Summary
Objective
To assess combined antidepressant and cognitive enhancer treatment in elderly patients presenting with depression plus cognitive impairment.
Methods
Twenty-three elderly (>50 years old) depressed, cognitively impaired (DEP-CI) patients participated in a pilot study. We evaluated whether, after 8 weeks of open antidepressant treatment, donepezil HCl (Aricept) would afford added cognitive benefit compared to placebo in a randomized 12-week trial. A subsample continued in an 8-month extension phase of open treatment with donepezil. Neuropsychological testing (NPT) was performed and antidepressant response monitored at baseline and the 8, 20, and 52-week time points.
Results
At 8-weeks, the antidepressant response rate was 61% (14/23). Improvement in SRT immediate recall (SRT-IR; e.g. episodic verbal memory) was observed in responders compared to non-responders. During the 12-week, placebo-controlled, donepezil add-on trial, patients on donepezil showed further improvement in SRT-IR versus patients on placebo. In the open extension phase, patients who continued open donepezil treatment (n = 6) maintained improvement in memory and tended to show an advantage over patients who never received donepezil and were evaluated at the 52-week time point (n = 6). There were no observed significant donepezil effects on non-memory cognitive domains.
Conclusion
These preliminary findings suggest that addition of a cholinesterase inhibitor (AChEI) following antidepressant medication treatment in elderly Dep-CI patients may improve cognition, and support the need for a confirmatory, larger randomized placebo-controlled trial.
Keywords: depression, cognitive impairment, memory decline, donepezil, randomized clinical trial, aging
Introduction
Depression in the elderly is a major cause of suffering and disability, and worsens outcome in patients with co-morbid medical illnesses (Charney et al., 2003). In the elderly, the most common neuropsychiatric disorders are depression (DEP) and cognitive impairment (CI; e.g. defined as a transitional phase between normal and dementia). Their co-occurrence (DEP-CI) may exceed chance (Burt et al., 1995). Clinical and epidemiological studies suggest that the presence of depression in patients with CI increases the likelihood of conversion to dementia, primarily Alzheimer's Disease (AD) (Devanand et al., 1996; Wilson et al., 2002). Therefore, treatment strategies for DEP-CI have longer-term implications beyond acute antidepressant treatment response (Alexopoulos et al., 1993).
In elderly depressed patients, greater severity of depression (Yaffe et al., 1999; Taylor et al., 2002), increased number of prior depressive episodes (Beats et al., 1996), increased age (Lyness et al., 1995; King et al., 1998), increased age of onset of depression (Salloway et al., 1996, Rapp et al., 2005), and increased vascular risk load (Hickie et al., 1995; Krishnan et al., 1998) appear to predict worse cognitive functioning on follow-up, but discrepant studies exist (Boone et al., 1994; Butters et al., 2004). In DEP-CI patients, physicians often empirically treat with antidepressant medications. Based on antidepressant response and the patient's subjective impression of cognitive improvement over time, the physician typically decides the diagnosis and treatment strategy. If cognition improves, the assumption is that the cognitive deficits are secondary to depression; if cognition does not improve, a work-up for dementia may be initiated. This approach remains to be validated by empirical research.
Extensive data support the use of acetylcholinesterase inhibitors (AChEIs), including donepezil, to treat mild to moderate AD (Burns et al., 1999; Tariot et al., 2000; Doraiswamy et al., 2002). Some treatment studies with AChEIs targeting non-demented patient populations with CI have shown cognitive benefits though the effect size is consistently small (Christodoulou et al., 2006; Demaerschalk and Wingerchuk, 2007) and the benefit may not last beyond a few months (Salloway et al., 2004; Petersen et al., 2005).
We previously reported limited to no improvement in cognition over a 12-week trial of open label antidepressant treatment with sertraline in 39 DEP-CI patients (Devanand et al., 2003). A new DEP-CI pilot sample was recruited to evaluate the added cognitive benefit of add-on donepezil versus add-on placebo in a 12-week randomized double-blind placebo-controlled trial that took place after 8 weeks of open antidepressant treatment. A sub-sample that continued in an extension phase of open treatment with donepezil for an additional 8 months was compared to a sub-sample that was never treated with donepezil over the course of the study (see Figure 1).
Figure 1.
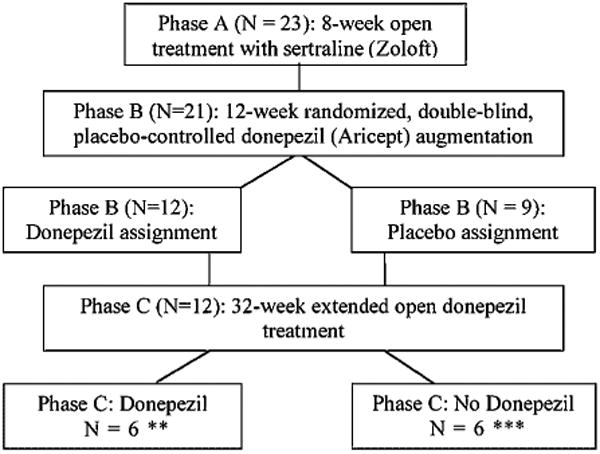
Flow diagram of patient treatment stratification during the trial.
* 2 patients dropped during Phase A and thus were excluded from Phase B analysis.
** 6 patients who had been assigned to donepezil during Phase B chose not to contuinue donepezil treatment during Phase C and thus were excluded form the Phases B + C analysis.
*** 3 patients who had been assigned to placebo during Phase B chose to begin donepezil treatment during Phase C and thus were excluded form the Phases B + C analysis.
Subjects and Methods
Outpatients 50 years or older were recruited from the Late Life Depression Clinic (65% of patients) and Memory Disorders Center (35% of patients) at the New York State Psychiatric Institute. These research clinics focus on depression and cognitive impairment/dementia, respectively. The study protocol was IRB-approved and written informed consent was obtained prior to study entry.
Inclusion/exclusion criteria
At baseline, the study psychiatrist (GHP or DPD) obtained a detailed medical history and conducted a general physical (including blood work), neurological, and psychiatric examination. A research social worker administered the SCID-P to evaluate Axis I disorders. Inclusion criteria for depression (DEP) were DSM-IV criteria for major depressive disorder, dysthymic disorder, or depression not otherwise specified (NOS), and a 24-item Hamilton Rating Scale for Depression (HRSD) ≥ 14. Inclusion criteria for cognitive impairment (CI) were subjective cognitive complaints and impaired performance (> 1 SD below standardized norms on at least two tests or >1.5 SD below standardized norms on at least one test) on a brief neuropsychological testing (NPT) battery. No activity of daily living (ADL) deficits were allowed.
Exclusion criteria were the diagnosis of other DSM-IV Axis 1 psychiatric conditions, dementia, Folstein Mini Mental State (MMS) score <20/30, or identified medical or neurological cause for mood or memory impairment. Patients on antidepressants were washed out for 7 days (n = 4).
Outcome measures
For depression, the study psychiatrist administered the 24-item HRSD and the Clinical Global Impression scale (CGI). Side effects were assessed by the Treatment Emergent Symptom Scale (TESS). A trained technician administered the NPT battery at baseline, 8-week, 20-week, and 52-week time points. For patients who exited any phase before completion, repeat NPT was completed at the time-point of exit. The NPT battery comprised the Buschke Selective Reminding Test (targeting memory), WAIS-III digit symbol and Trails B (executive function), CFL (verbal fluency), and Trails A (attention/psychomotor speed).
Treatment trial
Phase A: Open antidepressant treatment (baseline to week 8)
Based on a pilot study (Devanand et al., 2003), sertraline was the ‘first choice’ antidepressant; otherwise, ‘doctor's choice’ was administered. Medication dose was increased in a stepwise fashion, as clinically indicated, over the first four weeks or until an optimal tradeoff between efficacy and somatic side effects were reached.
Phase B: Donepezil vs placebo (week 8 to week 20)
The 12-week randomized, double-blind, placebo-controlled, add-on donepezil phase was initiated at week-8 for all patients with persistent subjective cognitive complaints or NPT scores >1 SD below standardized norms on any test at the 8-week time-point. Donepezil or placebo (in identical-looking tablets) was dispensed at 5 mg/day for the first 4 weeks and was increased to 10 mg/day (25 mg tablets) for the remainder of the trial.
Phase C: Extended open donepezil treatment (week 20 to week 52)
After the first 20 weeks (8-weeks antidepressant + 12-weeks donepezil/placebo add-on), all patients were offered open treatment with donepezil for an additional 32 weeks with monthly monitoring. Six Phase B donepezil treated patients wanted to continue on donepezil (‘donepezil’ group), and six Phase B placebo treated patients did not wish to receive open-extension donepezil (‘no-donepezil’). This was an open, naturalistic follow-up phase.
Statistical analyses
Intent-to-Treat analyses with the Last Observation Carried Forward (ITT-LOCF) was used in each phase of the trial. A mixed ANOVA was conducted for each a priori defined NPT outcome measure with time (Phase A: baseline and week-8; Phase B: weeks 8 and 20; Phase C: weeks 8, 20 and 52) as the within-subjects repeated measures factor, group (Phase A: antidepressant response; Phase B: donepezil vs placebo; Phase C: donepezil vs no-donepezil) as the between-subjects factor, and group by time as the interaction effect. During Phase A, a similar ANCOVA was conducted with age and education as covariates; and during Phase B and Phase C with age, education and week 8 HRSD scores as covariates.
As defined in the data analyses plan, tests of significance were alpha < 0.05 (one-tailed for all analyses), testing the hypothesis that donepezil is of therapeutic value (Ferris et al., 2007).
Results
The baseline demographic, clinical and neuropsychological features of the 23 DEP-CI patients are described in Table 1. No patient had an MMSE <22, one had 22, three had 23, and the average was 26.1 with SD 2.1. 95% (22/23) of the patients met Peterson amnestic-MCI criteria (SRT-IR, and/or SRT delayed recall >1.5 SD below age and ethnicity norms (Tabert et al., 2006)). Descriptive clinical and NPT results at the beginning and end of Phase B, and at the major time points in the subsample of patients included in the combined Phase B + C, are presented in Table 2.
Table 1. Baseline demographic and clinical features and neuropsychological raw scores of patients with depression and cognitive complaints (n = 23).
| Baseline features | Mean (SD) or % |
|---|---|
| Age (years) | 67.7 (8.7) |
| Sex (% female) | 56.5% |
| Education (years) | 13.3 (4.5) |
| Ethnicity* | |
| White non-Hispanic | 56.5% |
| African-American non-Hispanic | 21.7% |
| Hispanic | 17.4% |
| Other | 4.3% |
| Diagnosis of Major Depressive Disorder | 78.0% |
| 24-item HRSD | 22.2 (4.2) |
| Age at first depression (years) | 53.8 (19.4) |
| Duration of depression (months) | 30.4 (35.3) |
| Duration of memory complaints (months) | 37.2 (34.2) |
| Family history of dementia | 9.0% |
| Family history of depression | 48.0% |
| 30-item Folstein MMSE | 26.1 (2.1) |
| Selective Reminding Test Immediate Recall | 33.4 (9.5) |
| Verbal Fluency (CFL) | 36.7 (16.6) |
| WAIS-III Digit Symbol | 41.8 (20.4) |
| Trails A (seconds) | 78.8 (34.0) |
| Trails B (seconds) | 190.5 (95.1) |
At baseline, ethnicity was documented by self-report.
Table 2. Raw scores in clinical and neuropsychological measures in subjects included in Phase B and Phases B + C analyses.
| Measure | Phase B: week 8–week 20 Donepezil vs placebo | Combined phases B + C: week 8–week 52 Donepezil vs. no donepezil | |||||
|---|---|---|---|---|---|---|---|
| Donepezil (n = 12) Vs. Plac. (n = 9) | Pre-drug Mean (SD) | Post-drug Mean (SD) | Donepezil (n = 6) Vs. No Don. (n = 6) | Week 8 Mean (SD) | Week 20 Mean (SD) | Week 52 Mean (SD) | |
| Clinical | |||||||
| 24-item HRSD | Donepezil | 9.3 (7.1) | 10.4 (8.1) | Donepezil | 6.2 (3.3) | 6.0 (2.5) | 6.2 (2.6) |
| Placebo | 7.8 (3.9) | 4.6 (2.9) | No Don. | 13.5 (8.1) | 13.0 (10.1) | 10.3 (8.5) | |
| TESS total intensity | Donepezil | 4.3 (4.0) | 3.2 (3.3) | Donepezil | 2.8 (3.3) | 2.8 (2.3) | 2.5 (2.6) |
| Placebo | 3.4 (4.0) | 3.2 (3.2) | No Don. | 6.0 (3.3) | 3.5 (3.5) | 1.8 (1.9) | |
| Neuropsychological | |||||||
| Verbal memory | |||||||
| SRT Immediate Recall | Donepezil | 32.8 (11.9) | 37.8 (10.9) | Donepezil | 31.7 (7.7) | 41.7 (9.5) | 35.3 (3.6) |
| Placebo | 41.0 (13.6) | 39.0 (12.0) | No Don. | 36.5 (15.3) | 36.3 (12.9) | 35.0 (11.4) | |
| Verbal fluency | |||||||
| CFL | Donepezil | 35.3 (15.0) | 35.8 (15.1) | Donepezil | 37.0 (12.9) | 39.2 (13.8) | 35.0 (11.9) |
| Placebo | 40.0 (15.3) | 42.4 (17.9) | No Don. | 36.8 (9.8) | 33.2 (10.6) | 34.8 (13.5) | |
| Attention/psychomotor | |||||||
| Trails A | Donepezil | 69.1 (26.3) | 60.2 (19.8) | Donepezil | 76.5 (28.6) | 64.8 (19.2) | 61.7 (20.1) |
| Placebo | 103.0 (64.6) | 96.0 (47.6) | No Don. | 87.8 (67.3) | 77.3 (30.4) | 72.7 (46.3) | |
| Executive | |||||||
| Trails B | Donepezil | 183.5 (98.0) | 198.0 (95.1) | Donepezil | 178.3 (103.0) | 170.7 (108.7) | 175.2 (100.6) |
| Placebo | 201.0 (96.3) | 177.0 (89.9) | No Don. | 190.8 (99.8) | 195.6 (96.4) | 128.5 (16.5) | |
| WAIS-III digit symbol | Donepezil | 46.0 (21.1) | 46.8 (20.2) | Donepezil | 43.8 (22.5) | 46.3 (16.8) | 42.3 (17.5) |
| Placebo | 36.0 (20.6) | 39.0 (22.8) | No Don. | 42.7 (18.9) | 35.0 (17.6) | 38.0 (18.2) | |
Phase A: Open antidepressant treatment (baseline to week-8)
At 8 weeks, the antidepressant response rate was 61% (14/23) in the intent-to-treat sample and 67% (14/21) among completers. Baseline HRSD and NPT scores did not differ between responders and non-responders (p > 0.40).
Mixed ANOVA revealed a significant group (antidepressant responder vs. non-responder) by time interaction for SRT-IR (F = 4.42, p = 0.02), with responders showing an improvement. When age and education were included as covariates, the interaction remained significant (F = 3.3, p = 0.045).
There were no significant responder/non-responder effects on the NPT measures in the non-memory domains (p > 0.10).
Phase B: Donepezil vs placebo (week-8 to week-20)
In the week-8 to week-20 randomized, double-blind placebo-controlled trial, 12 patients received donepezil and nine patients received placebo. Week-8 age, HRSD, TESS and NPT scores did not differ between patients randomized to donepezil and placebo (p > 0.10).
In mixed ANOVA, there was a trend-level group (donepezil vs placebo) by time interaction for SRT-IR (F = 3.0, p = 0.05). Patients on donepezil tended to show an improvement in SRT-IR (t = 1.69, p = 0.05) in contrast to patients on placebo (t = 0.80, p = 0.23). When age and education, or age, education, and week-8 HRSD scores were included as covariates, the interaction did not demonstrably change (F = 3.3, p = 0.04; F = 2.9, p = 0.06, respectively).
There were no group by time effects (p-values > 0.50) on any non-memory domain measures (see Table 2 for means). Baseline MMSE did not correlate with change in performance on any NPT measures. The effect size, using Cohen's standardized mean difference (d) for the Group by Time interaction, (calculated by using the mean SRT-IR difference scores [20 - 8 week] for the donepezil and Placebo groups and dividing by the pooled standard deviation), and total SRT-IR as the dependent variable, was 0.78, which is considered in the medium to large range.
Combined Phases B + C: Extended open donepezil treatment (week-8 to week-52)
Week-8 mean HRSD score was significantly lower in the donepezil (n = 6) vs no-donepezil (n = 6) group (p = 0.04), but NPT scores did not differ (p > 0.10).
In mixed ANOVA, there was a significant group (donepezil/no-donepezil) by time interaction for SRT-IR (F = 3.3, p = 0.03). Post-hoc simple within-group effects revealed a significant effect for donepezil over time (F = 4.1, p = 0.03) but not for the no-donepezil group (F = 0.44, p = 0.3) (Figure 2). When age and education, or age, education, and week-8 HRSD scores were included as covariates, the interaction remained significant (F = 5.2, p < 0.01; F = 5.2, p < 0.01, respectively).
Figure 2.
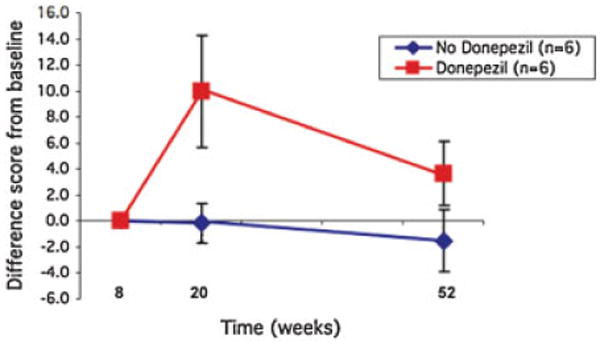
Change in SRT Immediate recall during the donepezil vs no-donepezil treatment.
There were no group by time effects (i-values > 0.20) on any non-memory domain measures (see Table 2 for means).
Somatic side effects
Donepezil treated patients reported the same level of side effects as the placebo treated patients in total TESS scores during Phase B (donepezil mean = 3.2 SD = 3.3 vs placebo mean = 3.2, SD = 3.2); and during Phase C (donepezil mean = 2.5 SD = 2.6 vs no-donepezil mean = 1.8, SD = 1.9).
Discussion
In this pilot study, elderly DEP-CI patients treated with an antidepressant plus donepezil had a short-term improvement in verbal memory compared to treatment with antidepressant plus placebo. This effect waned in patients who continued in open donepezil treatment for an additional 8 months. The time course and magnitude of improvement with donepezil are consistent with donepezil treatment studies of MCI (Petersen et al., 2005) and AD (Rogers et al., 1998), where measurable cognitive improvement was observed in the first few months, followed by a plateau or gradual decline.
Improvement limited to the memory domain is consistent with past AChEI studies in AD, which demonstrated benefits in memory more commonly than other domains (Burns et al., 1999). In studies of MCI patients, donepezil has shown superiority over placebo in the attention/psychomotor (Salloway et al., 2004), executive (Saykin et al., 2004) and language (Petersen, 2005) domains, but these findings have been inconsistent. The small sample size and large variance within the donepezil and placebo groups may account for our negative findings in non-memory domains. In addition, even though we had a placebo-control arm to address practice effects in Phase-B, a learning effect may also account for our negative findings in non-memory domains.
While our findings must be interpreted with caution, given the small sample size and limited length of follow-up, the cognitive improvement observed in this DEP-CI sample indirectly suggests that donepezil treatment is well tolerated and may delay cognitive decline and conversion to AD, an outcome of clear clinical value (Devanand et al., 1996; Petersen et al., 2001; Tabert et al., 2006). These pilot data also suggest that the cognitive benefit conferred to DEP-CI patients by AChEI treatment may be at least as substantial as that previously reported for CI patients without depression (Salloway et al., 2004; Petersen et al., 2005). Also, given the high prevalence of depression in the context of late-life CI, including depressed patients in future AChEI treatment trials for MCI and AD could increase the generalizability of such trials.
A limitation includes the heterogeneous inclusion criteria for depression, including depressions of varying severity and duration. A distinction within subgroups of depression (e.g. major depression vs dysthymia) may reveal significant differences in treatment response to an AChEI. However, small sample size precludes these analyses.
In the elderly DEP-CI patient, the beneficial effects of donepezil indirectly suggest that cholinergic neurotransmitter deficits contribute to the CI associated with DEP, similarly to MCI (Gron et al., 2006) and AD (Davis et al., 1985). Since deficits in the catecholamine neurotransmitter system (e.g. norepinephrine and serotonin) are also known to be associated with depression and to modulate cognitive performance (e.g. executive function; Schmitt et al., 2006), the cognitive improvements with combination antidepressant plus donepezil may involve independent but overlapping neurotransmitter systems.
In summary, the results of this pilot study raise the possibility that combined treatment with anti-depressant and AChEI may be considered in patients presenting with DEP-CI. It also provides a basis for a larger study that, if successful, may represent a possible approach to reducing conversion rates to a clinical diagnosis of dementia.
Acknowledgments
This work was supported in part by Federal Grants MH50513, MH55716, AG17761 and by an unrestricted educational grant from Pfizer, Inc. & Esai, Inc.
Pfizer, Inc. was not involved in the design, data collection, data analysis, interpretation or writing of this investigator-initiated study.
Footnotes
Conflict of Interest: None.
References
- Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with ‘reversible dementia’: a controlled study. Am J Psychiatry. 1993;150:1693–1699. doi: 10.1176/ajp.150.11.1693. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26:591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Boone KB, Lesser I, Miller B, et al. Cognitive functioning in a mildly to moderately depressed geriatric sample: relationship to chronological age. J Neuropsychiatry Clin Neurosci. 1994;6:267–272. doi: 10.1176/jnp.6.3.267. [DOI] [PubMed] [Google Scholar]
- Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer's disease–results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10:237–244. doi: 10.1159/000017126. [DOI] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- Butters MA, Bhalla RK, Mulsant BH, et al. Executive functioning, illness course, and relapse/recurrence in continuation and maintenance treatment of late-life depression: is there a relationship? Am J Geriatr Psychiatry. 2004;12:387–394. doi: 10.1176/appi.ajgp.12.4.387. [DOI] [PubMed] [Google Scholar]
- Charney DS, Reynolds CF, 3rd, Lewis L, et al. Depression and bipolar support alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, Melville P, Scherl WF, et al. Effects of donepezil on memory and cognition in multiple sclerosis. J Neurol Sci. 2006;245:127–136. doi: 10.1016/j.jns.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Davis BM, Mohs RC, Greenwald BS, et al. Clinical studies of the cholinergic deficit in Alzheimer's disease. I. neurochemical and neuroendocrine studies. J Am Geriatr Soc. 1985;33:741–748. doi: 10.1111/j.1532-5415.1985.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Demaerschalk BM, Wingerchuk DM. Treatment of vascular dementia and vascular cognitive impairment. Neurologist. 2007;13:37–41. doi: 10.1097/01.nrl.0000252919.46622.28. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pelton GH, Marston K, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry. 2003;18:123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Krishnan KR, Anand R, et al. Long-term effects of rivastigmine in moderately severe Alzheimer's disease: does early initiation of therapy offer sustained benefits? Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:705–712. doi: 10.1016/s0278-5846(01)00326-8. [DOI] [PubMed] [Google Scholar]
- Ferris S, Schneider L, Farmer M, et al. A double-blind, placebo-controlled trial of memantine in age-associated memory impairment (memantine in aami) Int J Geriatr Psychiatry. 2007;22:448–455. doi: 10.1002/gps.1711. [DOI] [PubMed] [Google Scholar]
- Gron G, Brandenburg I, Wunderlich AP, Riepe MW. Inhibition of hippocampal function in mild cognitive impairment: targeting the cholinergic hypothesis. Neurobiol Aging. 2006;27:78–87. doi: 10.1016/j.neurobiolaging.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Hickie I, Scott E, Mitchell P, et al. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- King DA, Cox C, Lyness JM, et al. Quantitative and qualitative differences in the verbal learning performance of elderly depressives and healthy controls. J Int Neuropsychol Soc. 1998;4:115–126. doi: 10.1017/s1355617798001155. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, George LK, Blazer DG. Six-month outcomes for mri-related vascular depression. Depress Anxiety. 1998;8:142–146. doi: 10.1002/(sici)1520-6394(1998)8:4<142::aid-da2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Conwell Y, King DA, et al. Age of onset and medical illness in older depressed inpatients. Int Psychogeriatr. 1995;7:63–73. doi: 10.1017/s1041610295001852. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment: where are we? Alzheimer Dis Assoc Disord. 2005;19:166–169. doi: 10.1097/01.wad.0000179417.95584.90. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691–698. doi: 10.1176/appi.ajp.162.4.691. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Bradshaw JL, Pantelis C, Phillips JG. Frontostriatal deficits in unipolar major depression. Brain Res Bull. 1998;47:297–310. doi: 10.1016/s0361-9230(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Wingen M, Ramaekers JG, et al. Serotonin and human cognitive performance. Curr Pharm Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The galantamine usa-10 study group. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Wagner HR, Steffens DC. Greater depression severity associated with less improvement in depression-associated cognitive deficits in older subjects. Am J Geriatr Psychiatry. 2002;10:632–635. [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes De Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, et al. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]


