Primary gastrointestinal malignant melanoma is an unusual clinical entity. Rarer still is primary gastric melanoma. Most melanomas found in the stomach are metastases from cutaneous sources.1 Primary gastric melanoma is underdiagnosed, its symptoms and signs are nonspecific, and specific staining techniques must be used to confirm the diagnosis. The following case of primary gastric melanoma involves a middle-aged white man who initially presented with upper gastrointestinal bleeding.
Case Report
A 50-year-old white man presented after 3 episodes of hematemesis and 1 episode of melena. He had a 9-month history of worsening epigastric pain and a 20-pound weight loss over the previous month, and his past medical history was significant for hypertension, pancreatitis, and peptic ulcer disease. In addition, the patient was a 40-pack-per-year smoker and a former alcoholic, and his family history was deemed noncontributory. On examination, the patient was hypotensive but not tachycardic, and pallor of the conjunctiva and mild epigastric tenderness were seen. Laboratory results were significant for a hemoglobin of 7.5 g/dL and hematocrit of 23.1%. Upper endoscopy revealed a large necrotic ulcerated mass extending from the antrum to the body of the stomach (Figure 1). Computed tomography scan of the abdomen was remarkable for thickening of the posterior wall of the body of the stomach, with the mass extending into the gastric lumen and the adjacent fat with 2 enlarged lymph nodes (Figure 2). Surgery demonstrated a mass measuring 12 cm × 10.5 cm × 2.5 cm involving 50% of the stomach, including the gastrocolic ligaments, and extending into the pancreas. Palliative resection was performed. Histopathology showed neoplastic cells in a sheet-like growth pattern showing no glandular differentiation (Figure 3). S100 protein, HMB-45 antibodies, and Melan A staining were strongly positive, thus confirming a diagnosis of gastric melanoma (Figure 4). The patient denied any history of cancerous lesions of the skin. A complete examination of his skin, including oral and anal mucosa, showed no suspicious lesions, and fundoscopic examination of the eye was normal. A diagnosis of advanced primary gastric melanoma was made. The patient was discharged to a correctional facility and subsequently lost to follow-up.
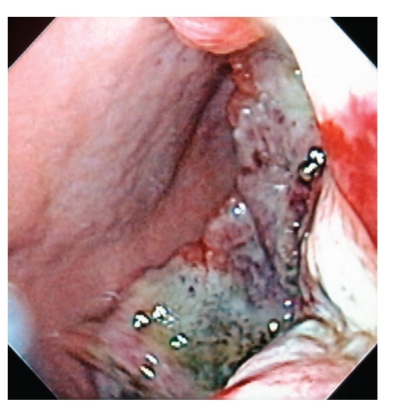
Figure 1.
Upper endoscopy revealed a large necrotic ulcerated mass extending from the antrum to the body of the stomach.
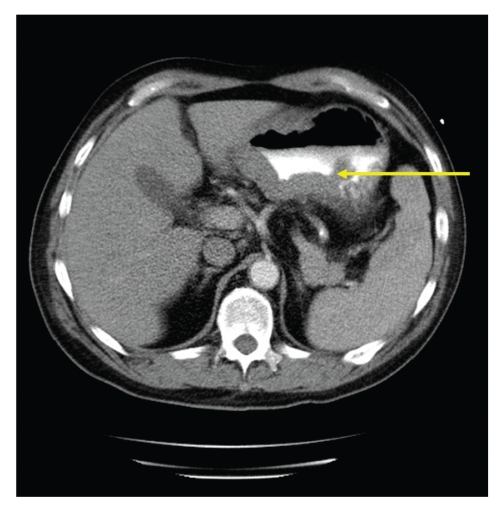
Figure 2.
Contrast-enhanced computed tomography scan of the abdomen demonstrated thickening of the posterior wall of the body of the stomach with extension of the mass into the gastric lumen and into the adjacent fat (yellow arrow) with 2 enlarged lymph nodes.
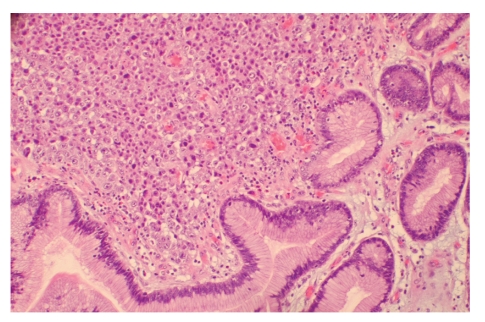
Figure 3.
Biopsy of the mass showed neoplastic cells in a sheet-like growth pattern showing no glandular differentiation (hematoxylin & eosin stain, ×200).
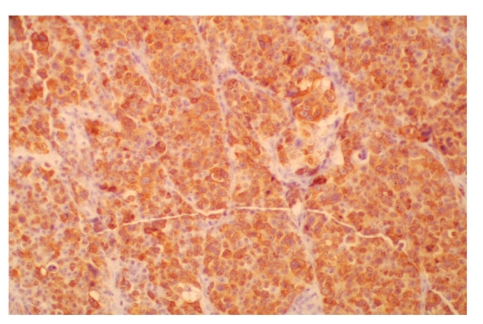
Figure 4.
Tumor cells were strongly positive for immunoperoxidase staining for Melan A (× 200).
Discussion
Malignant melanoma most commonly develops in the skin. The vast majority of gastrointestinal melanomas are metastases from a cutaneous primary tumor.1 In rare instances, primary malignant melanoma can arise from mucosa of the gastrointestinal tract, particularly from the esophagus, anorectum, and small bowel.2 Fewer than 15 cases of primary gastric melanoma have been documented in the literature.3–6
There has been speculation in the past as to whether primary melanoma can occur in the stomach, as benign melanocytes are absent in the normal gastric wall. However, melanosis of the stomach has been well documented in the case of anal and esophageal melanoma.6,7 Thus, it is possible that primary gastric melanoma can occur in unusual circumstances.
Criteria for the diagnosis of primary gastric melanoma include the absence of concurrent lesions and the lack of a history of melanoma or atypical melanocytic lesion removal from the skin or other organs.8 Disease-free survival of at least 12 months after curative surgical excision of the involved organ has been proposed as a criterion for the distinction of a primary lesion from a metastatic lesion, as 50% of patients with stage IV melanoma of the skin or visceral disease from an unknown primary lesion die 12 months after diagnosis.9
The clinical manifestations of primary gastric melanoma are similar to those of other gastric tumors, with weight loss, upper gastrointestinal bleeding, and anemia as the most common symptoms. Most patients are asymptomatic until the tumor becomes advanced. Computed tomography scan of the abdomen, upper endoscopy, and subsequent biopsy are the main diagnostic modalities. On upper endoscopy, a mass-like lesion with black pigmentation may be seen. Immunohistochemical stains with S100 protein, Melan-A, and HMB-45 antibodies have increased the diagnostic sensitivity of biopsy and cytology and play a key role in the diagnosis of these lesions.10 Chemotherapy options include interferon, interleukin-12, and other agents.8,11 Prognosis is extremely poor due to the frequent delay in diagnosis, the inherently more aggressive nature of the tumor, and earlier dissemination due to the rich lymphatic and vascular supply of the gastrointestinal mucosa.12
Primary gastric melanoma is an extremely uncommon clinical entity. Amelanotic melanoma such as the one in this case can be missed in poorly differentiated tumors unless appropriate staining tests are performed. Primary malignant melanoma of the stomach may be an underdiagnosed phenomenon. Early detection and surgical intervention is critical for long-term cure,4 though overall prognosis is very poor.
Footnotes
I would like to acknowledge Dr. Robert Osburne for providing guidance with the manuscript.
References
- 1.Washington K, McDonagh D. Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol. 1995;8:427–433. [PubMed] [Google Scholar]
- 2.Aagaard MT, Kristensen IB, Lund O, Hasenkam JM, Kimose HH. Primary malignant non-epithelial tumours of the thoracic oesophagus and cardia in a 25-year surgical material. Scand J Gastroenterol. 1990;25:876–882. doi: 10.3109/00365529008997607. [DOI] [PubMed] [Google Scholar]
- 3.Chandler AB, Jones GF. Malignant melanoma of the gastrointestinal tract: a case report. Am J Surg. 1951;17:719–721. [PubMed] [Google Scholar]
- 4.Alazmi WM, Nehme OS, Regalado JJ, Rogers AI. Primary gastric melanoma presenting as a nonhealing ulcer. Gastrointest Endosc. 2003;57:431–433. doi: 10.1067/mge.2003.120. [DOI] [PubMed] [Google Scholar]
- 5.Lagoudianakis EE, Genetzakis M, Tsekouras DK, Papadima A, Kafiri G, et al. Primary gastric melanoma: a case report. World J Gastroenterol. 2006;12:4425–4427. doi: 10.3748/wjg.v12.i27.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti JE, Bodor J, Torres AD, Pini N. Primary malignant melanoma of the esophagus associated with melanosis of the esophagus and stomach [in Spanish] Acta Gastroenterol Latinoam. 1984;14:139–148. [PubMed] [Google Scholar]
- 7.Horowitz M, Nobrega MM. Primary anal melanoma associated with melanosis of the upper gastrointestinal tract. Endoscopy. 1998;30:662–665. doi: 10.1055/s-2007-1001373. [DOI] [PubMed] [Google Scholar]
- 8.Elsayed AM, Albahra M, Nzeako UC, Sobin LH. Malignant melanomas in the small intestine: a study of 103 patients. Am J Gastroenterol. 1996;91:1001–1006. [PubMed] [Google Scholar]
- 9.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Blecker D, Abraham S, Furth EE, Kochman ML. Melanoma in the gastrointestinal tract. Am J Gastroenterol. 1999;94:3427–3433. doi: 10.1111/j.1572-0241.1999.01604.x. [DOI] [PubMed] [Google Scholar]
- 11.Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 12.Sachs DL, Lowe L, Chang AE, Carson E, Johnson TM. Do primary small intestinal melanomas exist? Report of a case. J Am Acad Dermatol. 1999;41:1042–1044. doi: 10.1016/s0190-9622(99)70273-2. [DOI] [PubMed] [Google Scholar]