite RA, and four of them used the American College of Rheumatology criteria for a diagnosis of RA. However, if some individuals who did not have RA were misclassified as having RA, then it would be expected to bias our results towards the null. All meta-analyses are also potentially subject to publication bias, whereby studies with negative or non-significant results tend to be published less often than those with positive results26. However, of these five studies, two did not find statistically significant associations between CCR5-Δ32 and RA,12,16 thus minimizing a publication bias. Furthermore, exclusion of the first published study by Cooke et al, and recalculating the pooled OR, did not change the results significantly (OR = 0.66; 95 % CI = 0.55 to 0.79).
Finally, the results of the present study suggest the possibility that CCR5 blockade could have clinical efficacy, at least in some subsets of patients with RA. In that respect, it is interesting that CCR5 blockade leads to inhibition of collagen-induced arthritis, an animal model of RA27,28.
Acknowledgments
Supported by grants from The National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23 AR50177), The National Center for Research Resources (M01-RR00064), The Val A Browning Charitable Foundation, and the Children's Health Research Center, Salt Lake City, UT. We would like to acknowledge Lynn Jorde PhD, Department of Human Genetics for critical review of the manuscript and suggestions for analysis.
References
- 1.Jawaheer D, Gregersen PK. The search for rheumatoid arthritis susceptibility genes: a call for global collaboration. Arthritis Rheum. 2002;46:582–4. doi: 10.1002/art.10169. [DOI] [PubMed] [Google Scholar]
- 2.Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33:177–82. doi: 10.1038/ng1071. [DOI] [PubMed] [Google Scholar]
- 3.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 4.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki N, Nakajima A, Yoshino S, Matsushima K, Yagita H, Okumura K. Selective accumulation of CCR5+ T lymphocytes into inflamed joints of rheumatoid arthritis. Int Immunol. 1999;11:553–9. doi: 10.1093/intimm/11.4.553. [DOI] [PubMed] [Google Scholar]
- 6.Ruth JH, Rottman JB, Katschke KJ, Jr, Qin S, Wu L, LaRosa G, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44:2750–60. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Katschke KJ, Jr, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, et al. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–32. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Robinson E, Keystone EC, Schall TJ, Gillett N, Fish EN. Chemokine expression in rheumatoid arthritis (RA): evidence of RANTES and macrophage inflammatory protein (MIP)-1 beta production by synovial T cells. Clin Exp Immunol. 1995;101:398–407. doi: 10.1111/j.1365-2249.1995.tb03126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, et al. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J Clin Invest. 1994;93:921–8. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 11.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 12.Garred P, Madsen HO, Petersen J, Marquart H, Hansen TM, Freiesleben Sorensen S, et al. CC chemokine receptor 5 polymorphism in rheumatoid arthritis. J Rheumatol. 1998;25:1462–5. [PubMed] [Google Scholar]
- 13.Gomez-Reino JJ, Pablos JL, Carreira PE, Santiago B, Serrano L, Vicario JL, et al. Association of rheumatoid arthritis with a functional chemokine receptor, CCR5. Arthritis Rheum. 1999;42:989–92. doi: 10.1002/1529-0131(199905)42:5<989::AID-ANR18>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Pokorny V, McQueen F, Yeoman S, Merriman M, Merriman A, Harrison A, et al. Evidence for negative association of the chemokine receptor CCR5 d32 polymorphism with rheumatoid arthritis. Ann Rheum Dis. 2005;64:487–90. doi: 10.1136/ard.2004.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapico I, Coto E, Rodriguez A, Alvarez C, Torre JC, Alvarez V. CCR5 (chemokine receptor-5) DNA-polymorphism influences the severity of rheumatoid arthritis. Genes Immun. 2000;1:288–9. doi: 10.1038/sj.gene.6363673. [DOI] [PubMed] [Google Scholar]
- 16.Cooke SP, Forrest G, Venables PJ, Hajeer A. The delta32 deletion of CCR5 receptor in rheumatoid arthritis. Arthritis Rheum. 1998;41:1135–6. doi: 10.1002/1529-0131(199806)41:6<1135::AID-ART24>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.John S, Smith S, Morrison JF, Symmons D, Worthington J, Silman A, et al. Genetic variation in CCR5 does not predict clinical outcome in inflammatory arthritis. Arthritis Rheum. 2003;48:3615–6. doi: 10.1002/art.11360. [DOI] [PubMed] [Google Scholar]
- 18.Venables PJ, Hajeer A. Delta32CCR5 and rheumatoid arthritis: comment on the article by Gomez-Reino et al. Arthritis Rheum. 1999;42:2732–3. doi: 10.1002/1529-0131(199912)42:12<2732::aid-anr37>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Mack M, Bruhl H, Gruber R, Jaeger C, Cihak J, Eiter V, et al. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 1999;42:981–8. doi: 10.1002/1529-0131(199905)42:5<981::AID-ANR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Zuniga JA, Villarreal-Garza C, Flores E, Barquera R, Perez-Hernandez N, Montes de Oca JV, et al. Biological relevance of the polymorphism in the CCR5 gene in refractory and non-refractory rheumatoid arthritis in Mexicans. Clin Exp Rheumatol. 2003;21:351–4. [PubMed] [Google Scholar]
- 21.Bakhiet M, Tjernlund A, Mousa A, Gad A, Stromblad S, Kuziel WA, et al. RANTES promotes growth and survival of human first-trimester forebrain astrocytes. Nat Cell Biol. 2001;3:150–7. doi: 10.1038/35055057. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez E, Bamshad M, Sato N, Mummidi S, Dhanda R, Catano G, et al. Race-specific HIV-1 disease-modifying effects associated with CCR5 haplotypes.PG - 12004-9. Proc Natl Acad Sci U S A. 1999;96 doi: 10.1073/pnas.96.21.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamshad MJ, Mummidi S, Gonzalez E, Ahuja SS, Dunn DM, Watkins WS, et al. A strong signature of balancing selection in the 5′ cis-regulatory region of CCR5. Proc Natl Acad Sci U S A. 2002;99:10539–10544. doi: 10.1073/pnas.162046399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K, et al. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA. Potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus.PG - 18946-61. J Biol Chem. 2000;275 doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- 25.Little J, Bradley L, Bray MS, Clyne M, Dorman J, Ellsworth DL, et al. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol. 2002;156:300–10. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- 26.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 27.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–20. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 28.Yang YF, Mukai T, Gao P, Yamaguchi N, Ono S, Iwaki H, et al. A non-peptide CCR5 antagonist inhibits collagen-induced arthritis by modulating T cell migration without affecting anti-collagen T cell responses. Eur J Immunol. 2002;32:2124–32. doi: 10.1002/1521-4141(200208)32:8<2124::AID-IMMU2124>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]


