Abstract
Collective cell polarization is an important characteristic of tissues. Epithelia commonly display cellular structures that are polarized within the plane of the tissue. Establishment of this planar cell polarity requires mechanisms that locally align polarized structures between neighbouring cells, as well as cues that provide global information about alignment relative to an axis of a tissue. In the Drosophila ovary, the cadherin Fat2 is required to orient actin filaments located at the basal side of follicle cells perpendicular to the long axis of the egg chamber. The mechanisms directing this orientation of actin filaments, however, remain unknown. Here we show, using genetic mosaic analysis, that fat2 is not essential for the local alignment of actin filaments between neighbouring cells. Moreover, we provide evidence that Fat2 is involved in the propagation of a cue specifying the orientation of actin filaments relative to the tissue axis. Monte Carlo simulations of actin filament orientation resemble the results of the genetic mosaic analysis, if it is assumed that a polarity signal can propagate from a signal source only through a connected chain of wild-type cells. Our results suggest that Fat2 is required for propagating global polarity information within the follicle epithelium through direct cell–cell contact. Our computational model might be more generally applicable to study collective cell polarization in tissues.
Keywords: Drosophila, follicle cell, Fat2, planar cell polarity, Monte Carlo simulation
1. Results and discussion
The planar polarization of structures like hairs or trichomes on the surface of epithelial sheets is common to animals [1–6]. Mathematical models of planar polarization based on complex feedback interaction schemes [7], feedback loops combined with external signalling of stochasticity [8] or mechanical tension [9] have been developed for vector cellular structures like the distally pointing hairs on the Drosophila wing.
The Drosophila follicle epithelium, a sheet of cells surrounding the germline cells within each egg chamber of the ovary, is a useful system to study planar polarity. Follicle cells display at their basal side bundles of contractile actin filaments. These actin filaments are oriented perpendicular to the anteroposterior (long) axis of the egg chamber, but they do not necessarily have a common polarity on this axis [10,11] (figure 1a,e and the electronic supplementary material, figure S1). The planar orientation of basal actin filaments appears to be important for the elongation of egg chambers along their anteroposterior axis that takes place during oogenesis [12]. We have recently shown that the planar orientation of actin filaments and egg chamber elongation depend on the activity of the protein Fat2 [13] (figure 1b,f and the electronic supplementary material, figure S1). Fat2 is a member of the cadherin superfamily of Ca2+-dependent cell adhesion molecules that is involved in cell–cell interactions [14]. The localization of Fat2 protein is polarized in the basal plane of follicle cells. Fat2 protein is enriched only on one of the two sides of follicle cells where the basal actin filaments terminate [13]. The mechanism by which Fat2 directs the orientation of basal actin filaments remains, however, poorly understood. Here we show, using a combination of mosaic genetic analysis and computational modelling, that Fat2 is dispensable for the local alignment of basal actin filaments between neighbouring cells, but that Fat2 instead is required for propagating global polarity information within the follicle epithelium through direct cell–cell contact.
Figure 1.
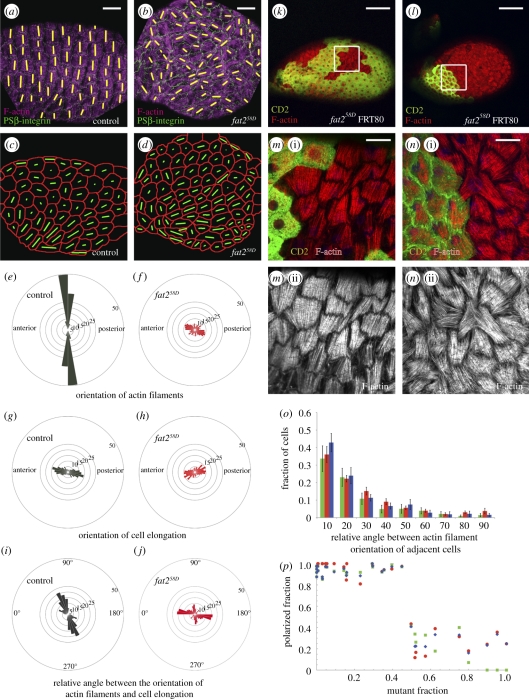
(a,b) Orientation of basal actin filaments and cell elongation in the follicle epithelium. Control (a) and a fat258D mutant (b) stage 12 egg chamber stained for F-actin (purple) and PSβ-integrin (green), a marker for basal cell outlines. Yellow bars highlight the orientation of actin filaments. (c,d) Cell meshwork based on PSβ-integrin staining for the egg chambers shown in (a) and (b), respectively. Green bars represent orientation and magnitude of cell elongation. (e,f) Angular distribution of actin filaments of control (e) and fat258D mutant (f) stage 12 egg chambers. The numbers refer to the fraction of cells in a bin as a percentage of the total number of cells analysed. (g,h) Angular distribution of cell elongation in the plane of the epithelium of control (g) and fat258D mutant (h) stage 12 egg chambers. (i,j) Distributions of relative angles between actin filaments and cell elongation of control (i) and fat258D mutant (j) stage 12 egg chambers. (k,l) Stage 12 egg chamber with a small (k) or high (l) fraction of fat258D homozygous mutant cells stained for F-actin (red). Mutant cells are marked by the absence of CD2 (green). (m,n) Magnified view of the basal actin filaments of the area indicated by a square in (k) and (l), respectively. (o) The angle in degrees between actin filaments of neighbouring cells measured in mosaic egg chambers containing more than 50% fat258D mutant cells for interfaces between wild-type cells (green columns), between fat258D mutant cells (red columns), and between wild-type and fat258D mutant cells (blue columns) as a fraction of the total number of cells analysed for each genotype. Mean and s.e.m. are shown. (p) The fraction of cells with actin filaments polarized ±10° perpendicular to the long axis of egg chambers for all cells (blue), wild-type cells (green) and fat258D mutant cells (red). A total of 3710 follicle cells in 32 egg chambers were analysed. Scale bars: (a,b) 30 µm; (k,l) 100 µm and (m,n) 20 µm.
2. The orientation of actin filaments is related to cell elongation
The contraction of basal actin filaments could contribute to the elongation of cells that drive the lengthening of the egg chamber along its anteroposterior axis. We therefore used automated image analysis [9] to test for a preferential elongation direction of follicle cells. Follicle cells were preferentially elongated along the anteroposterior axis of wild-type stage 12 egg chambers (figure 1c,g and the electronic supplementary material, figure S1). Interestingly, follicle cells were also preferentially elongated along this axis, though to a lesser extent, in fat258D, a null allele of fat2 [13], egg chambers (figure 1d,h and the electronic supplementary material, figure S1). These results indicate that Fat2 plays only a minor role in the oriented elongation of follicle cells. Moreover, in wild-type egg chambers, basal actin filaments were oriented roughly perpendicular to the long axis of follicle cells (figure 1i and the electronic supplementary material, figure S1). By contrast, in fat258D mutant egg chambers, follicle cells were preferentially elongated parallel to the actin filaments (figure 1j and the electronic supplementary material, figure S1). Thus, when the actin filaments are poorly aligned relative to the anteroposterior axis of the egg chamber, then there seems to be no contractile force exerted via the filaments and the cells end up longer on the axis of the filaments. However, when all actin filaments within egg chambers are aligned, cells are contracted on this axis and elongated perpendicular to the actin filaments. Proper actin alignment could thus facilitate the lengthening of the egg chamber during development by elongating cells along the anteroposterior axis.
3. Fat2 is required for the propagation of global polarity information
The alignment of basal actin filaments both depends on mechanisms that locally align the filaments between neighbouring cells and on mechanisms that align the filaments relative to the axis of the egg chamber. To test whether Fat2 is involved in one or both of these mechanisms, we generated mosaic egg chambers mutant for fat258D using the FRT-Flp system [15]. Small patches of follicle cells mutant for fat2 still aligned their actin filaments properly in respect to their neighbours and to the long axis of the egg chamber [13] (figure 1k,m). However, when large patches of follicle cells were mutant for fat2, then both mutant cells and the remaining wild-type cells failed to align their actin filaments perpendicular to the anteroposterior axis of the egg chamber [13] (figure 1l,n). Cells, however, still preferentially aligned their actin filaments parallel to their neighbours. The extent to which cells aligned their actin filaments to their neighbours was similar for interfaces between wild-type cells, between fat258D mutant cells, or between wild-type and fat258D mutant cells (figure 1o). Taken together, these results demonstrate that Fat2 is not required for locally aligning actin filaments between neighbouring cells, but rather suggest that Fat2 is involved in aligning actin filaments perpendicular to the anteroposterior axis of the egg chamber.
4. Actin filament alignment involves the orchestrated action of follicle cells
The proper alignment of basal actin filaments perpendicular to the anteroposterior axis of the egg chamber strictly depended on the fraction of fat2 mutant cells in the follicle epithelium. For mutant fractions smaller than approximately 50 per cent, actin filaments were oriented perpendicular to the anteroposterior axis of egg chambers (figure 1p). By contrast, when the mutant fraction exceeded approximately 50 per cent, actin filaments were no longer appropriately oriented with respect to the anteroposterior axis of egg chambers in either wild-type or fat258D mutant cells (figure 1p). These results show that the fraction of wild-type cells to mutant fat258D follicle cells is important for the alignment of actin filaments relative to the anteroposterior axis of the egg chamber and that this alignment involves the orchestrated action of a large number of follicle cells.
5. A monte carlo simulation to model the global alignment of actin filaments
The threshold for proper actin filament alignment near 50 per cent fat2 mutant fraction is reminiscent of the critical percolation threshold at this value that has been established theoretically for the node percolation problem on the triangular lattice [16]. The percolation theory describes the behaviour of connected clusters on lattices with randomly occupied sites, and the percolation threshold refers to the critical occupation probability at which long-range connectivity first occurs. To test whether the establishment of planar cell polarity in the follicle epithelium might rely on long-range connectivity, we used computational modelling employing the Monte Carlo (MC) algorithm. This method, widely used in various applications, does not attempt to reproduce actual dynamics but identifies preferred configurations by assigning to each cell a certain ‘energy’ E depending on the polarity of each cell and its neighbours, and evolving the system in the direction minimizing its overall energy.
We simulated the follicle epithelium as a honeycomb array consisting of 210 hexagonal cells. For convenience, the array is taken to be doubly periodic. The orientation of ‘actin filaments’ is reflected by one of three possible polarization directions normal to one of the three pairs of sides of a cell. A local source emanating a signal specifying global polarity is introduced as a certain row of cells. We assume that Fat2 is required for the propagation of the signal and that Fat2 does not impose directionality on the spreading of the signal. In our simulations, we therefore allow the signal to spread along the three polarization axes. In analogy to our experiments, two distinct types of cells are defined: ‘wild-type’ cells that transmit the signal propagating from the signal source and ‘mutant’ cells that are disabled to transmit such a signal. Mutant cells, like wild-type cells, tend to be aligned with neighbouring cells. Common alignment of adjacent cells is encouraged by assigning to each cell an energy gain E0 (taken as unity) when both cells are polarized normally to their common border. In addition, wild-type cells are assigned energy gain E1 when they are oriented in the preferred ‘vertical’ direction (to reflect the orientation of actin filaments perpendicular to the anteroposterior axis of egg chambers). This assignment is, however, conditioned on their connection to the signal source: we assume that wild-type cells adopt the vertical orientation only when they are reached by a signal propagating through other wild-type cells. Disconnected wild-type cells, similar to mutant cells, can acquire a proper polarization direction only under the influence of their neighbours.
The simulations have been carried out in the following way. First, locations of mutant cells are assigned. In order to imitate the experimental procedure, we generated cell patterns by seeding the lattice with a number of wild-type and mutant cells proportional to their chosen concentration and allowing the ‘seeds’ to grow in a random fashion by assigning the same cell type to neighbouring ‘empty’ cells until the type of every cell is assigned and a preset mutant fraction is reached. Second, we find the cluster of wild-type cells that is connected to the signal source. Initially, orientation of all cells is assigned at random, so that each orientation has the probability 1/3. After this, the MC algorithm [17] is implemented: at each step a randomly chosen cell is addressed, and its polarization is changed if it leads to energy gain and left unchanged otherwise. Typically, about 20 Monte Carlo cycles (MCC) are sufficient to reach a stationary configuration (MCC denotes the number of steps equal to the number of cells, e.g. 210 in our case).
6. Simulated configurations of actin filament orientation reveal a critical mutant fraction
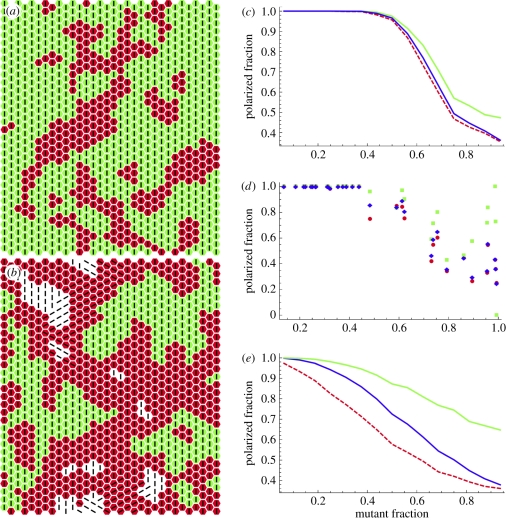
Some typical final configurations obtained in simulations are shown in figure 2a,b. Mutant cells are shaded red and connected wild-type cells are shaded green; disconnected wild-type cells are unshaded and lines show the polarization direction. Figure 2c shows the fraction of ‘vertically’ polarized cells as a function of mutant concentration. Each point representing mutant cell fractions from 1/16 to 15/16 is obtained as the average of 50 runs with different distributions of mutant cells and connected clusters. For mutant fractions between 1/16 and 7/16, the fraction of polarized cells was close to 1. For larger mutant fractions, the fraction of polarized cells decreased with increasing mutant fractions. Individual runs are rather noisy near and beyond the critical mutant concentration, as seen in figure 2d where, in imitation of figure 1p, results of 32 runs with randomly chosen mutant fractions are shown. Compared with the experimental data (figure 1p), the transition zone between polarized and non-polarized cells was broadened in the simulation, indicating that the simulation does not entirely capture the complexity of the biological system. We note that in our simulation attempts where all wild-type cells were assigned an energy gain when they were oriented vertically, irrespectively of their connection to the signal source, the polarized fraction decreased in a roughly linear fashion with growing mutant fraction and never exhibited a sharp transition (figure 2e). We have also tried various nonlinear energy functions dependent on the polarizations of closest neighbours, but in no case a sharp transition has been detected without taking account of the effect of a direct relay of a signal from a source. Our simulations, thus, stress the importance of a direct signal relay mechanism for the emergence of planar cell polarity and support our conclusion that Fat2 controls the orientation of actin filaments by propagating a polarity signal from a signal source by direct cell–cell interactions.
Figure 2.
Orientation of actin filaments as a function of mutant fraction in simulations. (a,b) Typical cell clustering and polarization patterns. Mutant cells are shaded red; connected wild-type cells are shaded green; disconnected wild-type cells are unshaded; lines show the polarization direction. (a) Mutant fraction 0.3; (b) mutant fraction 0.6. (c) Fraction of ‘vertically’ polarized cells as a function of mutant fraction (averages of 50 runs). Green, wild-type cells; red (dashed), mutant cells; blue, overall average. (d) Fraction of vertically polarized cells in 32 individual runs with randomly chosen mutant fractions. Colour code as in (c). (e) Same as (c) but simulated without taking into account wild-type cells connection to the signal source.
7. Conclusions
We demonstrate that Fat2 is not involved in the local alignment of actin filaments between neigbouring cells, but instead our results show that Fat2 is required for the global alignment of actin filaments relative to the anteroposterior axis of egg chambers. Mosaic genetic analysis revealed that the global alignment of actin filaments involves the orchestrated action of a large fraction of follicle cells. Our simulations indicate that this orchestrated action relies on the propagation of a signal from a localized signal source through direct cell–cell contact. The source and nature of the signal are currently unknown. Previously, graded molecular cues [18] or directed mechanical stresses [9,19] have been shown to specify global polarity in tissues. In the follicle epithelium, circumstantial evidence indicates that polar cells, specialized follicle cells located at the two poles of egg chambers, might act as a source of a molecular signal [20]. Alternatively, two groups of cells at the poles of the egg chamber show oscillating contractions that might act as sources of a mechanical signal [11]. Our modelling tools make it possible to deduce the position of the signal source from the spatial distribution of connected and unconnected wild-type cells, provided signal diffusion and polarization times are comparable. Further genetic mosaic analysis will be required to test this prediction.
Acknowledgements
We thank E. Knust and A. Oates for critical comments on the manuscript and anonymous referees for helpful suggestions. This work was supported by grants from the Deutsche Forschungsgemeinschaft (C.D.) and the Human Frontier Science Programme (L.P. and C.D.).
References
- 1.Adler P. N. 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2, 525–535 10.1016/S1534-5807(02)00176-4 (doi:10.1016/S1534-5807(02)00176-4) [DOI] [PubMed] [Google Scholar]
- 2.Saburi S., McNeill H. 2005. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr. Opin. Cell Biol. 17, 482–488 10.1016/j.ceb.2005.08.011 (doi:10.1016/j.ceb.2005.08.011) [DOI] [PubMed] [Google Scholar]
- 3.Seifert J. R., Mlodzik M. 2007. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126–138 10.1038/nrg2042 (doi:10.1038/nrg2042) [DOI] [PubMed] [Google Scholar]
- 4.Strutt H., Strutt D. 2005. Long-range coordination of planar polarity in Drosophila. Bioessays 27, 1218–1227 10.1002/bies.20318 (doi:10.1002/bies.20318) [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Nathans J. 2007. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647–658 10.1242/dev.02772 (doi:10.1242/dev.02772) [DOI] [PubMed] [Google Scholar]
- 6.Zallen J. A. 2007. Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 10.1016/j.cell.2007.05.050 (doi:10.1016/j.cell.2007.05.050) [DOI] [PubMed] [Google Scholar]
- 7.Amonlirdviman K., Khare N. A., Tree D. R., Chen W. S., Axelrod J. D., Tomlin C. J. 2005. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science 307, 423–426 10.1126/science.1105471 (doi:10.1126/science.1105471) [DOI] [PubMed] [Google Scholar]
- 8.Burak Y., Shraiman B. I. 2009. Order and stochastic dynamics in Drosophila planar cell polarity. PLoS Comput. Biol. 5, e1000628. 10.1371/journal.pcbi.1000628 (doi:10.1371/journal.pcbi.1000628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aigouy B., Farhadifar R., Staple D. B., Sagner A., Roper J. C., Jülicher F., Eaton S. 2010. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786 10.1016/j.cell.2010.07.042 (doi:10.1016/j.cell.2010.07.042) [DOI] [PubMed] [Google Scholar]
- 10.Gutzeit H. O. 1990. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur. J. Cell Biol. 53, 349–356 [PubMed] [Google Scholar]
- 11.He L., Wang X., Tang H. L., Montell D. J. 2010. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 12, 1133–1142 10.1038/ncb2124 (doi:10.1038/ncb2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutzeit H. O., Eberhardt W., Gratwohl E. 1991. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J. Cell Sci. 100, 781–788 [DOI] [PubMed] [Google Scholar]
- 13.Viktorinová I., König T., Schlichting K., Dahmann C. 2009. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development 136, 4123–4132 10.1242/dev.039099 (doi:10.1242/dev.039099) [DOI] [PubMed] [Google Scholar]
- 14.Shapiro L., Weis W. I. 2009. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 1, a003053. 10.1101/cshperspect.a003053 (doi:10.1101/cshperspect.a003053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu T., Rubin G. M. 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 [DOI] [PubMed] [Google Scholar]
- 16.Feng X., Deng Y., Blote H. W. 2008. Percolation transitions in two dimensions. Phys. Rev. E. 78, 031136. 10.1103/PhysRevE.78.031136 (doi:10.1103/PhysRevE.78.031136) [DOI] [PubMed] [Google Scholar]
- 17.Binder K., Heermann D. W. 1992. Monte Carlo simulation in statistical physics, 2nd edn Berlin, Germany: Springer [Google Scholar]
- 18.Strutt D. 2009. Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb. Perspect. Biol. 1, a000489. 10.1101/cshperspect.a000489 (doi:10.1101/cshperspect.a000489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olguin P., Glavic A., Mlodzik M. 2011. Intertissue mechanical stress affects frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr. Biol. 21, 236–242 10.1016/j.cub.2011.01.001 (doi:10.1016/j.cub.2011.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frydman H. M., Spradling A. C. 2001. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128, 3209–3220 [DOI] [PubMed] [Google Scholar]