Abstract
Reproductive experience (i.e. pregnancy and lactation) alters a number of physiological and behavioral endpoints, many of which are related to reproductive function and are regulated by oestrogen. For example, reproductive experience significantly attenuates the oestradiol-induced prolactin surge on the afternoon of proestrous and circulating oestradiol levels are reduced at this time. While parity-related effects on oestrogen receptor alpha (ERα) have been observed within the anterior pituitary, there are currently no data regarding possible parity-induced alterations in ERα in the brain. Thus, the purpose of the current study was to examine the effect of parity on the expression of ERα in reproductively relevant brain regions. Moreover, because previous findings have demonstrated that the long-term effects of reproductive experience are often oestrous cycle dependent, ERα was examined at two stages of the oestrous cycle (diestrous and proestrous). Finally, as the expression of ERα is significantly influenced by age, both young and middle-aged females were included in the present study. ERα status was determined using immunohistochemistry in select brain regions involved in the regulation of reproductive behavior in age-matched, cycling primiparous (one pregnancy and lactation) and nulliparous females as well as in age-matched, non-cycling (persistent estrus) 12 month-old primiparous and nulliparous females. Significant shifts in ERα cell numbers were observed in the medial preoptic area and medial amygdala as a consequence of reproductive experience in an oestrous-dependent manner. These findings indicate that significant changes in ERα activity occur in the brain as a function of reproductive experience.
Keywords: Parity, Proestrous, Medial Amygdala, Striatum, Immunohistochemistry
Introduction
Reproductive experience (pregnancy and lactation) results in significant changes in neuroendocrine function which can persist long after the female has weaned her young. In the female rat, these changes include reduced prolactin secretion and altered sensitivity to both dopamine antagonists and estrogen [1-3]. In addition, significant parity-induced changes in behavior have also been reported. For example, reproductively experienced females exhibit improved spatial memory, decreased stress responsiveness and reduced anxiety-like behavior [4-8]. Thus, reproductive experience induces both physiological and behavioral adaptations which continue even after the cessation of active maternal behavior.
The neural changes underlying the long-term effects of parity have yet to be determined. Interestingly, many of the processes influenced by reproductive experience are modulated by the activation of the estrogen receptor. For example, alterations in cognition and anxiety-like behavior have been documented following reproductive experience [4, 6, 9] and these changes are similar to the effects of increased estrogenic activity [10, 11]. Parity also leads to decreased circulating prolactin, an effect observed during periods of increased prolactin secretion [1, 2, 12]. Prolactin secretion is modulated by ERα activation within the anterior pituitary[13, 14] and arcuate nucleus (ARC) [15]. Thus, alterations in the number and/or function of ERα may underlie the observed effects of reproductive experience on both physiological and behavioral parameters.We have previously reported significant parity-related alterations on ERα mRNA within the anterior pituitary[1]. The objective of the present study was to determine whether parity might also alter the number of cells containing ERα in discrete brain regions. As previous findings suggest that the effects of reproductive experience are often oestrous cycle dependent, ERα expression was determined in both diestrous and proestrous females. Finally, as declining levels of estrogen associated with normal female aging can significantly alter ERα expression [16, 17], a second experiment was conducted to determine whether reproductive experience differentially effects age-dependent alterations in ERα expression.
The number of ERα-containing cells was determined using immunohistochemistry. In the first experiment, brain regions which both express high levels of ERα [18] and are known to regulate reproductive[19, 20] and parental behaviors were examined [21-23], including the medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST), ventromedial hypothalamus (VMH), arcuate nucleus (ARC), medial amygdala (MeA) and cortical amygdala (CoA). In middle-aged females, one additional brain region, the dorsal striatum (dSTR) was also included based on the preliminary observation of a significant number of ERα expressing cells in the dSTR in 12 month-old females, an occurrence not evident in young females. Overall, the data indicate that reproductive experience alters ERα expression in brain-region and age-dependent manners.
Methods
Animals
Thirty-six young, 175−200 g adult female Sprague-Dawley rats were obtained from Charles River Laboratories (Kingston, NY or CRL/Canada) and housed in our animal facility. Animals were maintained in temperature- (21−25°C) and light- (14:10 light-dark cycle) controlled rooms, and food and water were available ad libitum throughout the study. The animals used in these experiments were maintained in accordance with the National Reseach Council (NRC) Guide for the Care and Use of Laboratory Animals (© 1996, National Academy of Science). The research protocol was approved by Tufts University School of Veterinary Medicine Institutional Animals Care and Use Committee.
Procedures
Experiment 1
One week after arriving in our animal colony, one set of the females was mated with males from our colony. On postpartum day 1 (PPD1), litters from these mothers were culled to 5 males and 5 females. All litters were weaned on PPD21. Beginning 2 weeks after weaning, primiparous (n = 11) and age-matched, nulliparous females (n = 12) were monitored daily for oestrous cyclicity via vaginal lavage. Only females exhibiting at least 2 normal 4 day oestrous cycles were used. On either diestrous (1000 h) or proestrous (1600 h) females were overdosed with an intraperitoneal injection of ketamine/xylazine and perfused with 0.1M phosphate-buffered saline, pH 7.40 (PBS) for 3 min (∼30mls/min) followed by ice-cold 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB) for 3 min. Following perfusion, brains were extracted and placed into the PFA fixative. Brains were kept in fixative for 2−4 days at 4°C, and then placed in 30% sucrose in PBS (4°C) until immersed. Whole brains were placed in a cryoprotectant solution (30% ethylene glycol and 25% glycerol in 0.1M PB) and stored at −20°C. Brains were removed from the cryoprotectant and immersed 5 times for 15 min each in 0.1M PBS and then sectioned using a Vibratome (1000Plus Sectioning System; The Vibratome Company, St. Louis, MO). Forty μm sections were collected into a bath of ice-cold PBS from the decussation of the anterior commissure through the hypothalamus. All free-floating sections were placed in cryoprotectant and stored at −20°C until ERα immunohistochemistry was performed.
Experiment 2
Twelve to thirteen month old middle-aged primiparous (n=6) rats that were initially bred upon their arrival in our animal facility and raised one litter to weaning (see Experiemnt 1) and age-matched, nulliparous (n=6) females were checked daily by vaginal lavage for oestrous cyclicity. Only females exhibiting 7 days of constant oestrus were used in this experiment. Females were overdosed with ketamine/xylazine between 1400−1700 h and perfused as previously described. Following perfusion, brains were extracted and placed into fixative. Brains were kept in fixative for 2−4 days at 4°C, and then placed in 50mls of 30% sucrose in PBS (4°C) until immersed. Brains were then rapidly frozen in powdered dry ice and stored at −80°C. Forty μm sections of frozen brain tissues were collected using a cryostat. Sections were taken from the decussation of the anterior commissure through the hypothalamus and placed into a bath of ice-cold PBS. All free-floating sections were subsequently placed in cryoprotectant and stored at −20°C until ERα immunohistochemistry was performed.
ERα immunohistochemistry
Free-floating sections were removed from cryoprotectant and rinsed with PBS (3 × 5 min). To block endogenous peroxidase, sections were incubated for 15 min in 0.3% H2O2 at room temperature and then rinsed in PBS. To prevent non-specific binding, sections were placed into a blocking solution of 10% normal goat serum (NGS) in PBS with 0.2% Triton X-100 (TX) and then incubated for approximately 48 hours at 4°C in the primary antibody solution (anti-ERα 1:10,000 in PBS with TX and 2% NGS;). This ERα antiserum (cat.# 06−935, Chemicon, Temecula, CA) is a polyclonal antibody (C1355) raised in rabbit against the peptide representing the last 15 amino acids of rat ERα and does not cross-react with ERβ [24, 25]. Following incubation in the primary antibody, sections were rinsed with PBS and processed with an ABC Elite Kit (cat.# PK-6101; Vector Laboratories, Burlingame, CA) containing biotinylated goat antirabbit IgG according to the kit instructions. Sections were incubated with the secondary antibody for 90 min at room temperature. Following another rinse in PBS, sections were incubated for 2 hrs in the ABC solution. ERα protein was visualized using DAB intensified with nickel chloride (approx. 2−3 min) as the chromagen (DAB Substrate Kit, cat. # SK-4100; Vector Laboratories). Sections were mounted onto slides, dehydrated and coverslipped for subsequent analysis.
Data Analysis
Quantification of the number of ERα-immunoreactive (ERα-IR) cells was performed using light microscopy by an experimenter blind to the treatment group of each animal. ERα positive cells were defined as cells with intense black/purple staining confined to the nucleus. Two unilateral sections per animal were quantified in each anatomically defined region (see below). The limits of the region of analysis were defined by a 10×10 grid at 20X magnification.
Figure 1 shows a schematic of the areas analyzed. Based on the size of the region to be examined, some of the brain regions were divided into either anterior-posterior or medial-lateral segments as described below. In the MPOA, ERα-IR was quantified at −0.36 mm and −0.48 mm (all coordinates based on bregma; Paxinos and Watson, 2005). In the ARC, VMH, and MeA, ERα-IR was quantified at −2.52 mm and −3.00 mm. Both the MPOA and MeA were further subdivided into dorsal and ventral regions. In the VMH, ERα-IR was confined specifically to the ventrolateral portion of the nucleus, thus quantification was centered on this area of the nucleus. The MeA was too large to be quantified with one 10×10 grid of analysis, therefore two grid areas were quantified for the anterior MeA section, and 3 grids were used in the quantification of the posterior MeA (Fig. 1, bottom panels).
Figure 1.
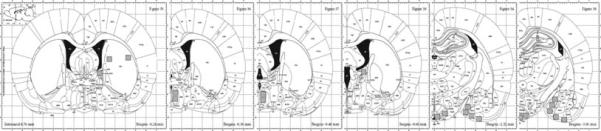
Schematic of brain regions included in the analyses.
Statistical Analysis
For experiment 1, each brain region was analyzed separately using a two-way ANOVA with reproductive experience and oestrous cycle stage as the factors. Post-hoc effects were determined using the Tukey's test. For experiment 2, t-test's were conducted comparing primiparous and age-matched nulliparous females. All differences were considered significant if p<0.05.
Results
Experiment 1
The findings from Experiment 1 are summarized in Table 1. Changes in ERα positive cell numbers were detected in select brain regions as a function of reproductive experience or oestrous cycle stage. First, a significant main effect of oestrous cycle stage was observed in the anterior-dorsal MPOA (F[1,22] = 4.92, p = 0.04). As shown in figure 2, this effect was due primarily to a significant increase in ERα positive cells on the afternoon of proestrous. Analysis of data from the anterior MeA revealed a significant interaction between cycle and reproductive experience (F[1,22] = 11.1, p < 0.01). As shown in figure 3, this effect was due to a significant increase in the number ERα positive cells in nulliparous females on the afternoon of proestrous when compared both to nulliparous female in diestrus as well as primiparous females in proestrous (both p's < 0.025). A photomicrograph demonstrating the differences in ERα staining in the MeA between a nulliparous and primiparous subject on the afternoon of proestrous are shown in figure 4. Finally, analysis of data from the CoA revealed a trend toward decreasing numbers of ERα positive cells in proestrous females (p<0.07). As illustrated in figure 5, this trend was due to a reduction in the number of ERα positive cells in proestrous, primiparous females when compared to diestrous, primiparous females. Differences in the VMH, BNST and ARC were not observed as a function of reproductive experience or stage of the oestrous cycle.
Table 1.
Mean (±SEM) number of ERα positive cells in young, cycling primiparous and age-matched, nulliparous females.
| Nulliparous | Primiparous | ||||
|---|---|---|---|---|---|
| DI | PRO | DI | PRO | ||
| Mean±SEM | Mean±SEM | Mean±SEM | Mean±SEM | ||
| aMPOA | Dorsal | 125.4 ± 11.1 | 147.3 ± 14.1 | 140.5 ± 20.4 | 185.6 ± 16.2 |
| Ventral | 215.0 ± 15.9 | 209.5 ± 16.5 | 232.4 ± 21.3 | 234.8 ± 22.3 | |
| pMPOA | Dorsal | 199.5 ± 34.6 | 227.6 ± 28.2 | 180.6 ± 22.1 | 211.7 ± 26.3 |
| Ventral | 226.2 ± 29.3 | 260.7 ± 26.2 | 249.4 ± 34.0 | 278.3 ± 31.2 | |
| aBNST | 27.5 ± 15.5 | 22.2 ± 13.5 | 91.7 ± 66.5 | 50.6 ± 23.3 | |
| pBNST | 42.4 ± 27.2 | 47.2 ± 12.8 | 249.4 ± 34.0 | 278.3 ± 31.2 | |
| aARC | 295.5 ± 32.3 | 327.5 ± 21.3 | 320.8 ± 23.6 | 354.0 ± 20.2 | |
| pARC | 392.0 ± 32.6 | 417.0 ± 30.9 | 405.6 ± 31.8 | 413.0 ± 22.1 | |
| aVMH | 297.6 ± 50.8 | 337.7 ± 40.6 | 349.6 ± 27.3 | 336.2 ± 36.9 | |
| pVMH | 404.5 ± 32.2 | 447.5 ± 71.0 | 218.8 ± 35.3 | 147.5 ± 17.8 | |
| aMeA | 243.3 ± 35.5 | 422.7 ± 53.9 | 352.4 ± 64.3 | 253.2 ± 34.6 | |
| pMeA | 821.5 ± 84.8 | 796.3 ± 136 | 874.4 ± 97.6 | 681.6 ± 87.2 | |
| CoA | 122.4 ± 29.9 | 113.8 ± 25.0 | 155.0 ± 5.1 | 76.0 ± 15.9 | |
Figure 2.
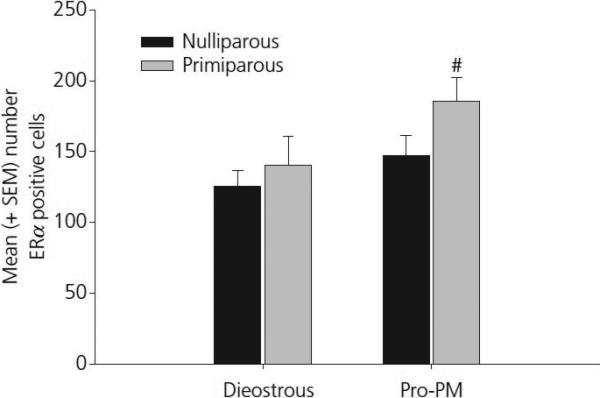
Mean (±SEM) ERα positive cells in the anterior-dorsal MPOA in young, cycling primiparous and age-matched, nulliparous females. # p< 0.05 as compared to primiparous, diestrous females.
Figure 3.
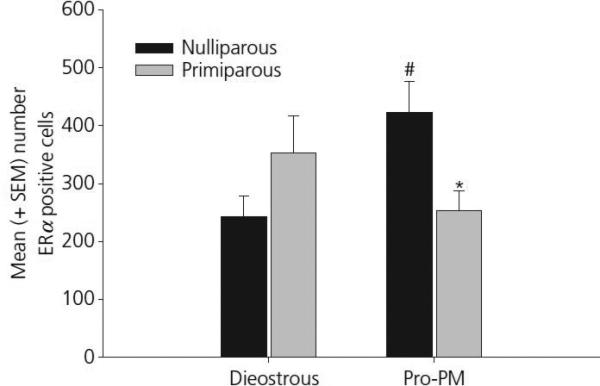
Mean (±SEM) ERα positive cells in the anterior MeA in young, cycling primiparous and age-matched, nulliparous females. # p< 0.05 as compared to primiparous, diestrous females. * p <0.05 as compared to nulliparous, Pro-PM females.
Figure 4.
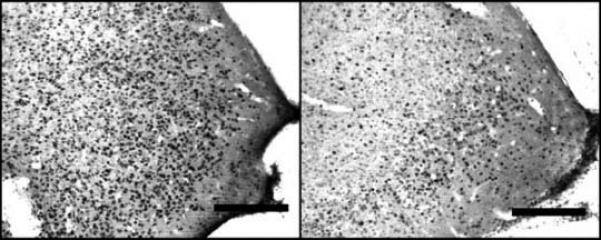
Photomicrograph of two representative samples of ERα positive cells in the anterior MeA of a young, cycling nulliparous female (panel A) and an age-matched, primiparous female (Panel B). Opt - Optic tract.
Figure 5.
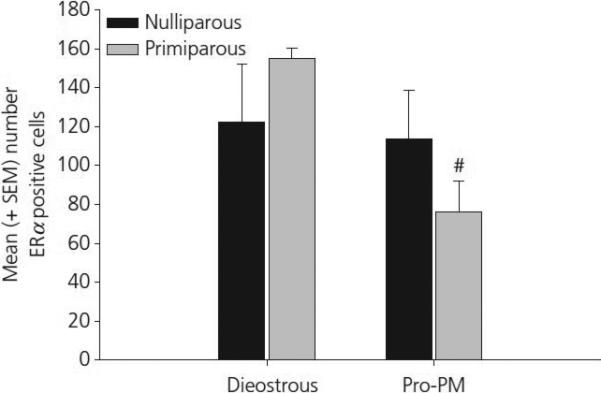
Mean (±SEM) ERα positive cells in the CoA in young, cycling primiparous and nulliparous females. # p< 0.05 as compared to primiparous, diestrous females.
Experiment 2
The findings from Experiment 2 are summarized in Table 2. No significant effects of reproductive experience were observed in any of the neural regions examined in Experiment 1. There was, however, a significant effect of reproductive experience on the number of ERα positive cells in the dSTR of 12−13 month old females. As shown in figure 6, middle-aged, primiparous females demonstrated increased numbers of ERα positive cells in the dSTR when compared with those present in age-matched nulliparous females (p < 0.05).
Table 2.
Mean (±SEM) number of ERα positive cells in middle-aged, constant estrous primiparous and age-matched, nulliparous females.
| Nulliparous | Primiparous | ||
|---|---|---|---|
| Mean±SEM | Mean±SEM | ||
| aMPOA | Dorsal | 120.5 ± 25.5 | 138.2 ± 24.7 |
| Ventral | 202.5 ± 38.3 | 251.7 ± 31.8 | |
| pMPOA | Dorsal | 273.0 ± 37.2 | 288.5 ± 12.2 |
| Ventral | 425.8 ± 47.6 | 455.8 ± 38.4 | |
| aBNST | 360.2 ± 36.9 | 485.3 ± 67.5 | |
| pBNST | 208.6 ± 39.8 | 192.6 ± 18.5 | |
| aARC | 218.0 ± 34.3 | 258.3 ± 37.4 | |
| pARC | 290.4 ± 50.3 | 323.4 ± 71.1 | |
| aVMH | 255.0 ± 47.4 | 257.5 ± 39.7 | |
| aMeA | 314.5 ± 34.5 | 349.5 ± 66.7 | |
| pMeA | 342.2 ± 64.4 | 430.3 ± 91.3 | |
| CoA | 180.0 ± 25.4 | 174.2 ± 24.6 | |
Figure 6.
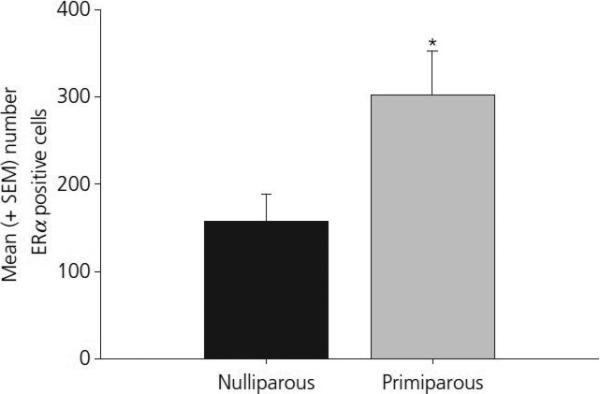
Mean (±SEM) ERα positive cells in the dSTR in middle-aged, constant oestrous primiparous and age-matched, nulliparous females. * p < 0.05 as compared to nulliparous females.
Discussion
Reproductive experience can exert significant long-term effects on the female neuroendocrine system. Suppression of prolactin secretion, which has been observed in both rodents and women, can last for months or years, respectively [1, 26]. In addition, reproductive experience can alter circulating estrogen levels [1, 27]. With regard to changes in neural function, reproductive experience alters synaptic morphology in both the hippocampus [28] and amygdala [29], shifts dopamine receptor sensitivity in the striatum [30-32] and pituitary [3], and alters μ-opioid receptor expression and function [33, 34]. Behaviorally, reproductive experience enhances learning and memory [6, 7, 9, 35, 36] and reduces stress responsiveness [8] and [4]. Thus, the effects of reproductive experience are widespread and include alterations in neural and endocrine systems that mediate a broad spectrum of physiological and behavioral processes that appear adaptive for the mother and offspring. Interestingly, all of these same processes can be modulated by ERα activation. For that reason, we examined potential alterations in ERα expression following reproductive experience. Overall, significant alterations in ERα expression as a function of reproductive experience were brain region specific as well as oestrous cycle and age-dependent.
In the present study alterations in the number of ERα positive cells as a function of reproductive experience were observed in select neural regions. In the anterior-dorsal MPOA increased numbers of ERα positive cells were observed on the afternoon of proestrous in females with reproductive experience. Previous studies examining the effects of oestrous cycle on ER mRNA and protein expression in the MPOA detected the highest levels of expression during metestrus with attenuated expression during diestrus and the lowest levels of expression during proestrus [37, 38]. These earlier studies, however, were conducted prior to the discovery of the ERα and ERβ subtypes, and, therefore, they in effect examined alterations in both subtypes rather than selective changes in ERα. In addition, prior studies did not examine oestrous cycle induced changes in the different regions of the preoptic area, but rather presented the MPOA as a unified brain region. In the current study, increased ERα expression in primiparous females on the afternoon of proestrus was observed in the anterior-dorsal portion of the MPOA, but not in more ventral regions. Interestingly, the dorsal MPOA is a brain region with particular relevance to maternal behavior [21, 39-41] and studies have demonstrated a critical role for ERα in the regulation of maternal care [42]. Thus, it is possible that the experience of mothering alters the regulation of ERα within this brain region which may impact her expression of maternal care. Such changes, however, may only be observed under particular endocrine conditions, for example, when estradiol, progesterone and/or prolactin are elevated, as occurs on the afternoon of proestrous.
Differential regulation of ERα as a function of parity was also observed in the MeA. Primiparous females had fewer ERα positive cells in the MeA on the afternoon of proestrous when compared to nulliparous females, while no significant differences were observed on diestrous. ERα expression was higher in nulliparous females in proestrous when compared to diestrous, with no significant differences in expression as a function of oestrous cycle observed in primiparous females. There was, however, a tendency toward decreased expression on proestrous as compared to diestrous in primiparous females. Thus, there was an oppositional effect on ERα expression in nullliparous and primiparous females on the afternoon of proestrous. In addition, primiparous females had reduced numbers of ERα positive cells on proestrous in the CoA when compared to diestrous, primiparous females, while no such effect was observed in nulliparous females. Thus, reproductive experience alters the manner in which the oestrous cycle modulates ERα in the MeA and CoA, with reduced expression observed on the afternoon of proestrous.
The MeA plays a broad role in the regulation of reproductive behaviors, including the modulation of both sexual and maternal behavior. A large body of research suggests that the anterior MeA is critical for categorizing odors based on social relevance. For example, lesions of the anterior MeA increase exploration of odors that are both socially relevant (conspecifics) and socially irrelevant (heterospecifics)[43]. In addition, males undergoing such lesions will spend equal amounts of time with oestrous and anestrous females[44]. They are not less attracted to oestrous females but rather appear equally interested in anestrous females. These findings, as well as many others, suggest that the role of the MeA is to determine the relative importance of social cues given the current conditions. In contrast, lesions of the posterior MeA decrease sexual attraction and/or motivation[44]. Similar effects are observed in rats, hamsters and mice. Interestingly, recent findings indicate a specific role for ERα within the MeA in male prosocial behavior. In the male prairie vole, a species that is highly social and has reduced expression of ERα in the MeA, enhancing ERα expression in the MeA via infusion of a viral vector results in reduced and/or altered prosocial behaviors [45]. Specifically, males with higher ERα expression display fewer alloparental behaviors. In addition, these males demonstrate increased preference for a novel female, suggesting a role for the MeA and in particular ERα expression in the MeA, in the balance between parental and sexual behavior.
Significant alterations in the MeA and MPOA as a function of sexual experience have been documented in both males [46] and females [47]. In males, this sexual experience effect is mediated in part by nitric oxide induced changes in the MPOA[48]. In females stimulation of the MPOA inhibits sex behavior[49]. Electrophysiology studies have shown that estrogen can modulate the firing dynamics of projections from the MPOA to the MeA [50]. Of the two ER subtypes, knock-out studies indicate that the ERα subtype is critical for sex behavior [51]. It is possible that sexual experience may alter the manner in which estrogen modulates projections from the MPOA to the MeA.
In females, the MeA is also involved in the regulation of maternal behavior. Excitotoxic lesions of the MeA lead to the rapid onset of maternal behavior in non-maternal, virgin female rats[52], indicating that this region normally inhibits this behavior. Similar to sex behavior, maternal behavior is differentially regulated by the MeA and MPOA. The specific roles of these brain regions in maternal behavior, however, are opposite that observed in sex behavior. That is, MPOA activity stimulates, while MeA activity inhibits maternal behavior. Once again, as is the case for feminine sexual behavior, of the two ER subtypes, ERα is apparently more critical for the display of maternal behavior [53]. Thus, the current finding that reproductive experience alters the expression of ERα specifically in these two brain regions may be telling. The fact that parity-induced alterations were observed on the afternoon of proestrous, but not during diestrous, suggests that prior experience interacts with current endocrine status to alter ERα-mediated activity. That these changes occur specifically in two brain regions involved in the critical aspects of reproduction, i.e. sex and maternal behavior, suggests a potential mechanism by which prior reproductive experience may influence future behavior. It is unclear, however, whether the effects of reproductive experience are due to the female's prior sexual experience or if pregnancy and/or maternal care are critical for the observed changes in ERα in the MPOA and MeA.
In reproductively senescent females (i.e. middle-aged, constant oestrous) no significant differences in the number of ERα postitive cells were observed in any of the brain regions examined in young, cycling females. There was, however, a significant effect of parity on the number of ERα positive cells in the striatum of middle-aged females. Reproductively experienced females had a significantly higher number of ERα positive cells in the striatum when compared to age-matched, nulliparous controls. No comparisons between young and middle-aged females were conducted as ERα expression was determined in two separate studies using slightly different methodologies. However, few very cells were labeled in the striatum of young, cycling females.
In regards to the effects of reproductive experience as a function of aging, a number of studies have demonstrated a neuroprotective effect of ERα in the striatum [54]. These protective effects have been observed in both stroke models [55] and in MPTP models of Parkinson's disease[56]. In addition, other research suggests that estrogen replacement therapy may ameliorate some of the neural dysfunctions caused by alzheimer's disease [57, 58]. The current findings raise the intriguing possibility that ERα-mediated neuroprotection may be modulated by prior reproductive experience. Few studies in aged women include parity as a factor. This may be due to the low number of nulliparous females in the general population. However, as greater numbers of women chose to forego motherhood, understanding the role of prior reproductive experience (or the lack thereof) may provide valuable information as to those life events that alter the aging female brain.
It is also important to evaluate the effects of reproductive experience in the context of the endpoints measured. Whereas ERα positive cell number provides one perspective of the status of the ERα system, it is also important to integrate factors such as receptor occupancy, sensitivity and ligand availability when developing a picture of the biological activity of this system. Additional studies are warranted to provide such perspectives. However, that ERα cell numbers are altered as a function of reproductive experience and aging indicate that meaningful biological changes occur in the female brain as a function of their reproductive and life histories.
In summary, reproductive experience results in alterations in ERα protein expression. In young, cycling females, these effects are observed in brain regions critical for reproductive behaviors, including both sex and maternal behavior. Moreover, ERα varies as a function of the oestrous cycle. In reproductively senescent females, significant parity-induced alterations in the number of ERα positive cells were observed in the striatum. Overall, these findings indicate that reproductive experience induces long-term changes in the regulation of neural ERα. Future studies are needed to determine the extent that these changes in ERα alter neural responsiveness and/or behavior in reproductively-experienced females.
Footnotes
Supported by PHS grant R01HD39895 from the NIH awarded to RSB
References
- 1.Bridges RS, Byrnes EM. Reproductive experience reduces circulating 17beta-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology. 2006;147:2575–2582. doi: 10.1210/en.2005-0917. [DOI] [PubMed] [Google Scholar]
- 2.Byrnes EM, Bridges RS. Lactation reduces prolactin levels in reproductively experienced female rats. Horm Behav. 2005;48:278–282. doi: 10.1016/j.yhbeh.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Byrnes EM, Bridges RS. Reproductive experience and expression of dopamine D(2) receptor mRNA: a possible mechanism for reduced prolactin secretion in primiparous rats. J Neuroendocrinol. 2007;19:773–778. doi: 10.1111/j.1365-2826.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 4.Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav. 2006;50:70–76. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- 7.Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 9.Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav. 2008;37:43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- 10.Walf AA, Frye CA. Conjugated equine estrogen enhances rats’ cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–792. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–260. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- 12.Bridges RS, Felicio LF, Pellerin LJ, Stuer AM, Mann PE. Prior parity reduces post-coital diurnal and nocturnal prolactin surges in rats. Life Sci. 1993;53:439–445. doi: 10.1016/0024-3205(93)90648-m. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Criado JE, Martin De Las Mulas J, Bellido C, Tena-Sempere M, Aguilar R, Blanco A. Biological role of pituitary estrogen receptors ERalpha and ERbeta on progesterone receptor expression and action and on gonadotropin and prolactin secretion in the rat. Neuroendocrinology. 2004;79:247–258. doi: 10.1159/000079100. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier G, Li S, Phaneuf D, Martel C, Labrie F. Morphological studies of prolactin-secreting cells in estrogen receptor alpha and estrogen receptor beta knockout mice. Neuroendocrinology. 2003;77:324–333. doi: 10.1159/000070898. [DOI] [PubMed] [Google Scholar]
- 15.Hou Y, Yang SP, Voogt JL. Changes in estrogen receptor-alpha expression in hypothalamic dopaminergic neurons during proestrous prolactin surge. Endocrine. 2003;20:131–138. doi: 10.1385/ENDO:20:1-2:131. [DOI] [PubMed] [Google Scholar]
- 16.Mehra RD, Sharma K, Nyakas C, Vij U. Estrogen receptor alpha and beta immunoreactive neurons in normal adult and aged female rat hippocampus: a qualitative and quantitative study. Brain Res. 2005;1056:22–35. doi: 10.1016/j.brainres.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- 18.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 19.Veening JG, Coolen LM. Neural activation following sexual behavior in the male and female rat brain. Behav Brain Res. 1998;92:181–193. doi: 10.1016/s0166-4328(97)00190-3. [DOI] [PubMed] [Google Scholar]
- 20.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 21.Numan M. Neural basis of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:47–62. doi: 10.1016/0306-4530(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 22.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 23.Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- 24.Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of Progestin Receptors by Estradiol in the Forebrain of Estrogen Receptor-alpha Gene-Disrupted Mice. The Journal of Neuroscience. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friend KE, Resnick EM, Ang LW, Shupnick MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steriod hormones. Molecular Cell Endocrinology. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 26.Musey VC, Collins DC, Musey PI, Martino-Saltzman D, Preedy JR. Long-term effect of a first pregnancy on the secretion of prolactin. N Engl J Med. 1987;316:229–234. doi: 10.1056/NEJM198701293160501. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein L, Pike MC, Ross RK, Judd HL, Brown JB, Henderson BE. Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst. 1985;74:741–745. [PubMed] [Google Scholar]
- 28.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 29.Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Byrnes EM, Byrnes JJ, Bridges RS. Increased sensitivity of dopamine systems following reproductive experience in rats. Pharmacol Biochem Behav. 2001;68:481–489. doi: 10.1016/s0091-3057(01)00449-x. [DOI] [PubMed] [Google Scholar]
- 31.Hucke EE, Cruz-Casallas PE, Sider LH, Felicio LF. Reproductive experience modulates dopamine-related behavioral responses. Pharmacol Biochem Behav. 2001;68:575–582. doi: 10.1016/s0091-3057(01)00458-0. [DOI] [PubMed] [Google Scholar]
- 32.Felicio LF, Florio JC, Sider LH, Cruz-Casallas PE, Bridges RS. Reproductive experience increases striatal and hypothalamic dopamine levels in pregnant rats. Brain Res Bull. 1996;40:253–256. doi: 10.1016/0361-9230(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 33.Mann PE, Bridges RS. Neural and endocrine sensitivities to opioids decline as a function of multiparity in the rat. Brain Res. 1992;580:241–248. doi: 10.1016/0006-8993(92)90950-e. [DOI] [PubMed] [Google Scholar]
- 34.Mann PE, Rubin BS, Bridges RS. Differential proopiomelanocortin gene expression in the medial basal hypothalamus of rats during pregnancy and lactation. Brain Res Mol Brain Res. 1997;46:9–16. doi: 10.1016/s0169-328x(96)00267-7. [DOI] [PubMed] [Google Scholar]
- 35.Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Shughrue PJ, Dorsa DM. Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology. 1995;61:276–283. doi: 10.1159/000126849. [DOI] [PubMed] [Google Scholar]
- 39.Terkel J, Bridges RS, Sawyer CH. Effects of transecting lateral neural connections of the medial preoptic area on maternal behavior in the rat: nest building, pup retrieval and prolactin secretion. Brain Res. 1979;169:369–380. doi: 10.1016/0006-8993(79)91037-0. [DOI] [PubMed] [Google Scholar]
- 40.Bridges RS, Hays LE. Steroid-induced alterations in mRNA expression of the long form of the prolactin receptor in the medial preoptic area of female rats: Effects of exposure to a pregnancy-like regimen of progesterone and estradiol. Brain Res Mol Brain Res. 2005;140:10–16. doi: 10.1016/j.molbrainres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci. 2007;36:121–131. doi: 10.1016/j.mcn.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 43.Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maras PM, Petrulis A. Chemosensory and steroid-responsive regions of the medial amygdala regulate distinct aspects of opposite-sex odor preference in male Syrian hamsters. Eur J Neurosci. 2006;24:3541–3552. doi: 10.1111/j.1460-9568.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- 45.Cushing BS, Perry A, Musatov S, Ogawa S, Papademetriou E. Estrogen receptors in the medial amygdala inhibit the expression of male prosocial behavior. Journal of Neuroscience. 2008;28:10399–10403. doi: 10.1523/JNEUROSCI.1928-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and estrous odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in male rats. Brain Res. 2005;1066:101–108. doi: 10.1016/j.brainres.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 47.Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007;1175:66–75. doi: 10.1016/j.brainres.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez JM, Brann JH, Gil M, Hull EM. Sexual experience increases nitric oxide synthase in the medial preoptic area of male rats. Behav Neurosci. 2006;120:1389–1394. doi: 10.1037/0735-7044.120.6.1389. [DOI] [PubMed] [Google Scholar]
- 49.Xiao K, Kondo Y, Sakuma Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology. 2005;81:56–62. doi: 10.1159/000084893. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida M, Suga S, Sakuma Y. Estrogen reduces the excitability of the female rat medial amygdala afferents from the medial preoptic area but not those from the lateral septum. Exp Brain Res. 1994;101:1–7. doi: 10.1007/BF00243211. [DOI] [PubMed] [Google Scholar]
- 51.Rissman EF, Wersinger SR, Fugger HN, Foster TC. Sex with knockout models: behavioral studies of estrogen receptor alpha. Brain Res. 1999;835:80–90. doi: 10.1016/s0006-8993(99)01452-3. [DOI] [PubMed] [Google Scholar]
- 52.Numan M, Numan MJ, English JB. Excitotoxic amino acid injections into the medial amygdala facilitate maternal behavior in virgin female rats. Horm Behav. 1993;27:56–81. doi: 10.1006/hbeh.1993.1005. [DOI] [PubMed] [Google Scholar]
- 53.Champagne FA, Curley JP. Maternal regulation of estrogen receptor alpha methylation. Curr Opin Pharmacol. 2008;8:735–739. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merchenthaler I, Dellovade TL, Shughrue PJ. Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci. 2003;1007:89–100. doi: 10.1196/annals.1286.009. [DOI] [PubMed] [Google Scholar]
- 56.Shughrue PJ. Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta). Exp Neurol. 2004;190:468–477. doi: 10.1016/j.expneurol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Kelly JF, Bienias JL, Shah A, Meeke KA, Schneider JA, Soriano E, Bennett DA. Levels of estrogen receptors alpha and beta in frontal cortex of patients with Alzheimer's disease: relationship to Mini-Mental State Examination scores. Curr Alzheimer Res. 2008;5:45–51. doi: 10.2174/156720508783884611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordberg A. Neuroprotection in Alzheimer's disease - new strategies for treatment. Neurotox Res. 2000;2:157–165. doi: 10.1007/BF03033791. [DOI] [PubMed] [Google Scholar]


