Abstract
Current theories of basal ganglia function emphasize their role in the integration of sensory information into motor activities, particularly in the control of movement timing. People with basal ganglia disorders such as Parkinson’s disease exhibit poor temporal control of movements, in general and articulation in particular, as demonstrated by irregular speaking rate, reduced stress contrasts, and reduced movement durations and velocities. Previous research has implicated sensory deficits as contributory factors in limb movement control in patients with Parkinson’s disease; however, the relation between sensory deficits and speech-movement abnormalities has not been documented. In the present study, the existence of perceptual processing difficulties of speaking rate was investigated in subjects with Parkinsonian dysarthria (PD). Comparisons in perception were made between subjects with PD, neurologically normal geriatrics (GN) and neurologically normal young adults (YN) for accuracy in identification of words presented at different speaking rates. We hypothesized that word-identification scores would be lower for PD and GN subjects compared to the YN subjects, an effect that was supported by the data. We also expected that there would be differences between the GN and PD subjects in their accuracy of word identification at a faster speaking rate, an hypothesis that was not supported by the data. Rather, GN and PD subjects differed in identification scores for words spoken at a slow rate. PD subjects who had faster habitual speaking rates (HSR) had significantly lower word-identification scores in the slow compared to conversational rate conditions, a relation that was significant r = +0.64). These data suggest the need to consider perceptual deficits as an additional factor that contributes to rate variations in PD speech.
Control of timing is a ubiquitous problem for people with Parkinson’s disease. In speech production, this disorder is manifested by accelerated speaking rate (Darley, Aronson, & Brown, 1975; Weismer, 1984), reduced stress contrasts (Darley et al., 1975; Murray, 1984), reduced movement amplitudes (i.e., hypokinesia), velocities, (i.e., bradykinesia), and duration (Caliguiri, 1989; Connor, Abbs, Cole, & Gracco, 1989; Forrest, Weismer, & Turner, 1989) and reduced segment durations (Weismer, 1984). Traditionally, these speech disorders of Parkinson’s disease have been attributed to motoric deficits caused by basal ganglia dysfunction, although more recent theories suggest that perceptual deficits may also contribute significantly to problems of temporal control (Artieda, Pastor, LaCruz, & Obeso, 1992; Brotchie, Iansek, & Horne, 1991a, 1991b; Freeman, Cody, & Schady, 1993; Georgiou, Iansek, Bradshaw, Phillips, Mattingley, & Bradshaw, 1993; Marsden & Obeso, 1994).
The basal ganglia form part of a complex loop within the central motor system. Although the precise role of the basal ganglia in motor control continues to be debated, recent theory, as well as empirical evidence, points to a sensory influence of the basal ganglia on movement initiation and continuation (Brotchie et al., 1991b; Schneider, Denaro, & Lidsky, 1982). Two major and related roles have been proposed: First, neurons in the basal ganglia discharge to environmental stimuli that are used to initiate movement (Rolls, 1990) and, second, the basal ganglia provide an internal cue that regulates the transition from one movement to the next in well-learned sequences (Georgiou et al., 1993). In animal preparations, the magnitude and phasic response of neurons in the globus pallidus, the efferent pathway from the basal ganglia, increase during the production of well-learned, predictable movement sequences (Brotchie et al., 1991a, 1991b). In contrast, the basal ganglia show only ill-defined activity during the learning of new sequences. Because speech is a well-learned behavior, globus pallidus units are likely to be active during articulatory sequences.
Empirical evidence pointing to the importance of the basal ganglia in movement timing comes from studies on parkinsonian patients’ use of timing cues for the generation of accurate tapping rhythms (Freeman et al., 1993; Georgiou et al., 1993). When asked to finger-tap in synchrony with an auditory cue, subjects with Parkinson’s disease were found to be less accurate than neurologically healthy individuals. Two patterns of inaccuracy were identified across the subjects: Some PD patients increased their tapping rate in response to low frequency cues (1–2 Hz) whereas other subjects decreased tapping speeds when required to follow a higher frequency pattern (4–5 Hz). These findings suggest that people with PD have difficulty using external cues for movement control. In an extension of this experiment, Freeman et al. (1993) found that tapping rate was further altered when the external pacing cue was removed. When parkinsonian patients were dependent on memory traces of the external cues to reproduce finger-tapping rates, their rates and accuracy varied significantly from the specified signal. Neurologically healthy subjects, by contrast, were able to reproduce rates accurately with or without external cues.
Finally, tests of temporal discrimination for tactile, auditory, and visual stimuli show increased difference limens in all three sensory modalities for subjects with PD compared to age-matched control subjects (Artieda et al., 1992). These elevated temporal difference limens correlated significantly with performance on finger-tapping tasks; subjects with higher temporal difference limens had slower tapping rates.
Taken together, these empirical data lend support to the hypothesis that the basal ganglia are involved in timing discrimination and control for movement sequences. When neuropathology such as Parkinson’s disease alters the status of the basal ganglia, internal timing for movements is disrupted and, as shown in many studies, people with Parkinson’s disease become dependent on external stimuli for movement guidance (Day, Dick, & Marsden, 1984; Flowers, 1975,1976,1978; Stern, 1986). However, the perceptual processing of external timing signals also is altered in Parkinson’s disease. As Freeman et al (1993) showed, movements either are accelerated in response to slow stimuli or are decelerated in response to higher frequency external pulses. This aberrant processing of externally generated timing information, combined with the greater dependence on this information for the guidance of movements in parkinsonian patients, may underlie some of the movement disorders that accompany the disease. Movements that are slow in the neurologically normal subject may be accelerated in some parkinsonian subjects, whereas some people with PD may reduce the speed of faster movements.
To date, studies have shown an interaction between deficits in the processing of internal and external tuning signals and disturbances in simple limb or digit movements. The relation between perceptual processing deficits and speech rate disturbances in Parkinson’s disease has not been investigated. Yet it is clear that some of the movement disorders found in the limbs are manifested in the speech production system. The classic studies by Darley et al. (1975) found that parkinsonian dysarthria was unique among the motor speech disorders in that it was characterized by a faster than normal speaking rate in a subset of PD patients. As with other motor behaviors, the control of articulatory rate also may be influenced by perceptual factors.
In the present investigation, the processing of simple speech stimuli (i.e., monosyllabic words) at different speaking rates was examined and related to Habitual Speaking Rate (HSR) in subjects with parkinsonian dysarthria. Two hypotheses were advanced. Based on studies by Sommers and his colleagues (Sommers, Humes, & Pisoni, 1993), we hypothesized that word-identification scores would be lower for PD and GN subjects compared to the YN subjects. Further, we expected that there would be differences between the GN and PD subjects in their accuracy of word-identification at a faster speaking rate, such that the PD subjects with faster HSRs would have higher word-identification scores for words produced at the fast rate than either PD subjects with lower HSRs or the GN subjects. This relation might account, in part, for the differences in speaking rate between GN and PD subjects, in general, and the heterogeneity within PD speakers that has been demonstrated in previous research (Weismer, 1984). That is, PD subjects who produce faster than normal speaking rates may do so because of perceptual factors. The subjects’ task was to identify words produced by a male speaker at normal, fast, and slow speaking rates. Identification accuracy was correlated with HSR from a spontaneous sample provided by each parkinsonian subject. Additionally, because normal aging may be expected to alter speech processing, neurologically normal young and older adults were tested using the same paradigm to assess the effect of age on processing information at different speaking rates.
METHODS
Subjects
Three groups of 10 subjects, participated in this study. One group (PD) was comprised of three males and seven females with idiopathic Parkinson’s disease. Subjects in this group ranged in age from 44 to 75 years (M = 64.7 years; SD = 9.8) and had moderate symptoms of Parkinson’s disease as indicated by ratings of stage 2 or 3 on the Hoehn and Yahr scale (1967). The Mini-mental Status Examination (Folstein, Folstein, & McHugh, 1975) was used to screen for cognitive problems and indicated that all subjects were free of dementia. Subjects in the PD group were on a regimen of Sinemet or Sinemet CR, in addition to other antiparkinson drugs, and participated in the experiment during the time of peak effect of their medication. As part of the dysarthria assessment, intelligibility was assessed with the sentence portion of the Assessment of Intelligibility of Dysarthric Speech (AIDS; Yorkston & Beukelman, 1981). Judges for the AIDS were three advanced graduate students in speech-language pathology. Each tape was transcribed orthographically by two students and agreement between these students was perfect for all but one tape. Listeners had disagreements on the tape from subject PD6, who was judged to have intelligibility deficits. Disagreements between listeners arose on words that were not intelligible.
Determination of dysarthria severity was made on the basis of the assessment which included sustained phonations, syllable repetition tasks, an oral mechanism evaluation, and a conversational speech sample. Judgments about each subject’s speech, including breath groups, voice quality, pitch and loudness, articulatory precision, and prosodic characteristics were determined from a review of the tape-recorded conversational sample. Each parameter that was evaluated was rated on a 4-point scale (1 = normal; 4 = severely disordered) by two graduate student clinicians.
Although most subjects with PD were highly intelligible, as measured by the AIDS sentences, all subjects exhibited signs of parkinsonian dysarthria including voice problems, articulatory imprecision, and/or fast rate on the conversational sample. These prosodic and articulatory disturbances impacted the naturalness of the PD subjects’ speech and required listeners to attend fully to the speakers to be able to maintain conversations. HSR for the PD and GN subjects was calculated from 3- to 5-minute samples of these informal conversations between graduate student clinicians and subjects. Table 1 presents a summary of the PD subject characteristics.
TABLE 1.
Summary characteristics of subjects in PD group.
| Subject | Age | Sex | Hoehn & Yahr Stage | Medications | Dysarthria (Percent Intelligibility on AIDS) | Dysarthria Characteristics | Speech Rate Syll/min |
|---|---|---|---|---|---|---|---|
| PD1 | 62 | M | 2 | Sinemet Parlodel Eldepryl |
Moderate (100) | Accelerated rate Imprecise consonants Glottal fry Reduced stress |
296 |
| PD2 | 71 | M | 3 | Sinemet | Moderate-severe (93) | Accelerated rate Phoneme and word repetitions Reduced stress Breathy voice Reduced pitch range Reduced loudness |
300 |
| PD3 | 65 | F | 3 | Sinemet Eldepryl Permax |
Moderate (95) | Reduced breath support Speaks on residual volume High fundamental frequency Breathy voice Word repetitions |
172 |
| PD4 | 74 | F | 2 | Sinemet CR Eldepryl |
Mild-moderate (97) | Glottal fry Low pitch Excessive pauses |
125 |
| PD5 | 75 | F | 2 | Sinemet CR Eldepryl Parlodel |
Moderate (100) | Monopitch Monoloud Aphonia Phonation breaks Reduced stress Syllable repetitions |
161 |
| PD6 | 56 | F | 3 | Sinemet | Severe (50) | Monopitch Monoloud Low intensity Reduced stress Imprecise consonants |
231 |
| PD7 | 44 | F | 2 | Sinemet Eldepryl Parlodel |
Mild-Moderate (100) | Speaks on residual volume Low pitch Breathy voice Glottal fry Reduced stress |
226 |
| PD8 | 67 | F | 3 | Sinemet Sinemet CR Eldepryl |
Moderate-severe (99) | Speaks on residual volume Low pitch Voice tremor Glottal fry Monopitch Imprecise consonants |
191 |
| PD9 | 60 | F | 2 | Sinemet Eldepryl Permax |
Mild-Moderate (100) | Glottal fry Word repetitions Excessive pauses Syllable repetitions |
149 |
| PD10 | 74 | F | 3 | Sinemet | Moderate-Severe (96) | Reduced respiratory support Monopitch Monoloud Breathy voice Low intensity Accelerated rate Phoneme repetitions |
185 |
The second group of subjects consisted of 10 neurologically healthy subjects who were age matched to the subjects in the PD group. These normal geriatric subjects (GN) ranged in age from 44 to 75 years with a mean age of 63.6 years (SD = 9.1). Attempts were made to match the PD and GN subjects by gender, as well as age and hearing acuity (see below). However, the people who responded to our calls for subjects were predominantly female. The males who did volunteer to participate in the study were found to be ineligible because of significant hearing loss. Because no differences in speech perception performance between male and female subjects was observed in earlier studies using the procedures of the present experiment (Sommers, Nygaard, & Pisoni, 1994), nor were differences found as a function of gender in the young normal subjects used in this study, attempts for gender matches were abandoned. Thus, all subjects in the GN group were female. Evaluation of these subjects followed the same procedures used for the PD subjects including administration of the AIDS, a hearing evaluation, and collection of a spontaneous speech sample.
Subjects in the third group (YN) included 10 neurologically healthy, young adults, equally divided between males and females. These subjects ranged in age from 22 to 30 years with a mean age of 24.1 years (SD = 2.3). Again, hearing was evaluated and a conversational speech sample was obtained to ensure that there were no speech impairments in this group of subjects.
Auditory thresholds were obtained from all subjects for octave frequencies between .25 and 8 kHz. Thresholds were obtained in an audiometric booth using a Grason-Stadler (Model 1704) audiometer with stimuli presented monaurally via TDH-39 headphones. Subjects used a response button to indicate the audibility of each tone. To be included in this study, subjects’ thresholds could not exceed 30 dB HL at frequencies of 4 kHz or below in the right ear. This criterion was based on the desire for comparable hearing sensitivity in the older and younger subjects, the need to test everyone in the same ear in case of laterality effects, and the cutoff frequency of the headphones which was about 4–5 kHz. Although hearing thresholds at 8 kHz were not used for selection criteria, no subject had thresholds in excess of 40 dB HL at that frequency.
Stimulus Materials
The stimuli for the word-recognition task consisted of single word tokens produced at three different speaking rates. These stimuli were a subset of the materials used by Sommers et al. (1994) in their investigations of the effects of stimulus variability on spoken word recognition. In the present experiment, 200 monosyllabic words taken from phonetically balanced word lists (ANSI, 1971) were recorded by one adult male who spoke with a standard American English dialect. During recording, each word was presented on a CRT screen and the talker produced the word in the carrier phrase “Please say the word _____.” Recordings were made in a sound-treated booth with a Shure (SM98) microphone. Utterances were low-pass filtered at 4.8 kHz, digitized at 10 kHz, and stored for later editing to extract each monosyllabic word. After the words were digitally edited from the carrier phrase, stimuli were equated for RMS amplitude level using a custom designed software package.
All 200 words were produced at each of three speaking rates: conversational, slow, and fast. The talker was not given a model for any rate. Instead, he was told the target rate (“conversational,” “fast,” or “slow”) and that the rates should be distinctly different from one another. Utterances were recorded in blocks of 100 words with speaking rate held constant for each block. Average durations for the tokens were 905 ms for the slow rate, 533 ms for the conversational rate, and 375 ms for the fast rate condition (see Sommers et al. [1994] for further details).
To ensure that all words were equally intelligible at all speaking rates, three sophisticated listeners (advanced graduate students in Speech-Language Pathology) were asked to listen to all the stimuli. These listeners were asked to type each word on a computer keyboard after the stimulus was presented via an insert earphone. Listeners’ responses were analyzed and only tokens that were identified correctly by all three listeners were used for the present experiment. Because each word was spoken at each rate, any word that was not identified correctly at one rate was eliminated from all rate conditions. This yielded 180 words that were used as stimuli in the present experiment.
Six different list formats, containing 30 words each, were prepared for aural presentation. For three of the lists, all words were produced at the same speaking rate (Independent rate condition, IR), fast, conversational, or slow. In the remaining three lists, speaking rate was varied from one stimulus word to the next (Mixed rate condition, MR). Each list in this condition contained one third of the words at each of the three speaking rates. Words were counterbalanced across the lists for different subjects within a group (i.e., for one subject in a group a word was presented at a slow rate whereas the same word may be presented to another subject at a fast or normal rate). No word was presented at more than one speaking rate for a given subject or in more than one list format (i.e., mixed versus independent). In total, 18 different word lists were constructed.
Procedures
Subjects were tested individually in a sound attenuated room. Stimuli were output from a 12 bit D/A converter at 10 kHz, filtered at 4.8 kHz and presented monaurally at 80 dB SPL via an insert earphone.1 Each subject was seated at a computer station facing a CRT monitor. A written warning signal was presented on the CRT screen prior to each stimulus item and a 500 ms silent interval was inserted between the warning signal and the stimulus word. After each word was presented, the subject was required to type the word that he or she heard. Subjects were asked to enter their responses as soon as the word was recognized, but there was no fixed time interval between stimuli. The next stimulus word was presented 2 seconds after the subject completed typing a response. Because people with Parkinson’s disease have been shown to have longer reaction times than neurologically healthy individuals (Stelmach, Worthington, & Strand, 1986) and because typing movements may be slower in the PD group than in the other groups, no time restrictions were placed on the subjects. Words with typographical or spelling errors were counted as correct if they resembled the target words.
All subjects listened to all six list formats (i.e., three IR lists: slow, conversational, and fast, and three MR lists). The order of list presentation was counterbalanced across subjects. After each list was completed, the subject was given a short break before starting on the next list of words. Because the experimental sessions were self-paced, and because breaks between lists varied somewhat in length, subject participation in this experiment took between 30 and 45 minutes.
Calculation of Habitual Speaking Rate
The term habitual speaking rate (HSR) is being used to designate the rate at which subjects produced speech during a conversational sample. This may not be their habitual rate because conversation was sampled for only 3–5 minutes per subject and HSR was calculated from a 1-minute segment of this sample. However, this term is being used operationally to define the rate at which subjects produced speech during a conversational sample.
During the initial dysarthria assessment, subjects were engaged in a conversation with the examiner. Topics ranged from a discussion of the subject’s health condition to descriptions of places that the subject had lived or visited, information about employment, spouses, children (i.e., whatever topic generated a 3–5 minute discussion during which the subject dominated the conversation). A 1-minute segment from the middle of the conversation, during which only the subject spoke (except for fillers, such as “uh-huh”) was extracted from the speech sample. HSR was determined from this sample by counting the number of words that were uttered. When possible, the segment was chosen to exclude any repetitions or part-word utterances. This criterion was met for all but two of the PD subjects (PD1 and PD2) who each produced part-word repetitions. For those subjects, only the full-word production was counted. Although syllables per minute might be a more precise measure of speaking rate, measurement of words per minute was used in this calculation of HSR so that comparisons to previous studies could be made (Goldman-Eisler, 1968; Yorkston & Beukelman, 1981).
Statistical Analysis
Preliminary evaluation indicated that the data were not distributed normally either within or across subject groups. For this reason, an Arcsine transform was applied and statistical analyses were conducted on those data. Because the transformation did not alter the outcome of the statistical tests of significance, only analyses of percent correct responses will be considered.
A three-way ANOVA was conducted on percent correct word identification to compare the effects of subject group (PD, GN, YN), speaking rate (slow, conversational, fast) and speaking rate variability (IR, MR). Post hoc t-tests were used to identify the comparisons that were significantly different. Per-experiment error rates of 0.05 were used for all tests, and where multiple t-tests were conducted, Bonferroni adjustments were made to maintain the probability of error at 0.05. Finally, correlation coefficients were calculated to determine the relation between HSR in the PD subjects and the accuracy of word-identification in the perception task for words presented at each of the three speaking rates.
RESULTS
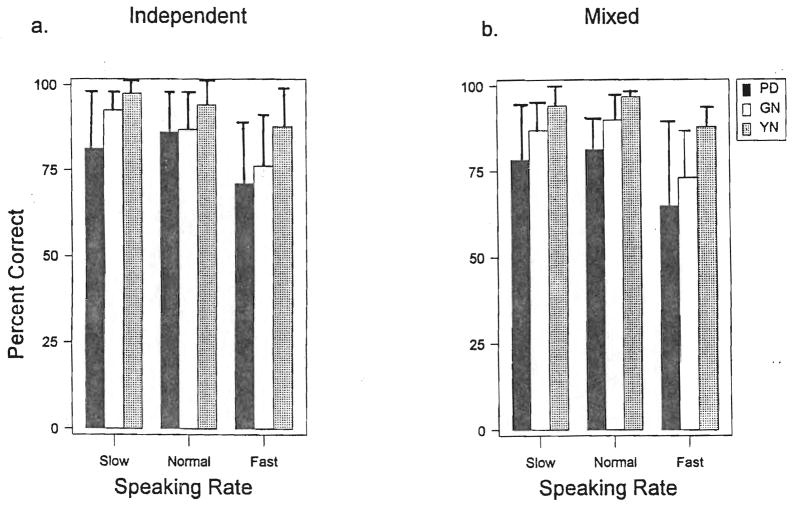
Figure 1 shows the percent correct word identification for the different speaking rates and subject groups. In Figure 1A, the results are presented from the independent rate condition in which each list was composed of words produced at a single speaking rate. Figure 1B presents the results from the mixed rate condition. The data in this graph have been parsed into the three speaking rates, even though the test session presented different rates mixed together within each list.
Figure 1.
Mean percent correct word identification for each subject group at each of the three speaking rates. Bars represent one standard deviation. The left panel presents results for the independent rate condition in which a full list of words was presented at a single speaking rate. The right panel shows data from the mixed rate condition in which speaking rate was mixed within each word list.
The three-way ANOVA indicated significant main effects of subject group (F(2,216) = 20.6) and speaking rate (F(2,216) = 45.8). As seen in Figure 1, identification accuracy was somewhat higher in the independent than in the mixed rate condition for all subject groups; however, this effect (i.e., presentation of words at a constant rate or varied rate within a single list) did not have a significant impact on word-identification scores (F(1,216) = 3.05; p = .082). Because there was no significant difference between IR and MR list conditions, data from the different list formats were combined for the post hoc analyses. There were no significant interaction effects between the factors of subject group, speaking rate or speaking rate variability.
Figure 1 presents the mean percent correct word-identification for each subject group and rate condition. Post-hoc t-tests on these data indicated that the PD and GN subjects had significantly lower word-identification scores for all speaking rates than the YN subjects. Further, significant differences in word identification were found between the neurologically normal GN subjects and the neurologically impaired PD subjects when stimulus words were presented at the slow rate. That is, the subjects with PD did significantly worse than the GN subjects in the identification of words presented at a slow speaking rate. No other significant differences existed between the PD and GN subjects as a function of speaking rate for word presentation. On average, the subjects with PD had identification scores for words presented at a slow speaking rate that were about 5% lower than their scores for words presented at a conversational rate. The GN subjects had identification scores on words presented at the slow rate that were 1.4% higher than their scores for words presented at the conversational rate. Mean scores for YN subjects were the same for words presented at the slow and conversational rates; however, this may have been do to ceiling effects.
The present findings are consistent with previous research (Sommers et al., 1994; Sommers et al., 1993); subjects in all three groups had lower identification scores for words presented in the fast rate condition than in the normal or slow rate condition. Post-hoc analysis indicated comparable scores for the slow and normal rate conditions (t(60) = −0.53) with significantly lower scores for the fast rate condition (t(60) = −5.41 for normal vs. fast and t(60) = −4.76 for slow vs. fast rates).
Habitual Speaking Rate
As noted in previous research (Darley et al., 1975; Weismer, 1984), there is considerable heterogeneity in the HSR of people with Parkinson’s disease. Some speakers with hypokinetic dysarthria are perceived to speak too quickly, whereas other parkinsonian patients seem to produce speech at a slower than normal rate. Such intersubject variability in HSR was noted in the present group of Subjects with PD, as seen by the data in Table 2. HSR ranged from 125 words per minute (PD4) to 300 words per minute (PD2) for the PD subjects (M = 203.6; SD = 59.2) and between 145 and 239 words per minute for the normal geriatric subjects (M = 195.5; SD = 41.6). Only PD1 and PD2 had HSRs that exceeded the range of HSR produced by the GN speakers in this study and one PD subject (PD4) spoke at a rate that was lower than the HSR rate of any GN subjects. However, there were no significant differences (t(12) = 0.88; p = 0.44) in HSR between the two groups of older subjects (GN and PD). As noted earlier, HSR was not calculated for subjects in the YN group.
TABLE 2.
Relation between PD speaking rate and relative accuracy of identification of words presented at slow versus conversational speaking rates. Relative accuracy of word identification is defined as the percent correct identification of words presented at a conversational rate minus the score for words presented at a slow rate. Positive numbers indicate better performance on the conversational rate whereas negative numbers mean higher accuracy when words were presented at a slow rate. PD speaking rates were determined from conversational speech samples and rankings are from slowest (1) to fastest (10) rates. Rankings of identification accuracy are from most negative number (identification of slow rate better than normal rate = 1) to most positive number (identifiction of normal rate better than slow rate = 10)
| Subject | Speaking Rate (wpm) | Speaking Rate Rank | Percent Difference Word Identification (Conversation-slow) | Rank of Percent Difference |
|---|---|---|---|---|
| PD1 | 296 | 9 | 3 | 4 |
| PD2 | 300 | 10 | 13 | 9 |
| PD3 | 172 | 4 | 3 | 4 |
| PD4 | 125 | 1 | −10 | 1 |
| PD5 | 161 | 3 | −7 | 2 |
| PD6 | 231 | 8 | 20 | 10 |
| PD7 | 226 | 7 | −3 | 3 |
| PD8 | 191 | 6 | 7 | 7 |
| PD9 | 149 | 2 | 3 | 4 |
| PD10 | 185 | 5 | 7 | 7 |
Correlational analysis revealed a significant relationship r = +0.64; t = 2.85; p<0.05) between HSR of the PD subjects and relative accuracy of identification of words presented at the slow versus normal rate. That is, those subjects with faster HSRs (PD1, PD2, PD6) had lower word-identification scores on words presented at the slow compared to the normal speaking rate. PD subjects with HSRs that fell within the range for the GN subjects had greater word-identification accuracy when words were presented at the slow, compared to the normal rate, the pattern that was typical in the GN subjects. No comparable analysis was undertaken for the GN subjects because they did not evidence differences in word-identification accuracy for the slow compared to the normal rate condition.
In summary, both groups of older subjects (PD and GN) had significantly lower identification accuracy than younger adults when words were presented at any of the three speaking rates: slow, conversational or fast. As a group, the PD subjects had significantly lower identification scores than GN or YN subjects for words spoken at a slow rate. This relative difficulty with the identification of words spoken at a slow rate was related to the HSR of the PD subjects.
DISCUSSION
The hypothesis posed at the outset of this investigation was that perceptual deficits would have an impact on speech produced by people with parkinsonian dysarthria. Such an impact was demonstrated by a relationship between characteristics of hypokinetic dysarthria in the subjects with Parkinson’s disease and their ability to perceive changes associated with speech timing. A major source of variance in speech timing is speaking rate, which influences the duration of syllables and pauses (Kent & Read, 1992). As with many elements of speech motor control, the neuromuscular mechanisms that regulate speech timing are unclear; however, models that emphasize the integration of sensory information into timed, sequential actions are gaining favor (Edelman, 1989; Kent, Adams, & Turner, 1996). In the context of the present research, a model in which sensory and perceptual input are used in speech motor activity may predict the relationship between HSR of PD speakers and the accuracy of word identification as temporal characteristics vary.
One prediction of the present experiment was that people with parkinsonian dysarthria would have higher word-identification scores than GN subjects for words presented at fast speaking rates. We reasoned that people with Parkinson’s disease may have fast speaking rates because that is the rate at which speech monitoring is most efficient for them. This relationship was not confirmed by the data. Instead, PD subjects had lower identification scores for words presented at the fast rate compared to the conversational or slow rates. An inverse relationship between word-identification accuracy and rate at which the presented words were spoken has been noted in previous investigations using neurologically normal young and older adults (Sommers et al., 1993) as subjects and was replicated by the YN and GN subjects in the present study. Although we found a trend for subjects in the YN and GN groups to have the highest identification scores in the slow rate condition, the PD speakers performed best on words spoken at a conversational rate. Comparison of the GN and PD subjects showed the greatest difference in word-identification of words spoken at a slow rate (10% on average), a difference that was statistically significant.
Inaccuracies of spoken word recognition at different speaking rates may present challenges to rate-reduction therapies used to treat hypokinetic-dysarthric speech. Although different procedures may be used, all rate-reducing treatment programs depend on the client’s perceptual skills; treatment centers on some external signal to guide speaking rate. A goal of these treatments is to promote the generalization of slowed speaking rate when the external cue is removed (Yorkston et al., 1988). Unfortunately, this goal rarely is met (Ramig, 1990); speakers with Parkinson’s disease are able to control speaking rate when an external signal is available, but they do not generalize this rate reduction when the external cue is removed (Castor & Hammen, 1996). Of course, the perceptual demands used to monitor speaking rate in conversation may not utilize the same strategies needed in the word-recognition task used in this experiment. However, the relatively poor performance by PD speakers in this simple task suggests that perceptual deficits may be a relevant factor to HSR control in PD.
In addition to rate fluctuations, other prominent speech features of parkinsonian dysarthria, such as stress neutralization and imprecise consonants, can be viewed as an effect of deficits in timing perception. For example, contrasts between stressed and unstressed syllables are realized by duration differences. Articulatory precision is effected by temporal coordination of orofacial, respiratory, and phonatory structures. If perceptual accuracy is reduced by temporal changes, as is found in rate alterations, control of speech parameters associated with these events is likely to be affected.
It should be remembered that changes in basal ganglia function are a normal consequence of aging. If these neurons are critical to the control of timing through the interaction of sensory and motor information, as current theory suggests (Artieda et al., 1992; Brotchie et al., 1991b; Freeman et al., 1993; Georgiou et al., 1993; Marsden & Obeso, 1994), then both speech perception and production would be expected to be affected adversely. It is clear from the present data that normal aging, as well as Parkinson’s disease, impacts word-identification accuracy. Compared to the normal young adults, both the PD and GN subjects had lower word-identification scores for words presented at all speaking rates, a finding that also was noted earlier by Sommers et al. (1993) and Sommers (1997). However, word-identification accuracy of subjects in the GN group in the present study was not affected by stimulus variability as Sommers et al. (1993) found. The basis for this disparity in experimental findings is not clear (see also Sommers, Kirk, & Pisoni, 1997).
Subjects in all three groups of the present study had comparable auditory thresholds in the speech-frequency range. However, other factors probably influenced the performance of the older subjects in the identification of words, independent of speaking rate. It has been shown that older adults have decreased temporal processing abilities, as measured by increased duration difference limens (Fitzgibbons & Gordon-Salant, 1995); speech recognition (Gordon-Salant & Fitzgibbons, 1993); and temporal gap detection (Gordon-Salant & Fitzgibbons, 1993). The implications of these perceptual effects on speech production changes with age remain to be investigated.
A sensory-motor view of parkinsonian dysarthria is parsimonious with the present results and with known characteristics of the speech disorder. Accepting such a view provides a unifying theoretical framework to understand and explain some of the deficits associated with Parkinson’s disease. However, treatments that rely on the integrity of the perceptual system for their success may need to be amended in accordance with the limitations imposed by basal ganglia dysfunction. The present study provides a preliminary examination of the role of speech perception in the control of HSR in hypokinetic disorders. Clearly, more research into the nature of perceptual deficits and their role in speech motor control in parkinsonian dysarthria is needed. For example, the subjects in the present study had relatively mild-moderate parkinsonian symptoms that classified as stage II or III on the Hoehn and Yahr scale (1967) and were tested at the peak effectiveness of their medication. One might expect greater perceptual deficits with more advanced stages of the disease or in subjects who are not medicated. However, the results of the present study demonstrate that the effects of PD extend beyond speech motor control to processes employed in speech perception and spoken word recognition, as well as perceptual-motor interactions between these systems.
Acknowledgments
This work was supported by NIDCD grants DC00111-20, DC-00012-17, and DC00783.
Footnotes
These settings of D/A rate, filter cutoff frequency and signal level were chosen for two reasons; first, previous research (Sommers, Nygaard, & Pisoni, 1994; Sommers, 1997) found that normal young and elderly subjects could accurately identify words sampled at 10 kHz, thereby making any higher sampling rate unnecessary. Second, the insert earphone has a cutoff frequency around 5 kHz, so any spectral information above that would be eliminated by the earphone.
Contributor Information
Karen Forrest, Department of Speech and Hearing Sciences, Indiana University, Bloomington, Indiana
Lynne Nygaard, Department of Psychology, Emory University, Atlanta, Georgia
David B. Pisoni, Department of Psychology, Indiana University, Bloomington, Indiana
Eric Siemers, Movement Disorders Clinic, Department of Neurology, Indiana University Medical School, Indianapolis, Indiana
References
- American National Standards Institute. Method for measurement of monosyllabic word intelligibility. New York: American National Standards Institute; 1971. S3.2-1960 (R1971) [Google Scholar]
- Artieda J, Pastor MA, LaCruz F, Obeso JA. Temporal discrimination is abnormal in Parkinson’s disease. Brain. 1992;115:199–210. doi: 10.1093/brain/115.1.199. [DOI] [PubMed] [Google Scholar]
- Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. 1. Neuronal discharge and parameters of movement. Brain. 1991a;114:1667–1683. doi: 10.1093/brain/114.4.1667. [DOI] [PubMed] [Google Scholar]
- Brotchie P, Iansek R, Horne MK. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity. Brain. 1991b;114:1685–1702. doi: 10.1093/brain/114.4.1685. [DOI] [PubMed] [Google Scholar]
- Caligiuri MP. The influence of speaking rate on articulatory hypokinesia in parkinsonian dysarthria. Brain and Language. 1989;36:493–502. doi: 10.1016/0093-934x(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Castor KL, Hammen VL. Direct magnitude production versus Pacer: Generalization of speaking rate in speakers with Parkinson’s disease. Paper presented at the Conference on Motor Speech; Amelia Island. 1996. Feb, [Google Scholar]
- Connor N, Abbs JH, Cole K, Gracco V. Parkinsonian deficits in serial multi-articulate movements for speech. Brain. 1989;112:997–1009. doi: 10.1093/brain/112.4.997. [DOI] [PubMed] [Google Scholar]
- Darley F, Aronson A, Brown J. Motor speech disorders. Philadelphia: W, B. Saunders; 1975. [Google Scholar]
- Day BL, Dick JPR, Marsden CD. Patients with Parkinson’s disease can employ a predictive motor strategy. Journal of Neurology, Neurosurgery, and Psychiatry. 1984;47:1299–1306. doi: 10.1136/jnnp.47.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. The remembered present. New York: Basic Books; 1989. [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Age effects on duration discrimination with simple and complex stimuli. Journal of the Acoustical Society of America. 1995;98:3140–3145. doi: 10.1121/1.413803. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Ballistic and corrective movements in an aiming task: Intention tremor and parkinsonian movement disorders compared. Neurology. 1975;25:413–421. doi: 10.1212/wnl.25.5.413. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Lack of prediction in the motor behavior of parkinsonism. Brain. 1978;101:19–34. doi: 10.1093/brain/101.1.35. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the mental state of patients for the clinician. Journal of Psychiatric Research. 1975;12:184–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forrest K, Weismer G, Turner G. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric adults. Journal of the Acoustical Society of America. 1989;85:2608–2622. doi: 10.1121/1.397755. [DOI] [PubMed] [Google Scholar]
- Freeman JS, Cody FWJ, Schady W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1993;56:1078–1084. doi: 10.1136/jnnp.56.10.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA. An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- Goldman-Eisler F. Psycholinguistics: Experiments in spontaneous speech. New York: Academic Press; 1968. [Google Scholar]
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. Journal of Speech and Hearing Research. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Kent RD, Adams SG, Turner G. Models of speech production. In: Lass NJ, editor. Principles of experimental phonetics. New York: Academic Press; 1996. [Google Scholar]
- Kent RD, Read C. The acoustic analysis of speech. San Diego: Singular Publishing Group; 1992. [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson’s disease. Brain. 1994;117:877–879. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Murray T. The production of stress in three types of dysarthric speech. In: Berry WR, editor. Clinical Dysarthria. San Diego: College-Hill Press; 1984. pp. 69–84. [Google Scholar]
- Ramig LO. Speech therapy for patients with Parkinson’s disease. In: Koller W, Paulsen G, editors. Therapy of Parkinson’s disease. New York: Marcel Dekker; 1990. [Google Scholar]
- Rolls ET. Experimental psychology: Function of different regions of the basal ganglia. In: Stern GM, editor. Parkinson’s disease. Baltimore: Johns Hopkins University Press; 1990. pp. 151–184. [Google Scholar]
- Schneider JS, Denaro FJ, Lidsky TI. Basal ganglia: Motor influences mediated by sensory interactions. Experimental Neurology. 1982;77:534–543. doi: 10.1016/0014-4886(82)90226-6. [DOI] [PubMed] [Google Scholar]
- Sommers MS. Stimulus variability and spoken word recognition. II: The effects of age and hearing impairment. Journal of the Acoustical Society of America. 1997;101:2278–2288. doi: 10.1121/1.418208. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Humes LE, Pisoni DB. The effects of speaking rate and stimulus variability on spoken word recognition by young and elderly listeners. Research on Spoken Language Processing. 1993;19:91–100. [Google Scholar]
- Sommers MS, Kirk KI, Pisoni DB. Some considerations in evaluating spoken word recognition by normal-hearing, noise masked normal-hearing, and cochlear implant listeners. I: The effects of response format. Ear and Hearing. 1997;18:89–99. doi: 10.1097/00003446-199704000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers MS, Nygaard LC, Pisoni DB. Stimulus variability and spoken word recognition: I. Effects of variability in speaking rate and overall amplitude. Journal of the Acoustical Society of America. 1994;96:1314–1324. doi: 10.1121/1.411453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Worthington CJ, Strand EA. Movement preparation in Parkinson’s disease: The use of advance information. Brain. 1986;109:1179–1194. doi: 10.1093/brain/109.6.1179. [DOI] [PubMed] [Google Scholar]
- Stern Y. Patients with Parkinson’s disease can employ a predictive motor strategy. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:107–108. doi: 10.1136/jnnp.49.1.107-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer G. Articulatory characteristics of Parkinsonian dysarthria: Segmental and phrase-level timing, spirantization, and glottal-supraglottal coordination. In: McNeil MR, Rosenbek JC, Aronson AE, editors. The dysarthrias: Physiology, acoustics, perception, management. San Diego: College-Hill Press; 1984. pp. 101–130. [Google Scholar]
- Yorkston KM, Beukelman DR. Assessment of intelligibility of dysarthric speech. Tigard, OR: CC Publications; 1981. [Google Scholar]
- Yorkston KM, Beukelman DR, Bell KR. Clinical management of dysarthric speech. Austin, TX: Pro-Ed; 1988. [Google Scholar]