Abstract
Fecal incontinence is a common condition that often impairs quality of life. It is generally caused by a variety of conditions that are associated with anorectal sensorimotor dysfunction and/or diarrhea. A detailed characterization of symptoms, particularly bowel habits, is useful for assessing symptom severity and guiding management. A careful digital rectal examination is invaluable for gauging anal resting and squeeze pressures and anorectal evacuation. Tests should be tailored to age, symptom severity, and response to previous therapy. Anorectal manometry and sphincter imaging are useful starting tests. Depending upon the clinical circumstances, additional testing and therapeutic options may be useful. Fecal continence can be improved by measures to regulate bowel habits and pelvic floor retraining. Surgical repair of anal sphincter defects improves fecal continence in the short but not in the long term. Newer surgical therapies and devices are of limited and/or unproven utility.
Keywords: Fecal incontinence, management, diagnosis, anal incontinence
Fecal incontinence (FI) is defined as the recurrent uncontrolled passage of fecal material for at least 1 month's duration in an individual with a developmental age of at least 4 years.1 FI is a common condition, often impairing quality of life and generally caused by a variety of conditions associated with anorectal sensorim-otor dysfunction and/or diarrhea.2–4 Unless specifically questioned, most people with FI will not mention the condition to a healthcare provider. Therefore, it is essential that patients, in particular those at risk for FI (eg, those with diarrhea), be questioned regarding the condition. This review will highlight salient features of the etiology and pathophysiology of FI, discuss the role of diagnostic assessments, and detail various therapies.
Mechanisms of Fecal Continence
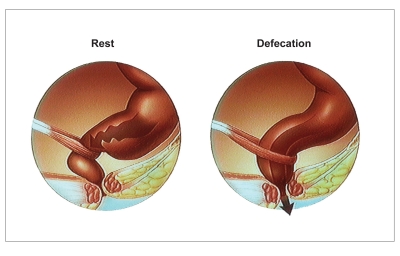
Fecal continence is normally associated with anatomic factors such as the pelvic barrier, rectal curvatures, and transverse rectal folds; recto-anal sensation; and rectal compliance.5 Rectal distension by stool induces rectal contraction, the sensation of urgency, reflex relaxation of the internal anal sphincter, and semivoluntary relaxation of the pelvic floor muscles, the puborectalis and external anal sphincter, prompting defecation if it is socially convenient (Figure 1). If not, rectal contractions and the sensation of urgency generally subside as the rectum accommodates to continued distention. This accommodation, together with voluntary contraction of the external anal sphincter and puborectalis muscles, allows defecation to be postponed when necessary.6,7 The factors that determine whether rectal distention is interpreted as a desire to defecate or to pass flatus are unclear.
Figure 1.
Anorectal configuration at rest and during defecation. At rest, the puborectalis maintains a relatively acute anorectal angle and the anal sphincters ensure that the canal is closed. During defecation, the puborectalis relaxes, allowing the anorectal angle to widen. The anal sphincters also relax, allowing defection to occur.
Etiology and Pathophysiology of Fecal Incontinence
FI is caused by anorectal dysfunctions and/or disordered bowel habits that result from a variety of conditions (Table 1). Moreover, the etiology influences the pathophysiologic mechanisms of FI.2 More than one pathophysiologic mechanism may contribute to FI in the same patient. In women without systemic or neurologic disease, FI is generally caused by anorectal sensorimotor dysfunctions and/or bowel habit disturbances. Although anal sphincter damage due to obstetric or iatrogenic injury and pudendal neuropathy have been implicated as a cause of FI in women, the contribution of these factors to FI is incompletely understood due to the following reasons: among women in the community, the median age of onset of FI is 55 years (ie, years after vaginal delivery of children)3; “minor” sphincter defects are common in asymptomatic women after vaginal delivery,8 thereby making it challenging to ascertain the precise contribution of anal sphincter injury to anal weakness; and prolonged pudendal nerve latencies, which are widely used to diagnose pudendal neuropathy, are flawed indices of pudendal nerve function.9
Table 1.
Etiology of Fecal Incontinence
|
Reprinted with permission from Bharucha A.2
Anorectal Sensorimotor Dysfunctions
The cardinal features of anorectal sensorimotor dysfunctions in FI are summarized in Table 2. A majority of women with FI have reduced anal resting and/or squeeze pressures, reflecting the weakness of the internal and/or external anal sphincters, respectively10,11 (Figure 2). In addition to anal sphincter injury, FI is also associated with atrophy, denervation, and impaired function of the puborectalis muscle.11–13 Some patients with FI have more generalized pelvic floor weakness, known as descending perineum syndrome, which is often associated with pelvic organ prolapse affecting the anterior and/or middle compartments.14 In contrast to anal weakness, the contribution of rectal sensorimotor dysfunctions to FI is underrecognized, perhaps because techniques for evaluating rectal sensorimotor functions are not widely available. Reduced rectal capacity and rectal hypersensitivity may contribute to the symptom of urgency in FI.11 Conversely, other patients with FI have reduced rectal sensation. When rectal sensation is reduced, the external anal sphincter may not contract promptly when the rectum is distended by stool.15,16
Table 2.
Cardinal Aspects of Anorectal Sensorimotor Dysfunctions in Fecal Incontinence
|
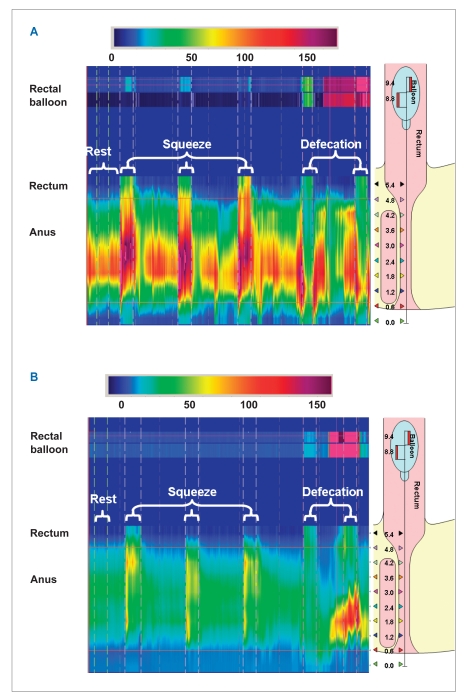
Figure 2.
Anorectal pressures measured by high-resolution manometry in a healthy subject (A) and a patient with fecal incontinence (B). Pressures were measured at rest, during squeeze (3 times), and during simulated defecation, which was performed before and after inflation of a rectal balloon. The right side of each panel shows the location of the sensors: 10 sensors at 0.6-cm intervals in the rectum and anal canal, and 2 sensors in the rectal balloon. Figure 2A shows normal anal resting pressure (81 mmHg), normal and sustained squeeze pressure (196 mmHg for 30 sec), and normal anal relaxation during simulated defecation. In contrast, the patient with fecal incontinence (B) has low anal resting pressure (30 mmHg), a poorly sustained squeeze response (82 mmHg for only approximately 5 sec), and paradoxical contraction during simulated defecation with an inflated balloon.
FI may also be associated with features of disordered evacuation. Impaired rectal evacuation with retention of feces is typically associated with fecal soiling or seepage.11,17,18 Such patients may benefit from biofeedback retraining to improve abdominopelvic coordination during defecation. In the long term, excessive straining may cause increased perineal descent, or descending perineum syndrome, which can stretch and thus damage the pudendal nerve. This straining also increases the obtuseness of the anorectal angle, thereby impairing the flap valve normally responsible for maintaining fecal continence during increased intra-abdominal pressure.
Disordered Bowel Habits
Clinical observations and epidemiologic studies suggest that disordered bowel habits, particularly diarrhea and rectal urgency, are risk factors for FI.19 After controlling for frequency, consistency, and obstetric trauma, the symptom of rectal urgency is an independent, and perhaps the most important, risk factor for FI in women.20 On average, women with rectal urgency have an 8-fold increased risk for FI even after controlling for a history of obstetric anal sphincter injury and other bowel symptoms such as constipation and diarrhea.20 Bowel habits, rectal urgency, and a sense of incomplete evacuation also explain why women may be incontinent for some but not all bowel movements.21 Thus, women with FI are more likely to be incontinent when they have frequent stools, loose stools, and rectal urgency. Together, these observations reinforce the importance of managing bowel disturbances in FI. Finally, in addition to normal anorectal functions and stool consistency, mental faculties and mobility are also necessary to preserve continence.
Assessment of Fecal Incontinence
Clinical Assessment
In patients with FI, a detailed history can provide an indication of the severity, etiology, and pathophysiology of this symptom, establish rapport with the patient, and guide diagnostic testing and treatment. Bowel habits and stool consistency should preferably be characterized by pictorial stool scales such as the Bristol scale.22 As with incontinence, the symptom of urgency, which can be gauged by questioning patients regarding the length of time they have to reach the toilet, can also be very distressing to people with FI. Leakage of solid stool likely reflects more severe anal weakness than isolated leakage of liquid stool. However, patients are usually more troubled by leakage of liquid than solid stool.23 Although patients with FI are also distressed by involuntary passage of flatus, incontinence for flatus alone does not suffice for the characterization of FI, partly due to the difficulty of determining when passage of flatus is abnormal. Symptom severity can be quantified by validated instruments.3,4,24–26 With one exception,4 most scales, including the Wexner instrument, which is widely used in surgical trials, quantify the consistency and frequency but not the amount of stool loss3,4 (Table 3). The severity of FI is strongly correlated with the impact of this condition on quality of life.27
Table 3.
Symptom-Severity Scale in Fecal Incontinence
| Symptoms | Symptom-severity score | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Frequency | <1/month | >1/month to several times/week | Daily | |
| Composition | Mucus/liquid stool | Solid stool | Liquid and solid stool | |
| Amount | Small (ie, staining only) | Moderate (ie, requiring change of underwear) | Large (ie, requiring change of all clothes) | |
| Urgency or passive incontinence | Neither | Passive incontinence | Urge incontinence | Combined (ie, passive and urge) incontinence |
The symptom-severity score was formulated by applying a physician-assigned score (ie, the symptom-severity score) to each of the 4 self-reported symptoms of fecal incontinence. The maximum total score was 13. Scores of 1–6, 7–10, and 11–13 were categorized as mild, moderate, and severe fecal incontinence, respectively.
Reproduced with permission from Bharucha AE, et al.3
After anal inspection, a digital rectal examination is very useful for gauging anal sphincter and puborectalis functions. The resistance to anal digital insertion provides a measure of anal resting pressure. When patients squeeze their anal sphincter, the sphincter and puborectalis should contract, lifting the examining finger anterosuperiorly, or toward the umbilicus. Conversely, simulated defecation should be accompanied by 2–4 cm of perineal descent and puborectalis relaxation. Finally, examination in the seated position on a commode may reveal rectal prolapse or excessive perineal descent, which may not be evident when the patient is supine.
Diagnostic Testing
The extent of diagnostic testing is tailored to the patient's age, probable etiologic factors, symptom severity, impact on quality of life, response to conservative medical management such as loperamide or stool softeners, and availability of tests. The strengths and limitations of these tests have been reviewed in detail in the literature.2,9,28
Endoscopy. Endoscopic assessment of the rectosigmoid mucosa should be considered in most patients, particularly those with constipation and/or diarrhea; a colonoscopy may be desirable in certain circumstances, for example, if the differential diagnosis includes colon cancer.
Anal Manometry. Assessment of anal pressures by manometry is a starting point for diagnostic testing in FI. Manometry should employ rigorous techniques, and pressures should be interpreted with reference to normal values in age- and gender-matched subjects measured by the same techni que,9,29,30 particularly because anal resting and squeeze pressures decline with age, even in asymptomatic people.31
Anal resting and squeeze pressures are frequently reduced in FI.10,11 Among patients with weak or normal anal pressures, other explanations for FI, including diarrhea, disturbances of rectal compliance, and/or rectal sensation, should be sought. Anorectal testing such as anal manometry or rectal balloon expulsion tests can also identify a rectal evacuation disorder, which may coexist with FI.32,33 However, it is important to recognize that the anal sphincter or puborectalis may not relax during defecation in up to 20% of asymptomatic subjects.34 Thus, a rectal evacuation disorder should be diagnosed by clinical features and manometry rather than manometry alone.
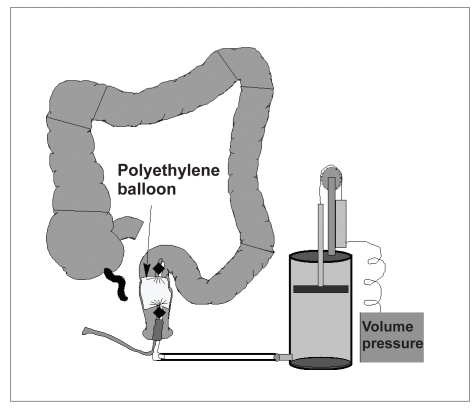
Rectal Sensation. Rectal sensation is assessed by progressively distending a latex balloon manually or by distending a polyethylene balloon with a barostat (Figure 3). Thresholds for first perception, desire to defecate, and severe urgency are measured during distention. Generally, only volume thresholds are measured during latex balloon distention, a test that is widely available. A barostat, which is only available in certain centers, can also assess balloon pressure and therefore also characterize compliance.
Figure 3.
Rectal barostat assembly. Rectal compliance and sensation are measured by distending a highly compliant polyethylene balloon with a barostat.
Reprinted with permission from Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology. 2004;126:S90-S98.
Thresholds for rectal sensation may be normal, reduced, or increased in FI.10,11 When rectal sensation is reduced, stool may leak before the external sphincter contracts.7,35 By improving rectal sensation, sensory retraining can restore coordinated contraction of the external sphincter and improve fecal continence.35,36 However, patients require at least some rectal perception to participate in sensory retraining. In uncontrolled studies, preserved rectal sensation before biofeedback therapy and improved sensation after biofeedback therapy predicted improved continence.37 Conversely, some incontinent patients have exaggerated rectal sensation, perhaps resulting from rectal hypersensitivity and/or reduced rectal capacity.11
Rectal Compliance. Rectal compliance is optimally measured by assessing rectal pressure-volume relationships with a barostat. Rectal compliance can also be measured with a latex balloon. Because a latex balloon is not infinitely compliant, it is necessary to measure pressures at different volumes outside the rectum and subtract these pressures from pressures at comparable volumes during rectal distension. Reduced compliance may cause symp toms of rectal urgency and frequent defecation in diarrhea-predominant irritable bowel syndrome, ulcerative colitis, or radiation injury. The rectal capacity, or the balloon volume at the maximum imposed pressure, is also reduced in a subset of women with idiopathic FI.11 Moreover, reduced rectal capacity is associated with the symptom of urgency and with rectal hypersensitivity.
Endoanal Ultrasound. Endoanal ultrasound is widely used to identify anal sphincter abnormalities in FI. Ultrasound identifies anal sphincter thinning and defects, which are often clinically unrecognized and may be amenable to surgical repair38 (Figure 4). Although endoanal ultrasound reliably identifies anatomic defects or thinning of the internal sphincter, interpretation of external sphincter images is much more subjective, operator-dependent, and confounded by normal anatomic variations in the external sphincter. The external sphincter and perirectal fat are both echogenic and frequently indistinguishable, which can preclude accurate characterization of external sphincter thickness and identification of external sphincter atrophy. The asymmetry of the external sphincter—often in the upper anal canal and particularly in women—can also impair differentiation of normal variants from sphincter defects.
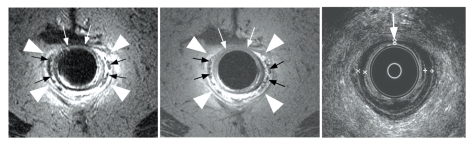
Figure 4.
Endoanal fast spin-echo T2-weighted (left panel) and spin-echo T1-weighted (center panel) magnetic resonance images demonstrate marked atrophy of the external anal sphincter (arrowheads) in a 75-year-old incontinent patient, resulting in the prominence of the internal anal longitudinal muscle (black arrows). Corresponding endoanal ultrasound images (right panel) identified patchy thinning of the internal sphincter also seen on magnetic resonance imaging (white arrows), but no external sphincter atrophy.
Reprinted with permission from Bharucha AE, et al.11
Dynamic Proctography (Defecography). Dynamic proctography is useful for selected patients with FI when clinical features suggest excessive perineal descent, internal rectal intussusception, rectoceles, sigmoidoceles, or enteroceles. Puborectalis dysfunction during squeeze and evacuation can also be characterized.39,40 During dynamic proctography, anorectal anatomy and pelvic floor motion are recorded with the patient at rest, coughing, squeezing, and straining to expel barium paste from the rectum; the anorectal angle and position of the anorectal junction are tracked during these maneuvers, as well as retention and evacuation of contrast material.
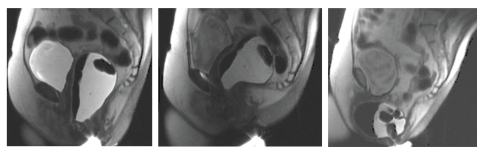
Pelvic Magnetic Resonance Imaging. Pelvic magnetic resonance imaging (MRI) is the only imaging modality that can visualize both anal sphincter anatomy and global pelvic floor motion (anterior, middle, and posterior compartments) in real-time without radiation exposure41 (Figures 5 and 6). The sphincters can be imaged by an endoanal coil or phased-array imaging. Pelvic floor motion can be visualized by dynamic imaging (image acquisition every 1.4–2.0 sec) in the desired cross-sectional plane while patients squeeze their pelvic floor muscles, evacuate ultrasound gel from the rectum, and perform a Valsalva maneuver.
Figure 5.
Anorectal imaging by dynamic magnetic resonance imaging at rest (left panel), during squeeze (center panel), and simulated defecation (right panel). Prominent contraction of the puborectalis was observed, with a reduction in the anorectal angle from 98° at rest to 53° during squeeze. During simulated defecation, there was increased perineal descent (the anorectal junction dropped from 4.2 cm below to 9.1 cm below the pubococcygeal line) and a very large anterior rectocele (5.1 cm × 6.4 cm × 6.1 cm, which did not empty during defecation, was observed.
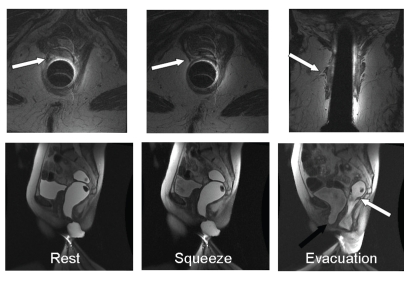
Figure 6.
Endoanal and dynamic magnetic resonance proctogram in a 70-year-old woman with urinary and fecal urgency. Endoanal magnetic resonance images show a partial tear and atrophy of the right puborectalis in axial and coronal sections (upper panel, arrow). Dynamic images reveal accentuation of the puborectalis indentation on the posterior rectal wall but little anterior or superior movement of the anorectal junction, which is consistent with puborectalis injury. During defecation, a cystocele (black arrow) and a small rectal intussusception (white arrow) were observed.
Reprinted with permission from Bharucha AE, Fletcher JG. Recent advances in assessing anorectal structure and functions. Gastroenterology. 2007;133:1069-1074.
Although there is disagreement regarding which technique is superior for evaluating the internal sphincter, MRI performs as well as or better than ultrasound for assessment of the external sphincter.42 In one study, endoanal MRI, unlike ultrasound, revealed external sphincter atrophy in 20% of women with idiopathic FI.11 Baseline external sphincter atrophy may identify those patients who will not fare well after repair of external sphincter defects.43 Endoanal MRI also revealed puborectalis atrophy in FI. Dynamic MRI provides a unique appreciation of global pelvic floor motion due to the visualization of bladder and genital organs.11
Pudendal Nerve Terminal Motor Latency. Pudendal nerve terminal motor latency has been widely used but is inaccurate for identifying pudendal nerve injury.9 Indeed, a position statement issued by the American Gastroenterological Association recommended that pudendal nerve terminal motor latency not be used for evaluating patients with FI.9
Needle Electromyography of the External Sphincter. Needle electromyography (EMG) provides a sensitive measure of denervation (fibrillation potentials) and can usually identify myopathic damage (small polyphasic motor unit potentials), neurogenic damage (large poly-phasic motor unit potentials), or mixed injury affecting the enteral anal sphincter.11
Anal EMG should be considered in patients with clinically suspected neurogenic sphincter weakness, particularly if there are features suggestive of proximal (sacral root) involvement. Neurogenic changes isolated to the external anal sphincter may be caused by injury at any level along the lower motor neuron (ie, from motor neurons in the sacral spinal cord to the nerve fascicles entering the anal sphincter, caused, for example, by local or obstetric trauma). Therefore, pudendal neuropathy can be diagnosed with certainty only when neurogenic changes affect anterior and posterior quadrants of the anal sphincter or when they affect the anal sphincter and ischiocavernosus muscle. When performed by an experienced professional, EMG is not associated with severe discomfort.
Summary and Role of Testing in Clinical Practice
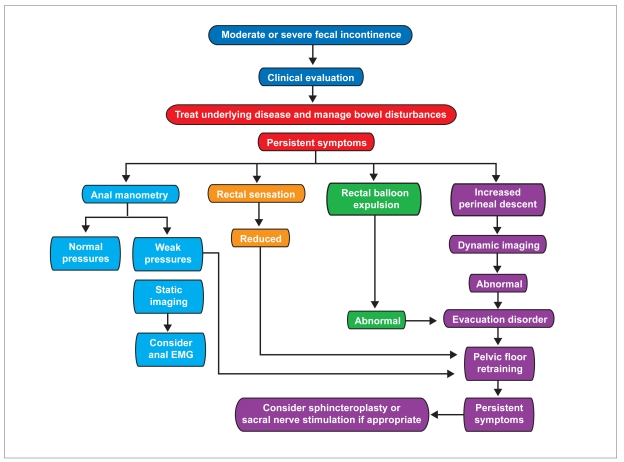
Although it is useful, anorectal testing is not mandatory in every patient with FI. Testing should be considered in patients with symptoms of moderate severity (Figure 7). The extent of testing is guided not only by the clinical features, as detailed above, but also by the availability of the tests and therapeutic options under consideration.44–46 Anorectal testing can guide management particularly when surgical options are being considered.47 Test results should be interpreted together with clinical features. The indications, modalities, and utility of testing will continue to evolve as newer therapeutic options become available.
Figure 7.
Simplified algorithm for managing fecal incontinence. Treatment choice is guided by clinical features, as detailed in the text, and by response to conservative measures, particularly management of bowel disturbances. Thereafter, further measures (eg, pelvic floor retraining) may then be necessary.
- EMG
- electromyography.
Reprinted with permission from Bharucha AE, Fletcher JG. Investigation of fecal incontinence. In: Stoker J, Taylor SA, DeLancey JOL, eds. Diagnostic Imaging: Imaging Pelvic Floor Disorders. 2nd vol. New York, New York: Springer-Verlag; 2008:229-244.
Management
The management of FI must be tailored to its clinical manifestations, address treatment of underlying disease, and be guided by diagnostic testing.
Dietary and Pharmacologic Approaches
Modifying irregular bowel habits is often the cornerstone to effectively managing FI. Bowel habits must be characterized accurately to enable the tailoring of therapy to the bowel disturbance. This tailoring is particularly important for patients who have intermittent diarrhea, where the challenge lies in reducing diarrhea while avoiding constipation.
A detailed dietary history is useful for identifying excessive ingestion of natural or processed foods (eg, prunes or beverages, respectively) that contain fructose or sorbitol, which may cause or aggravate diarrhea.48 Loperamide reduces diarrhea and slightly increases internal sphincter tone, thereby reducing FI.49 It is important to ensure that adequate doses are administered (2–4 mg, 30 min before meals, up to 16 mg daily). Taking loperamide before social occasions may reduce the risk of having an accident outside the home.
Diphenoxylate and amitriptyline are alternative options for treating diarrhea, and amitriptyline may also reduce rectal urgency.50,51 In addition, cholestyramine resin may improve postcholecystectomy diarrhea,52 and the 5-HT3 antagonist alosetron (Lotronex, Prometheus) is useful for treating refractory functional diarrhea. Conversely, patients with constipation, fecal impaction, and overflow incontinence may benefit from a regular evacuation program using timed evacuation by digital stimulation and/or bisacodyl or glycerol suppositories, fiber supplementation, and selective use of oral laxatives.53
The α1-adrenergic agonist phenylephrine, administered topically, increased anal resting pressure, though it did not improve fecal continence or anal resting pressure in a randomized, double-blind, placebo-controlled, crossover study.54
Pelvic Floor Exercises and Biofeedback Therapy
Pelvic floor exercises refer to the process by which patients are taught to squeeze the muscles surrounding the anal canal without contracting the abdominal wall. Patients are encouraged to perform these exercises several times a day. During each session, patients repetitively contract (for 10 seconds) and then relax the muscles, initially while sitting and then while standing.
Biofeedback therapy is generally performed by providing patients with visual feedback from rectal balloon and anal manometric or surface EMG sensors. Patients learn to coordinate sphincter contraction during rectal distention and also to recognize rectal distention with progressively smaller volumes, generally beginning with 50 mL and declining to 10 mL or lower.
A systematic review of mostly uncontrolled studies found that 72% of patients' symptoms resolved or improved after biofeedback therapy.55 There are 2 large randomized trials of biofeedback therapy for FI. In the St. Marks' study, 171 patients with FI were randomized to 1 of 4 groups: standard medical or nursing care (“advice only”); advice plus verbal instruction on sphincter exercises; hospital-based computer-assisted sphincter-pressure biofeedback; or hospital-based biofeedback plus use of a home electromyography biofeedback device.56 Overall, there was no significant difference among the groups, with 54% of patients in the biofeedback groups reporting improved symptoms compared to 53% in the advice-only group. Symptoms, as well as resting and squeeze pressures, improved to a similar degree in all 4 groups; this improvement was sustained for 1 year after therapy. These results appear to emphasize the utility of conservative measures—instruction of patients, emotional support, lifestyle modifications such as diet and fluids, and management techniques such as improvement of evacuation, a bowel-training program, and antidiarrheal medication—for treatment of FI.
Another study assessed the utility of biofeedback therapy for patients with FI who did not respond to other conservative measures. Of 168 patients with FI, 36 patients (21%) reported adequate relief during a 4-week run-in treatment period during which medication, education, and behavioral strategies for the prevention of FI were implemented. After excluding 24 patients (14%) who withdrew during the run-in period, the remaining 108 patients were randomized to pelvic floor exercises alone (64) or with EMG-assisted biofeedback therapy (44). Training was provided during 6 biweekly, 1-hour sessions. Three months after completing therapy, a higher proportion (77% vs 41%) of biofeedback-treated patients reported adequate symptom relief. These data demonstrated that biofeedback is more effective than pelvic floor exercises alone for patients with FI who do not respond to routine medications, educational programs, or behavioral strategies.57
Surgical Approaches
Sphincteroplasty. Reconstructive surgery is reserved for a small number of patients with FI, particularly when anal sphincter injury is recognized shortly after vaginal delivery. Whether anal sphincter defects that are recognized incidentally several years after a presumed obstetric insult should be repaired is not clear because the initial improvement in fecal continence after overlapping anterior sphincteroplasty is not often sustained. Although short-term improvements in fecal continence have been reported in up to 85% of patients, continence deteriorates thereafter, and there is a 50% failure rate after 40–60 months.58 Indeed, a recent Cochrane review concluded “that there was not enough evidence from trials on which to judge whether surgery does more good than harm in comparison with nonsurgical management, nor whether one type of surgical operation is better or worse than another one.”59
Other Surgical Approaches. Dynamic graciloplasty involves continuous electrical stimulation of the gracilis muscle, which is surgically transposed around the anal canal. Electrical stimulation facilitates anal tone by converting type II (fast-twitch, fatigue-prone) to type I (slow-twitch, fatigue-resistant) muscle fibers. The hardware for dynamic graciloplasty is approved in Europe, though not in the United States. Although fecal continence may improve in approximately 50% of patients, this procedure may be complicated by mortality (2% in 1 study) and significant morbidity (infections, 28%; device problems, 15%; leg pain, 13%), for which reoperation may be required. Even obstructed defecation has been reported.27
Clinical experience of implantation of an artificial anal sphincter, which is approved for use in the United States, is similar to that of dynamic graciloplasty. A systematic review of 14 artificial anal sphincter studies observed that most studies were case series with little or no follow-up of patients in whom the device failed.60 Moreover, complications were common, and the device was removed in approximately one third of patients. However, most patients with a functioning device reported clinically significant improvements in continence and quality of life.
A colostomy is the last resort for patients with severe FI.
Incontinence Products
Perineal protective devices include disposable and reusable bodyworn products (diaper-type garments or pads) and disposable and reusable underpads (also known as bedpads). A Cochrane review concluded that disposable bodyworn products with superabsorbent materials were more effective than reusable bodyworn products at preventing skin problems, though these studies predominantly focused on urinary incontinence rather than FI.61 Many patients with mild FI line their underwear with toilet paper and then progress to a panty liner, pad, or diaper for symptoms of increasing severity.
Anal plugs are used for the management of FI in Europe, but they are not available in the United States.62 Anal plugs can be difficult to tolerate and must be removed before bowel movements. However, when tolerated, they are effective. In a study of 14 patients, 64% were continent for feces when they used the plug63; however, the plug occasionally slipped out in 43% of patients, and 71% of patients experienced discomfort to a varying degree.
Newer Therapeutic Approaches
Predominantly uncontrolled studies suggest that sacral nerve stimulation improves fecal continence and augments anal squeeze more than resting pressure.64 Sacral stimulation may also modulate rectal sensations in incontinent patients.65 This procedure is conducted in stages; patients whose symptoms respond to temporary stimulation over approximately 2 weeks proceed to permanent subcutaneous implantation of the device. The procedure for device placement is straightforward, and device-related complications are less frequent and significant than the more invasive artificial sphincter devices discussed above. A multicenter US study assessing sacral nerve stimulation for FI has been completed, and results are awaited. Uncontrolled, predominantly small studies have used a variety of agents such as autologous fat,66 col-lagen,67 and, more recently, implantable microballoons,68 carbon-coated beads,69,70 and silicone71 to augment the pelvic barrier, with variable efficacy for managing FI. The largest study to date, in which silicon was injected into 82 patients with severe FI and low anal resting pressure, was uncontrolled. Moreover, the improvement in symptoms and resting anal pressure was modest up to 6 months after the procedure, with limited follow-up thereafter.71 The long-term efficacy and safety of these agents is presently unknown. At this stage, these approaches must be considered investigational.
Summary
FI is a common and often devastating condition. Because people with FI are often reluctant to acknowledge their condition, it behooves physicians to question at-risk patients whether they have FI. Clinical assessment is extremely useful for understanding the circumstances and severity of FI and also provides considerable information regarding the pathophysiology. Diagnostic testing should be guided by clinical features and response to previous therapy, and management of FI should focus on regulating bowel habits. Pelvic floor retraining and surgery are also helpful in selected patients.
Footnotes
This work was supported in part by Grant R01 HD41129 and DK78924 from the National Institutes of Health, United States Public Health Service.
References
- 1.Whitehead WE, Wald A, Norton NJ. Treatment options for fecal incontinence. Dis Colon Rectum. 2001;44:131–142. doi: 10.1007/BF02234835. discussion 142-144. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha A. Fecal incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Zinsmeister AR, Locke GR, Seide B, McKeon K, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Zinsmeister AR, Locke GR, Schleck C, McKeon K, Melton LJ. Symptoms and quality of life in community women with fecal incontinence. Clin Gastroenterol Hepatol. 2006;4:1004–1009. doi: 10.1016/j.cgh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18:507–519. doi: 10.1111/j.1365-2982.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Read M, Read N. Role of anorectal sensation in preserving continence. Gut. 1982;23:345–347. doi: 10.1136/gut.23.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun WM, Read NW, Miner PB. Relation between rectal sensation and anal function in normal subjects and patients with faecal incontinence. Gut. 1990;31:1056–1061. doi: 10.1136/gut.31.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;329:1905–1911. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]
- 9.Diamant NE, Kamm MA, Wald A, Whitehead WE. American Gastroenterological Association medical position statement on anorectal testing techniques. Gastroenterology. 1999;116:732–760. doi: 10.1016/s0016-5085(99)70194-0. [DOI] [PubMed] [Google Scholar]
- 10.Sun WM, Donnelly TC, Read NW. Utility of a combined test of anorectal manometry, electromyography, and sensation in determining the mechanism of ‘idiopathic’ faecal incontinence. Gut. 1992;33:807–813. doi: 10.1136/gut.33.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharucha AE, Fletcher JG, Harper CM, Hough D, Daube JR, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartolo DC, Jarratt JA, Read MG, Donnelly TC, Read NW. The role of partial denervation of the puborectalis in idiopathic faecal incontinence. Br J Surg. 1983;70:664–667. doi: 10.1002/bjs.1800701108. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Fraga X, Azpiroz F, Malagelada JR. Significance of pelvic floor muscles in anal incontinence. Gastroenterology. 2002;123:1441–1450. doi: 10.1053/gast.2002.36586. [DOI] [PubMed] [Google Scholar]
- 14.Bartolo DC, Read NW, Jarratt JA, Read MG, Donnelly TC, Johnson AG. Differences in anal sphincter function and clinical presentation in patients with pelvic floor descent. Gastroenterology. 1983;85:68–75. [PubMed] [Google Scholar]
- 15.Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am J Gastroenterol. 2006;101:1140–1151. doi: 10.1111/j.1572-0241.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 16.Andrews CN, Bharucha AE, Seide B, Zinsmeister AR. Rectal sensorimotor dysfunction in women with fecal incontinence. Am J Physiol Gastrointest Liver Physiol. 2007;292:G282–G289. doi: 10.1152/ajpgi.00176.2006. [DOI] [PubMed] [Google Scholar]
- 17.Parellada CM, Miller AS, Williamson ME, Johnston D. Paradoxical high anal resting pressures in men with idiopathic fecal seepage. Dis Colon Rectum. 1998;41:593–597. doi: 10.1007/BF02235265. [DOI] [PubMed] [Google Scholar]
- 18.Rao SS, Ozturk R, Stessman M. Investigation of the pathophysiology of fecal seepage. Am J Gastroenterol. 2004;99:2204–2209. doi: 10.1111/j.1572-0241.2004.40387.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar JS, Howell S, Talley NJ. Prevalence of faecal incontinence and associated risk factors; an underdiagnosed problem in the Australian community? Med J Aust. 2002;176:54–57. [PubMed] [Google Scholar]
- 20.Bharucha AE, Zinsmeister AR, Locke GR, Seide B, McKeon K, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101:1305–1312. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 21.Bharucha AE, Seide B, Zinsmeister AR, Melton LJ., 3rd Relation of bowel habits to fecal incontinence in women. Am J Gastroenterol. 2008;103:1470–1475. doi: 10.1111/j.1572-0241.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaton KW, Ghosh S, Braddon FE. How bad are the symptoms and bowel dysfunction of patients with the irritable bowel syndrome? A prospective, controlled study with emphasis on stool form. Gut. 1991;32:73–79. doi: 10.1136/gut.32.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood TH, Church JM, Fleshman JW, Kane RL, Mavrantonis C, et al. Patient and surgeon ranking of the severity of symptoms associated with fecal incontinence: the fecal incontinence severity index. Dis Colon Rectum. 1999;42:1525–1532. doi: 10.1007/BF02236199. [DOI] [PubMed] [Google Scholar]
- 24.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 25.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44:77–80. doi: 10.1136/gut.44.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockwood TH. Incontinence severity and QOL scales for fecal incontinence. Gastroenterology. 2004;126:S106–S113. doi: 10.1053/j.gastro.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 27.Andrews CN, Bharucha AE. The etiology, assessment, and treatment of fecal incontinence. Nat Clin Pract Gastroenterol Hepatol. 2005;2:516–525. doi: 10.1038/ncpgasthep0315. [DOI] [PubMed] [Google Scholar]
- 28.Bharucha AE. Anorectal manometry and imaging are necessary in patients with fecal incontinence. Am J Gastroenterol. 2006;101:2679–2681. doi: 10.1111/j.1572-0241.2006.00900_2.x. [DOI] [PubMed] [Google Scholar]
- 29.Rao SS, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha AE, Seide B, Fox JC, Zinsmeister AR. Day-to-day reproducibility of anorectal sensorimotor assessments in healthy subjects. Neurogastroenterol Motil. 2004;16:241–250. doi: 10.1111/j.1365-2982.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- 31.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49:1726–1735. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 32.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 33.Minguez M, Herreros B, Sanchiz V, Hernandez V, Almela P, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Voderholzer WA, Neuhaus DA, Klauser AG, Tzavella K, Muller-Lissner SA, Schindlbeck NE. Paradoxical sphincter contraction is rarely indicative of anismus. Gut. 1997;41:258–262. doi: 10.1136/gut.41.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buser WD, Miner PB., Jr Delayed rectal sensation with fecal incontinence. Successful treatment using anorectal manometry. Gastroenterology. 1986;91:1186–1191. doi: 10.1016/s0016-5085(86)80015-4. [DOI] [PubMed] [Google Scholar]
- 36.Wald A, Tunuguntla AK. Anorectal sensorimotor dysfunction in fecal incontinence and diabetes mellitus. Modification with biofeedback therapy. N Engl J Med. 1984;310:1282–1287. doi: 10.1056/NEJM198405173102003. [DOI] [PubMed] [Google Scholar]
- 37.Chiarioni G, Bassotti G, Stanganini S, Vantini I, Whitehead WE, Stegagnini S. Sensory retraining is key to biofeedback therapy for formed stool fecal incontinence. Am J Gastroenterol. 2002;97:109–117. doi: 10.1111/j.1572-0241.2002.05429.x. [DOI] [PubMed] [Google Scholar]
- 38.Stoker J, Halligan S, Bartram CI. Pelvic floor imaging. Radiology. 2001;218:621–641. doi: 10.1148/radiology.218.3.r01mr26621. [DOI] [PubMed] [Google Scholar]
- 39.Shorvon PJ, McHugh S, Diamant NE, Somers S, Stevenson GW. Defecography in normal volunteers: results and implications. Gut. 1989;30:1737–1749. doi: 10.1136/gut.30.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agachan F, Pfeifer J, Wexner SD. Defecography and proctography. Results of 744 patients. Dis Colon Rectum. 1996;39:899–905. doi: 10.1007/BF02053989. [DOI] [PubMed] [Google Scholar]
- 41.Fletcher JG, Busse RF, Riederer SJ, Hough D, Gluecker T, et al. Magnetic resonance imaging of anatomic and dynamic defects of the pelvic floor in defecatory disorders. Am J Gastroenterol. 2003;98:399–411. doi: 10.1111/j.1572-0241.2003.07235.x. [DOI] [PubMed] [Google Scholar]
- 42.Malouf AJ, Halligan S, Williams AB, Bartram CI, Dhillon S, Kamm MA. Prospective assessment of interobserver agreement for endoanal MRI in fecal incontinence. Abdom Imaging. 2001;26:76–78. doi: 10.1007/s002610000100. [DOI] [PubMed] [Google Scholar]
- 43.Briel JW, Stoker J, Rociu E, Lameris JS, Hop WC, Schouten WR. External anal sphincter atrophy on endoanal magnetic resonance imaging adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327. doi: 10.1046/j.1365-2168.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 44.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130:1510–1518. doi: 10.1053/j.gastro.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 45.Rao SS. A balancing view: fecal incontinence: test or treat empirically—which strategy is best? Am J Gastroenterol. 2006;101:2683–2684. doi: 10.1111/j.1572-0241.2006.00900_3.x. [DOI] [PubMed] [Google Scholar]
- 46.Wald A. Anorectal manometry and imaging are not necessary in patients with fecal incontinence. Am J Gastroenterol. 2006;101:2681–2683. doi: 10.1111/j.1572-0241.2006.00900_2.x. [DOI] [PubMed] [Google Scholar]
- 47.Liberman H, Faria J, Ternent CA, Blatchford GJ, Christensen MA, Thorson AG. A prospective evaluation of the value of anorectal physiology in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1567–1574. doi: 10.1007/BF02234373. [DOI] [PubMed] [Google Scholar]
- 48.Skoog SM, Bharucha AE. Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol. 2004;99:2046–2050. doi: 10.1111/j.1572-0241.2004.40266.x. [DOI] [PubMed] [Google Scholar]
- 49.Read M, Read NW, Barber DC, Duthie HL. Effects of loperamide on anal sphincter function in patients complaining of chronic diarrhea with fecal incontinence and urgency. Dig Dis Sci. 1982;27:807–814. doi: 10.1007/BF01391374. [DOI] [PubMed] [Google Scholar]
- 50.Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-over study comparing loperamide, codeine and diphenoxylate in the treatment of chronic diarrhea. Gastroenterology. 1980;79:1272–1275. [PubMed] [Google Scholar]
- 51.Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676–1681. doi: 10.1007/BF02236848. [DOI] [PubMed] [Google Scholar]
- 52.Sciarretta G, Furno A, Mazzoni M, Malaguti P. Post-cholecystectomy diarrhea: evidence of bile acid malabsorption assessed by SeHCAT test. Am J Gastroenterol. 1992;87:1852–1854. [PubMed] [Google Scholar]
- 53.Locke GR, 3rd, Pemberton JH, Phillips SF. American Gastroenterological Association medical position statement: guidelines on constipation. Gastroenterology. 2000;119:1761–1766. doi: 10.1053/gast.2000.20390. [DOI] [PubMed] [Google Scholar]
- 54.Carapeti EA, Kamm MA, Phillips RK. Randomized controlled trial of topical phenylephrine in the treatment of faecal incontinence. Br J Surg. 2000;87:38–42. doi: 10.1046/j.1365-2168.2000.01306.x. [DOI] [PubMed] [Google Scholar]
- 55.Norton C, Kamm MA. Anal sphincter biofeedback and pelvic floor exercises for faecal incontinence in adults--a systematic review. Aliment Pharmacol Ther. 2001;15:1147–1154. doi: 10.1046/j.1365-2036.2001.01039.x. [DOI] [PubMed] [Google Scholar]
- 56.Norton C, Chelvanayagam S, Wilson-Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329. doi: 10.1016/j.gastro.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 57.Heymen S, Scarlett Y, Jones K, Ringel Y, Drossman D, Whitehead W. AGA Institute abstracts: randomized controlled trial shows biofeedback to be superior to alternative treatments for patients with fecal incontinence. Gastroenterology. 2007;132:A–83. [Google Scholar]
- 58.Cheung O, Wald A. Review article: the management of pelvic floor disorders. Aliment Pharmacol Ther. 2004;19:481–495. doi: 10.1111/j.1365-2036.2004.01886.x. [DOI] [PubMed] [Google Scholar]
- 59.Brown SR, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database Syst Rev. 2007;(2):CD001757. doi: 10.1002/14651858.CD001757.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Mundy L, Merlin TL, Maddern GJ, Hiller JE. Systematic review of safety and effectiveness of an artificial bowel sphincter for faecal incontinence. Br J Surg. 2004;91:665–672. doi: 10.1002/bjs.4587. [DOI] [PubMed] [Google Scholar]
- 61.Shirran E, Brazzelli M. Absorbent products for the containment of urinary and/or faecal incontinence in adults. Cochrane Database Syst Rev. 2000;(2):CD001406. doi: 10.1002/14651858.CD001406. [DOI] [PubMed] [Google Scholar]
- 62.Deutekom M, Dobben A. Plugs for containing faecal incontinence. Cochrane Database Syst Rev. 2005;(3):CD005086. doi: 10.1002/14651858.CD005086.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Christiansen J, Roed-Petersen K. Clinical assessment of the anal continence plug. Dis Colon Rectum. 1993;36:740–742. doi: 10.1007/BF02048363. [DOI] [PubMed] [Google Scholar]
- 64.Jarrett ME, Mowatt G, Glazener CM, Fraser C, Nicholls RJ, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569. doi: 10.1002/bjs.4796. [DOI] [PubMed] [Google Scholar]
- 65.Vaizey CJ, Kamm MA, Turner IC, Nicholls RJ, Woloszko J. Effects of short term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut. 1999;44:407–412. doi: 10.1136/gut.44.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shafik A. Perianal injection of autologous fat for treatment of sphincteric incontinence. Dis Colon Rectum. 1995;38:583–587. doi: 10.1007/BF02054115. [DOI] [PubMed] [Google Scholar]
- 67.Kumar D, Benson MJ, Bland JE. Glutaraldehyde cross-linked collagen in the treatment of faecal incontinence. Br J Surg. 1998;85:978–979. doi: 10.1046/j.1365-2168.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- 68.Feretis C, Benakis P, Dailianas A, Dimopoulos C, Mavrantonis C, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609. doi: 10.1007/BF02234379. [DOI] [PubMed] [Google Scholar]
- 69.Davis K, Kumar D, Poloniecki J. Preliminary evaluation of an injectable anal sphincter bulking agent (Durasphere) in the management of faecal incontinence. Aliment Pharmacol Ther. 2003;18:237–243. doi: 10.1046/j.1365-2036.2003.01668.x. [DOI] [PubMed] [Google Scholar]
- 70.Altomare DF, La Torre F, Rinaldi M, Binda GA, Pescatori M. Carbon-coated microbeads anal injection in outpatient treatment of minor fecal incontinence. Dis Colon Rectum. 2008;51:432–435. doi: 10.1007/s10350-007-9170-7. [DOI] [PubMed] [Google Scholar]
- 71.Tjandra JJ, Lim JF, Hiscock R, Rajendra P. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum. 2004;47:2138–2146. doi: 10.1007/s10350-004-0760-3. [DOI] [PubMed] [Google Scholar]