Abstract
Lung infections are important risk factors for an increased morbidity and mortality in prematurely-delivered babies. Immaturity of the innate immune components makes them extremely susceptible to infection. Recently, we isolated lung dendritic cell (DC)-precursor cells from preterm fetal baboons. The isolated cells were found to be defective in phagocytosing Escherichia coli under basal conditions. In this study, we investigated the effects of exogenously-added purified native lung surfactant protein (SP)-A and recombinant Toll-like receptor (TLR)-4-MD2 proteins on phagocytic uptake and cytokine secreting ability of fetal baboon lung DC-precursor cells. The cells were pulsed with SP-A and/or TLR4-MD2 proteins and the phagocytic function was investigated by incubating the cells with fluorescent-labeled E. coli bioparticles and analyzed by spectrofluorometry. The amounts of TNF-α secreted in cell-free supernatants were measured by ELISA. Our results demonstrate that SP-A and TLR4-MD2 proteins, whether added alone or together, induce phagocytosis of E. coli (p<0.05). The SP-A does not affect TNF-α secretion, while the TLR4-MD2 protein induces TNF-α. However, simultaneous addition of SP-A with TLR4-MD2 protein reduces the TLR4-MD2-protein induced TNF-α to basal level. In conclusion, our results indicate that an exogenous administration of SP-A can potentially induce phagocytic activity and anti-inflammatory effect in preterm babies, and help control infection and inflammation.
Keywords: Dendritic cells, Surfactant protein-A, Toll-like receptor-4, Preterm babies, Innate immunity
Introduction
Preterm infants are highly susceptible to infections; this increased susceptibility to infections is associated with perturbed development and extreme immaturity of immune system. The antigen-presenting cells have an important role in pathogen-uptake, processing and in regulating the inflammatory and adaptive host immune responses. Among various types of antigen-presenting cells, dendritic cells (DCs) have been recognized as the most potent antigen-presenting cells [1–4]. In the past, several studies have confirmed immunomodulatory role of lung DCs against a variety of antigens in adult humans and animal models with a mature immune system [5–10]. However, the phenotypes and functions of lung DCs remained poorly known in preterm babies till recently. For the first time, we isolated unique low-density lung DC population from preterm baby baboons [11]. The cells have density similar to adult baboon lung DCs, are lineage-negative and defective in responding to infectious stimuli. Overall our results suggest that despite having similar isolation characteristics, this unique cell-population harvested from fetal lung does not belong to conventional immature or mature DC category [11]. Recent results from our lab, further demonstrate that the fetal DC-precursor cells express low level of DC-markers, and incubation with DC-promoting cytokines (GM-CSF, IL-4 and TNF-α) induces differentiation of these fetal cells into typical DCs (unpublished results). Based on these properties, we identified them as DC-precursor cells [11].
The DCs are well known to co-ordinate innate and adaptive immunity via pathogen-pattern recognition receptors, such as Toll-like receptors (TLR), mannose receptors, scavenger receptors and collectins (such as surfactant protein-A, SP-A) [12]. Deficiencies or functional defects of pathogen-pattern recognition receptors can negatively affect the DC functions, compromise the host defense and lead to serious consequences in early life-periods of preterm babies [13]. We focus on SP-A, a major protein of lung surfactant that lines the alveoli, and TLR-4, a potent membrane-receptor that senses both pathogen-associated and damage-associated molecular patterns (PAMP and DAMPs) during infection [14–15].
Earlier we observed that the expression of SP-A and TLR4 is undetectable or negligible in lung tissues of fetuses (at 67%–75% of complete gestation term) under steady-state condition, and increases to the levels equivalent to adult counterparts as the gestation period reaches closer to term [16–18]. However, preterm birth, mechanical injury (ventilation-associated) and infection significantly influence the lung-homeostasis and decrease the alveolar SP-A pools to significantly low levels [16–17]. In contrast, the expression of TLR4 is increased in preterm babies having lung infection [18].
To this end, recent understanding in the field suggests that SP-A and TLR4 both enhance the phagocytosis. The lack of SP-A in alveoli may compromise the uptake of pathogens. However, an exaggerated activation of TLR4 can lead to chronic inflammatory response or “cytokine storm” [19–23]. This pattern correlates well with fulminating infection (low SP-A = low phagocytic uptake) and inflammatory response (increased TLR4 = increased amounts of pro-inflammatory cytokines) in preterm babies having lung infection. These results led us to hypothesize that the introduction of SP-Abased clinical surfactants and TLR4-antagonists may compensate for the loss of SP-A and downregulate an exaggerated TLR4-mediated inflammatory response, respectively. It has also been learned recently that SP-A interacts with TLR4 in vitro [24–25] and in lung (manuscript under revision) [26]. Thus, introduction of SP-A may have an effect on TLR4-mediated immune responses. Here, we investigated the immunomodulatory effects of native SP-A and recombinant TLR4-MD2 proteins on selected immune functions (phagocytosis and cytokine response) of fetal baboon lung DC-precursor cells and compared with those of adult baboon lung DCs. Our results suggest that in both adult and fetal systems, pulsing of cells with SP-A and TLR4-MD2 proteins increases the phagocytic uptake of Escherichia coli bioparticles. When added together, no additive effect was demonstrated on phagocytic function of DCs. Co-incubation of cells with SPA and TLR4-MD2 proteins, however, significantly inhibits the TLR4-MD2-induced release of TNF-α against E. coli.
Overall, our results support a significant role of SP-A in improving innate phagocytic function and in suppressing the TLR4-mediated deleterious inflammatory response against infectious stimuli.
Materials and Methods
Baboon lung tissues
The animal studies were approved by Institutional Animal Care and Use Committees, Environmental Health and Safety or Institutional Biosafety Committee of the University of Oklahoma Health Science Center, Oklahoma City, OK (OUHSC). Baboon (Papio anubis) colonies are maintained at Baboon Resources, OUHSC, Oklahoma City, OK. At the time of necropsy, whole fresh lung or a lobe of lung from fetal (delivered at 125 ± 4 days of gestation; 125dGA, complete term = 185dGA) and adult baboons (age range 10–22 years) were collected in RPMI 1640 medium containing 2 mM glutamine, 1 mM N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum (low endotoxin <10EU/ml, FBS; Invitrogen, Carlsbad, CA). None of the animals recruited in this study showed any clinical sign of infection or lung pathology. Gross examination of all major viscera and the placenta revealed no signs of inflammation or infection.
Purification of baboon lung SP-A
We purified SP-A from bronchoalveolar lavage fluid of a normal healthy adult baboon by a slight modification of the procedure described previously [27]. The bronchoalveolar lavage fluid was collected from an adult baboon lung by instilling endotoxin-free, sterile normal saline (endotoxin-free 0.9% NaCl, 1.9–2 L with approximately 90% recovery). The lavage fluid was centrifuged, and the supernatant was concentrated using a tangential flow filtration technique (10 kDa hollow fiber filter; GE Healthcare BioSciences Corp, NJ). The surfactant lipids were removed using isobutyl alcohol (1:5 ratio lavage: isobutyl alcohol). The delipidated protein was centrifuged at 5,000 × g for 15 min at room temperature, dried under nitrogen gas, and subsequently completely dried in a lyophilizer (Labconco, MO). The dried lavage residue was rehydrated in extraction buffer (25 mM Tris (pH 7.5), 0.15 M NaCl, and 20 mM octyl-β-D-glucoside) overnight at 4°C. Rehydrated surfactant was extracted six times with extraction buffer by vortex mixing and centrifugation at 20,000 × g for 30 min at 4°C. Insoluble SP-A was then suspended in solubilization buffer (5 mM HEPES, pH 7.5, 0.02% sodium azide) and dialyzed for 72 h against four changes of the solubilization buffer. Insoluble protein was removed by centrifugation at 50,000 × g for 30 min at 4°C, and supernatant was adjusted to 20 mM CaCl2 and 1 M NaCl to re-precipitate SP-A. Precipitated SP-A was pelleted by centrifugation at 50,000 × g for 30 min at 4°C, and washed two times in 5 mM HEPES (pH 7.5), 20 mM CaCl2 and 1 M NaCl. The SP-A was suspended in 5 mM HEPES, 5 mM EDTA (pH 7.5) and dialyzed for 72 h against four changes of the solubilization buffer to remove EDTA. The purified SP-A was dialyzed against four changes of the endotoxin-free, highly-purified water (Invitrogen, CA) for 72 h to remove any remaining EDTA or salts (CaCl2 and NaCl). Finally, purified SP-A was lyophilized completely and resuspended in endotoxin-free Dulbecco’s phosphate buffered saline. The purified protein was filter-sterilized using a 0.2 μm low-protein binding, HT Tuffryn membrane filter (Pall Life Sciences, NY) and stored frozen at −80°C. The protein concentration of purified SP-A was measured by microBCA method (Pierce, IL).
All the purification steps were performed under aseptic conditions using endotoxin-free solutions and reagents. The endotoxin concentration was measured using the End-point chromogenic Limulus Amebocyte Lysate (LAL) assay (Charles River Laboratories, MA). The purity of the SP-A protein was confirmed by SDS-PAGE and Western blotting using the procedures described earlier [16–17]. The isolated protein was further characterized by high performance liquid chromatography (HPLC) on a Phenomenex C-18 reverse phase column using solvents acetonitrile/water/trifluoroacetic acid (60:40:1) at 1ml/min, with the UV detector set at 280 nm. The retention time of SP-A was determined to be 1.3 min.
Culture of KG-1-derived DCs and isolation of primary lung DCs
KG-1 cells (Bone marrow myeloblast cells derived from a leukemia patient; ATCC, VA) were cultured in the presence of recombinant human-GM-CSF (100 ng/ml), IL-4 (100 ng/ml) and TNF-α (40 ng/ml) (all the cytokines were purchased from PeproTech, NJ) for a period of 5 days [28–30]. The phenotype and morphology of the KG-1-derived DCs were confirmed by flow cytometry and light microscopy, respectively [31]. The KG-1-derived DCs were included as model system to optimize the amounts of effector molecules (purified baboon lung SP-A and recombinant TLR4-MD2 proteins).
Isolation of adult baboon lung DC or fetal baboon lung DC-precursor population
Freshly collected lobe of the lung or whole lung samples of adult and fetal baboons were transported on ice in RPMI 1640 medium containing 2 mM glutamine, 1 mM HEPES, 10 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS. After a mild mechanical disruption, the single cell suspension was seeded in tissue culture flask (Nalge-Nunc International Corp, NY) at a density of 30–50×106 leukocytes/175 cm2 flask in RPMI 1640 medium containing 2 mM glutamine, 1 mM HEPES, 10 μg/ml gentamicin, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS. The light-density DC-populations were harvested using OptiPrep cell-separation solution (density 1.32 g/ml, Accurate Chemicals, NY) [31] [11]. Basic characteristics of lung DC-precursor cells and DCs isolated from fetal and adult baboons have been described earlier [11]. Here, first we compared the immunophenotype of KG-1-derived DCs and fetal baboon lung DC-precursors or adult baboon lung DCs by flow cytometry. Briefly, cells were suspended in Dulbecco’s phosphate buffered saline (DPBS) containing 1% FBS and 0.09 % sodium azide and incubated with fluorochrome-conjugated antibodies to HLA-DP, DQ, DR, CD11c, CD40, CD80, CD86 (typical DC-markers) as described earlier [11].
Expression of endogenous TLR4 by flow-cytometry and western blotting
Flow cytometry
The harvested cells were suspended in 100 μl DPBS containing 1% FBS and 0.09 % sodium azide. Previously titrated phycoerythrinin (PE)-conjugated anti-human TLR4 antibody (BD Biosciences, CA), was added at the ratio of 1 μg antibody per 1×106 cells. Cells and antibody were incubated for 30 min on ice in the dark. The cells were washed thrice with PBS containing 1% FBS and 0.09% sodium azide and fixed in freshly prepared 0.5% paraformaldehyde. The cells were run through an automated dual laser excited FACS Calibur at the Flow and Imaging Core Facility (OUHSC, Oklahoma City). The histogram and dot-plot charts were obtained and analyzed using Summit V4.3 software (Dako Colorado Inc, CO). The isotype control antibody-stained cells served as controls for background staining.
Western immunoblotting
For western immunoblotting, whole cell lysates were prepared in lysis buffer (50 mM Tris-HCl, pH 7.4) containing 1% Igepal, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM phenyl methyl sulfonyl fluoride (PMSF), and 1 μg/ml each of leupeptin and pepstatin. After protein estimation, about 15 μg of total proteins were fractionated by Novex 4–20% Tris–glycine gradient SDS-PAGE gel (Invitrogen, CA). Separated proteins were electro-transferred onto a nitrocellulose membrane using iBlot gel transfer device (Invitrogen, CA). The non-specific sites were blocked by incubating the membrane with 7% skimmed milk in Tris-buffered saline with 0.4 % Tween-20 (TBST). The blocked membrane was incubated overnight at 4°C with monoclonal antibody against TLR4 (clone HTA125; ebioscience, CA) diluted 1:1000 in TBST. The membrane was washed and incubated with horse-radish peroxidase (HRP)-conjugated-anti-rabbit-IgG antibody (Sigma–Aldrich, MO) diluted 1:1000 in TBST. The immunoreactive bands were detected by SuperSignal West Femto detection reagent (Thermo Fischer Scientific, IL).
Cellular distribution of exogenously added TLR4-MD2 protein
Since KG-1-derived-DCs, adult baboon lung DCs and fetal baboon lung DC-precursor cells expressed negligible amounts of TLR4 protein, the cells were pulsed with recombinant human TLR4-MD2 proteins (RnD Systems, MN). The cellular distribution of exogenously-added recombinant TLR4-MD2 protein was investigated by confocal microscopy and flow cytometry.
Labeling of recombinant TLR4-MD2 protein with Alexa-fluor 594 fluorescent dye
Recombinant TLR4-MD2 protein was labeled with Alexa-fluor 594 fluorescent dye using a microscale protein labeling kit (Invitrogen-Molecular Probes, CA) optimized for labeling proteins with molecular weights between 12 and 150 kDa, as per the manufacturer’s directions. Briefly, 40 μg of recombinant TLR4-MD2 protein (at the concentration of 1 mg/ml in DPBS) was labeled with Alexa-Fluor 594 carboxylic acid, succinimidyl ester (Excitation/Emission wavelengths: 590/617nm). The pH of the protein was adjusted to 8.3 using 1/10 volume of 1M sodium bicarbonate, and Alexa-Fluor 594 reactive dye stock solution (12.2 nmol/μl) was added to the protein. The protein:dye mixture was incubated for 15 min at room temperature. Fluorochrome-conjugated protein was then separated from unconjugated dye using a spin filter conditioned with gel resin. The spin filter loaded with reaction mixture was centrifuged at 16,000 × g for 1 min. The absorbance of the Alexa-Fluor 594 dye-conjugated TLR4-MD2 protein was read at 280 nm and 590 nm using UV/Vis NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, DE) and degree of labeling (DOL) was determined using following formula:
Where A280 and A590 are the protein’s absorbance at 280 nm and at 590 nm, respectively. The value of 0.56 is a correction factor for the fluorophore’s contribution to the A280, and 90,000 cm−1M−1 is the approximate molar extinction coefficient of the Alexa-Fluor 594 dye.
Cellular distribution of Alexa-fluor 594-conjugated TLR4-MD2 protein by confocal microscopy
The KG-1-derived DCs were seeded at a density of 2.5×105 cells per well in an 8-well chamber slide (Nalge Nunc international, NY) in serum-free Opti-MEM medium (Invitrogen, CA). The cells were then incubated with 10 μg of Alexa fluor 594-conjugated recombinant TLR4-MD2 protein (DOL ~4.0) for 1h and 4h at 37°C in 5% CO2 atmosphere. Thirty minutes prior to the completion of incubation period, the Vybrant DiO cell labeling solution (5 nM final concentration, Invitrogen-Molecular Probes, CA) and Hoechst 33342 dye (0.3 μg/ml final concentration, Invitrogen-Molecular Probes, CA) were added to the cells for staining cell-cytoplasm and nucleus, respectively. Finally, the cells were washed twice in Opti-MEM medium, fixed using Vectashield-antifade mounting medium (Vector Laboratories, CA) and observed under Leica TCS SP2 AOBS (Acousto Optical Beam Splitter) multi-photon laser confocal microscope at the Flow and Image Cytometry laboratory (OUHSC, Oklahoma City). Images were acquired with 63× lense objective (at excitation/emission wavelengths: 405/410–550 nm for Hoechst 33342 dye, 488/500–550 nm for DiO dye, and 594/610–660 nm for Alexa-fluor 594 dye) and were analyzed using the Leica TCS software (Leica Microsystems CMS, Mannheim, Germany). Finally, the images acquired were merged and composite pictures were obtained.
Localization of Alexa-fluor 594-conjugated TLR4-MD2 protein by flow cytometry
The KG-1-derived DCs were suspended in Opti-MEM medium at the density of 2.5×105 cells per 100 μl and incubated with 10 μg Alexa-fluor 594-conjugated recombinant TLR4-MD2 protein. After 1h and 4h of incubation, cells were washed thrice in fresh Opti-MEM medium, re-suspended in 500 μl of 37°C pre-warmed DPBS, and run on Influx Cell Sorter (BD Biosciences, CA) at the Flow and Image Cytometry laboratory (OUHSC, Oklahoma City). The cells were gated to remove debris and histogram charts were obtained at 624–40 nm emission wavelengths. Cell-staining with Alexa-fluor 594-conjugated TLR4-MD2 protein was analyzed using Summit V4.3 software (Dako Colorado Inc, CO).
Phagocytosis assay
In this study, we employed pHrodo-conjugated, heat-killed E. coli K12 (encapsulated) bioparticles (Invitrogen-Molecular Probes, CA). The pHrodo-fluorescent label offers advantage over other conventional methods, that it fluoresces only in acidic conditions i.e. after the bioparticles are taken inside the intracellular lysosomes (Invitrogen-Molecular Probes, CA). To ensure that the fluorescence relates to phagocytosed pHrodo-conjugated E. coli bioparticles only, the phagocytosis reaction mix was imaged by confocal microscopy. Briefly, the KG-1-derived DCs were seeded at a density of 2.5 × 105 cells/well in an 8-well chamber slide and incubated with pHrodo-conjugated E. coli K12 bioparticles (one cell-to-~300 bacterial bioparticles) for 3h. The Hoechst 33342 dye was added to the cells (0.3 μg/ml final concentration, Invitrogen-Molecular Probes, CA). The cells were washed once, re-suspended in Opti-MEM medium, fixed with Vectashield-antifade mounting medium (Vector Laboratories, CA), and observed under Leica TCS SP2 AOBS (Acousto Optical Beam Splitter) multi-photon laser confocal microscope (at excitation/emission wavelengths: 550/600 nm for pHrodo-labeled bioparticles, 405/410–550 nm for Hoechst 33342 dye) and under brightfield. Images taken at different wavelengths were merged, and composite pictures were obtained.
After confirming that the fluorescence is of phagocytosed bioparticles, comprehensive experiments were performed in presence and absence of effector molecules (SP-A and TLR4-MD2 proteins). The cells were pulsed with purified, native baboon lung SP-A and recombinant human TLR4-MD2 or MD2 proteins (RnD Systems, MN) for an hour prior to phagocytosis assay with pHrodo-conjugated, heat-killed E. coli K12 bioparticles (Invitrogen-Molecular Probes, CA). We also included MD2 protein because SP-A is known to interact with MD2, and questioned if MD2 can influence the immune functions of DC-population. The assay was performed in serum-free Opti-MEM medium (Invitrogen, CA), as described by the manufacturer (Invitrogen-Molecular Probes, CA). The assay mixtures were incubated for another 3h at 37°C in 5% CO2 incubator. The fluorescence readings were taken at 550 nm excitation and 600 nm emission wavelengths using SpectraMax M2 spectrofluorometer (Molecular Devices, CA).
The phagocytosis index was calculated by subtracting the average fluorescence intensity of the reaction with bioparticles alone from the control (basal without any effector molecules) and experimental wells. Finally, the percent effect was calculated using the following formula:
The percent phagocytosis of E. coli bioparticles was also confirmed by fluorescence microscopy in representative reaction wells. The cell-free supernatants were collected after taking the fluoremetric readings, and stored at −80°C for further analysis.
Cytokine (TNF-α) measurement
The TNF-α levels were measured in cell-free supernatants by enzyme linked immunosorbent assay (ELISA) using a commercially available kit (eBioscience, CA), as per the manufacturer’s instructions. Briefly, the microwells of a 96 well plate were coated with diluted purified anti-human TNF-α antibody. The wells were washed and nonspecific sites were blocked. Diluted recombinant human TNF-α (7.8–500 pg/ml standard) and cell-free-supernatant (1:10) were added to the antibody-coated wells and the plate was incubated overnight at 4°C. Next day, the plate was washed and incubated with biotin-conjugated anti-human TNF-α antibody followed by avidin-horseradish peroxidase and substrate solution. The reaction was stopped by adding 2 N H2SO4 and read at 450 nm (Molecular Devices, CA).
Statistical Analysis
The results were analyzed by Student’s t-test for statistical significance using Prism software (Graphpad, San Diego, CA). p<0.05 was considered significant.
Results
The fetal baboon lung DC-population collected from top of the density gradient were unique to fetal baboons, and were not identified in adult baboon. Details on morphologic, phenotypic characteristics and functions of primary lung DC-population have been reported earlier [11]. We identify the fetal lung DC population as DC-precursors [11].
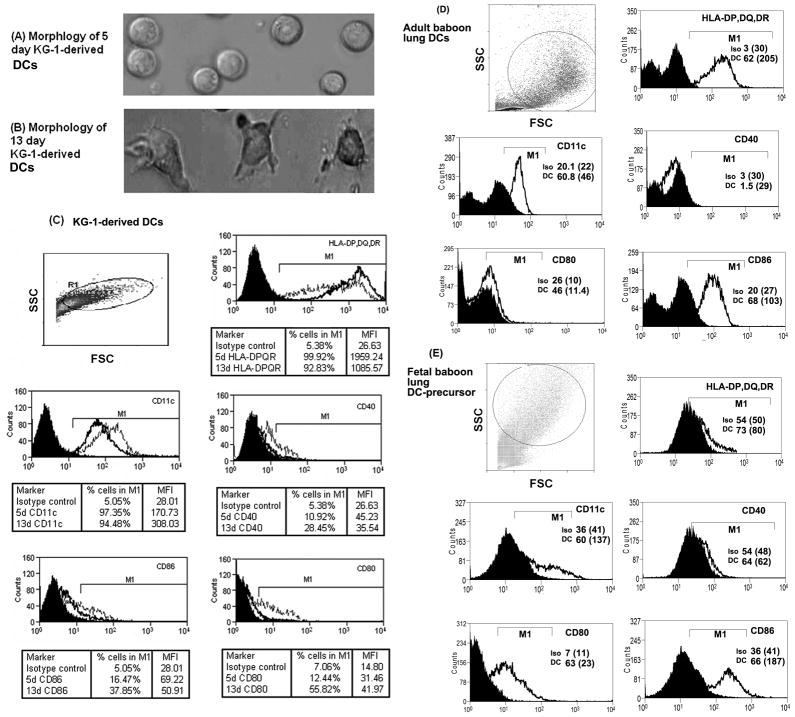
Prior to conducting experiments with adult baboon lung DCs or fetal baboon lung DC-precursor cells, the KG-1-DCs (harvested on 6th day of culture) were used in the initial experiments to optimize the concentration of effector molecules. Morphologically, KG-1-derived DCs harvested on day 5 of culture were round and did not show any tentacles or dendrites (typical of immature DCs). However, after 13 days, the DCs developed dendrites and irregular shape, characteristic features of mature DCs (Figures 1A and B). Flow cytometry analysis of cell-surface-antibody-stained cells showed that KG-1 cells transform into DCs after 13 days and express HLA-DP,DQ,DR, CD11c, CD40, CD80 and CD86 cell-surface markers, characteristics of typical DCs (Figure 1C). On comparison, we found that the KG-1-derived DCs express DC-markers to levels similar to those in adult baboon lung DCs (Figures 1C and 1D). The fetal baboon lung DC-precursor cells showed negligible levels of DC-markers except CD80 and CD86 (Figure 1E). Later, we used primary lung DCs isolated from healthy adult baboons and DC-precursor cells isolated from fetal baboons [11] to study the immunomodulatory effects of SP-A and TLR4-MD2 proteins against infectious stimuli.
Figure 1.
Photomicrographs of wet mount of KG-1-derived DCs after days (A) 5 and (B) 13, of in vitro culture in presence of recombinant human-GM-CSF, IL4 and TNF-α. Flow-cytometric histogram charts of (C): KG-1-derived DCs on days 5 (dark line) and 13 (faded line), (D): adult baboon lung DCs, and (E): fetal baboon lung DC-precursor cells. The cells were stained with DC-markers-specific, fluorescent-conjugated antibodies or isotype control antibody (black area). The cells with high forward scatter (FSC) and side scatter (SSC) were gated, and histogram charts were obtained. The percent number and mean fluorescent intensity (MFI) values of DC-marker positive KG-1-derived DCs are shown in tabulated form. The percent number of primary lung DC or DC-precursor cells positive for DC-markers (DC) is shown within the chart itself. Values shown within parenthesis indicate MFI values. The percent number and MFI values of isotype control (iso) stained cells in M1 region are also shown. The results presented here are representative of at least three experiments.
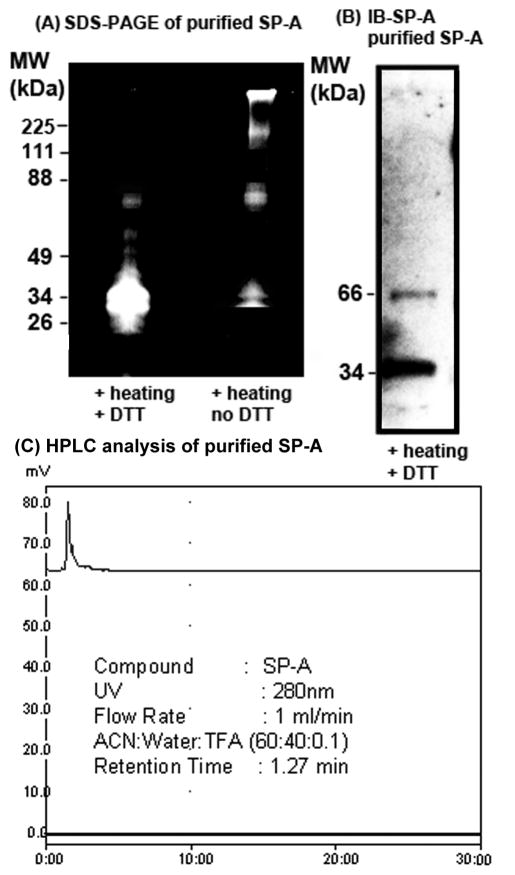
Characterization of purified native baboon lung SP-A
To elucidate the effects of SP-A, first we purified the native SP-A protein from bronchoalveolar lavage fluid specimens of a normal, healthy adult baboon [16–17]. The purity and identity of the native baboon lung SP-A was confirmed by SDS-PAGE, western blotting and HPLC. Under partially-reducing conditions (heating and no DTT), SP-A separated as an oligomer, ~ 100 kDa trimer, and a 66 kDa dimer on the SDS-PAGE gel. Under reducing condition (heating + DTT), SP-A ran as 32–34 kDa monomer and 66 kDa partially reduced dimer (Figure 2A). The purified baboon lung SP-A protein was also immunoblotted with SP-A antibody which identified the SP-A-specific protein bands (Figure 2B). The HPLC chromatogram further confirmed the purity of SP-A (Figure 2C). Since the TLR4-MD2 proteins are less abundant in biological system, and it is difficult to obtain native TLR4-MD2 protein in sufficient quantity, recombinant human TLR4-MD2 protein (RnD Systems, MN) was used.
Figure 2.
Characterization of purified native baboon lung SP-A. (A): Purified baboon SP-A (5 μg in each lane) protein was run under reducing (+ heating, + DTT) and partially-reducing (+ heating, no DTT) conditions on SDS-PAGE gel and stained. (B): Immunoreactivity of purified SP-A by western blotting with SP-A-specific antibody (IB-SP-A). The SP-A protein was run on reducing SDS-PAGE gel prior to western blotting. (C) HPLC chromatogram of purified lung SP-A.
Since TLR4 is a potent receptor for endotoxin, the presence of endotoxin can significantly influence the results. We prepared all the solutions and reagents in endotoxin-free water and performed all the assays in an aseptic environment. We measured the endotoxin concentration in the purified baboon SP-A and in the reconstituted TLR4-MD2 and MD2 proteins by chromogenic LAL method (Charles River Lab, MA). The endotoxin concentration was negligible in purified baboon SP-A (0.0003 ng/μg protein) and in recombinant TLR4-MD2 and MD2 protein suspensions (≤ 0.006 ng/μg protein).
KG-1-derived DCs and primary DCs express negligible TLR4 protein under normal conditions; exogenously added TLR4-MD2 protein localizes mainly on the cell surface
The basal cell-surface expression of TLR4 was negligible in primary adult baboon lung DCs and KG-1-derived DCs (Figure 3). The expression of TLR4 was also undetectable in fetal lung DC-precursor cells (not shown). Thus, we pulsed the DCs with recombinant TLR4-MD2 protein prior to the phagocytosis assay. We tracked and studied the localization of Alexa-fluor 594-conjugated TLR4-MD2 protein in the KG-1-derived DCs by confocal microscopy. Confocal images showed that the TLR4-MD2 protein localized mainly on the cell membrane (Figures 4A and 4B). Flow-cytometric analysis of DCs pulsed with Alexa-fluor 594-labeled TLR4-MD2 protein showed that there is an increase in MFI values and percent number of cells staining positive for Alexa-fluor 594 stain. These findings further confirmed that the TLR4-MD2 protein localizes on the cell-surface (Figure 4C).
Figure 3.
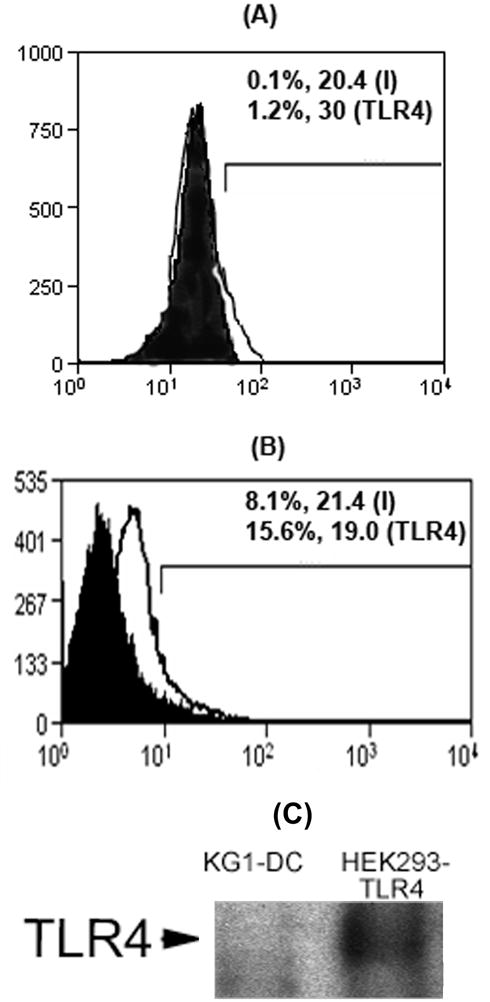
Basal TLR4 expression by (A) primary adult baboon lung DCs and (B) KG-1-derived DCs under steady-state condition. Cell-surface expression of TLR4 was detected by flow cytometry after staining the cells with TLR4-specific antibody. The percent number and MFI values of cells stained with TLR4-specific antibody (TLR4) are compared with isotype control antibody-stained cells (I) in M1 region. (C): Western blot showing undetectable expression of TLR4 in 5 μg cell lysate protein of KG-1-derived DCs (KG1-DC). An equal amount of cell lysate protein of HEK293 cells stably transfected with TLR4 (HEK-TLR4) served as positive control.
Figure 4.
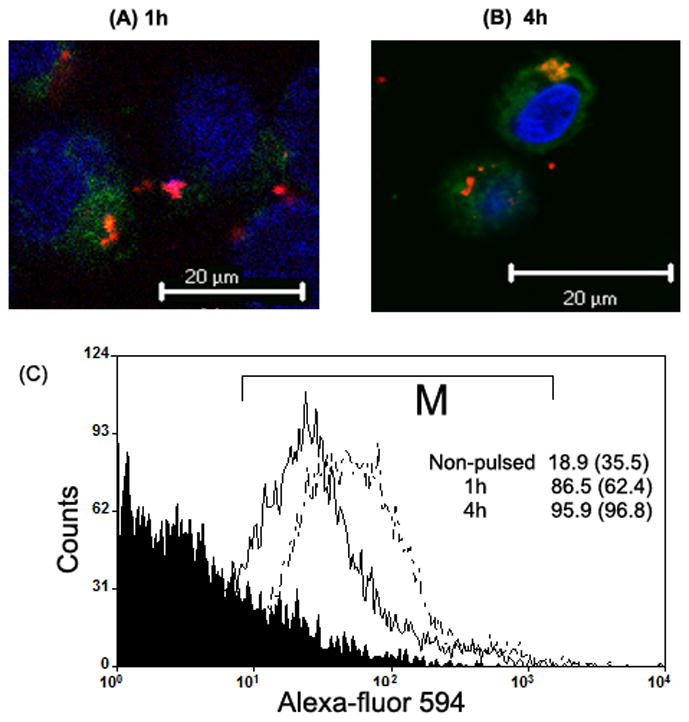
Localization of exogenously-added recombinant TLR4-MD2 protein by confocal microscopy and flow-cytometry. Confocal microscopic images of KG-1-derived DCs pulsed with Alexa-fluor 594-conjugated recombinant TLR4-MD2 protein for (A) 1h and (B) 4h. Vybrant DiO (green) dye stains the cytoplasm, and Hoechst 33342 (blue) dye stains the nucleus of the cell. The images were acquired using 63 X objective. (C) Flow-cytometric charts of KG-1-derived DCs pulsed with Alexa-fluor 495-conjugated recombinant TLR4-MD2 protein after 1h (dark line) and 4h (dotted line). The histogram chart of non-pulsed cells (negative control) is shown under the black area. Cells were gated in M region. Percent number of cells (and MFI values) positive for fluorescence are shown within the chart. Results are representative of two experiments.
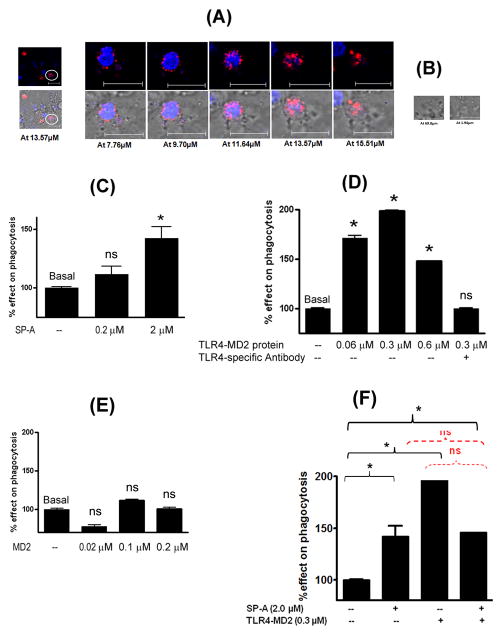
Exogenous addition of native SP-A and recombinant TLR4-MD2 proteins increases the phagocytic uptake of E. coli bioparticles
In this study, we utilized pHrodo-labeled, heat-killed encapsulated E. coli K12 bioparticles for investigating the phagocytic ability of DCs. First, we confirmed the fluorescence of phagocytosed bioparticles in KG-1-derived DCs by confocal microscopy. The images showed that only the phagocytosed bioparticles fluoresce (Figure 5A). In Figure 5A, a fluorescent cell is focused that has taken up the bioparticles. In contrast, the extracellular bioparticles in the same field, settled at the bottom of the well (z-stack slice # 69.8 μm) or floating towards the top (z-stack slice #1.94 μm) do not emit any fluorescence at all (Figures 5A and 5B).
Figure 5.
Effect of purified native SP-A, recombinant TLR4-MD2 protein and MD2 protein on phagocytic function of KG-1-derived DCs. (A): Confocal microscopic images of KG-1-derived DCs incubated with pHrodo-labeled E. coli bioparticles for 3h. Phagocytosed bioparticles fluoresce red. Cells without phagocytosed particles and extracellular bacteria do not fluoresce. Enlarged images of a cell (shown as circle) are also shown in the figure, at different z-stack slices. (B): The extracellular bacteria that are either settled at the bottom or lie towards the top do not emit any fluorescence. These images confirm that fluorescence is of phagocytosed bioparticles. Next, KG-1-derived DCs were incubated with (C): purified baboon lung SP-A (0.2 and 2 μM), (D): recombinant TLR4-MD2 protein (0.06–0.6 μM) and functional-grade anti-human TLR4 antibody (HTA125 clone, Imgenex, CA; control reaction), (E): recombinant MD2 protein (0.02–0.2 μM), and (F): purified baboon lung SP-A (2 μM) and TLR4-MD2 protein (0.6 μM), for an hour prior to addition of pHrodo-labeled E. coli bioparticles. The phagocytic uptake of E. coli bioparticles was measured spectrofluorometrically at 550 nm excitation and 600 nm emission wavelengths. Results are mean (SEM) of three different experiments. * p<0.05 or ns: not significant as compared to basal phagocytosis.
In comprehensive phagocytosis experiments, the fluorescence signal reflecting the red fluorescence emitted by phagocytosed pHrodo-labeled E. coli bioparticles, was measured by spectrofluorometry using identical wavelengths setting. Briefly, the KG-1-derived DCs were incubated with purified baboon lung SP-A protein ± TLR4-MD2 protein. The % net effect on phagocytosis was calculated in the presence of effector molecules (TLR4-MD2, MD2 and SP-A) after normalizing with the basal phagocytosis in the absence of the effector molecules. The percent phagocytosis calculated by fluorescence microscopy (number of fluorescing cells/total number of cells in a composite of 5 different fields) correlated with the phagocytosis indices calculated by the spectrofluorometry methods. Our data suggest that both SP-A and TLR4-MD2 proteins increase the phagocytic uptake of E. coli bioparticles by 1.5–2 fold in concentration-dependent manner (p<0.05, Figures 5C and 5D). The MD2 protein alone did not affect the phagocytosis (Figure 5E). Next, the phagocytosis assay was performed with primary lung DC or DC-precursor population in presence of purified SPA (2 μM) and TLR4-MD2 (0.3 μM) proteins at concentrations that provided maximum phagocytic uptake in KG1-derived DCs (Figures 5F and 6). The results suggest that, similar to KG-1-derived DCs, the phagocytic uptake of E. coli is increased in the presence of exogenous SP-A (p<0.05) and TLR4-MD2 protein in primary baboon lung DCs. When SP-A and TLR4-MD2 proteins were added together, the phagocytic uptake of E. coli remained increased as compared to basal level, however, no additive effect was observed (Figure 6).
Figure 6.
Effect of simultaneous addition of purified SP-A and recombinant TLR4-MD2 protein on phagocytic function of primary (A) adult baboon lung DCs and (B) fetal baboon lung DC-precursor cells. The DCs were incubated with respective proteins for an hour prior to addition of pHrodo-labeled E. coli bioparticles. The phagocytic uptake of E. coli bioparticles was measured spectrofluorometrically. * p<0.05, ns: not significant or otherwise indicated. Results are mean (SEM) of three different experiments performed at different times.
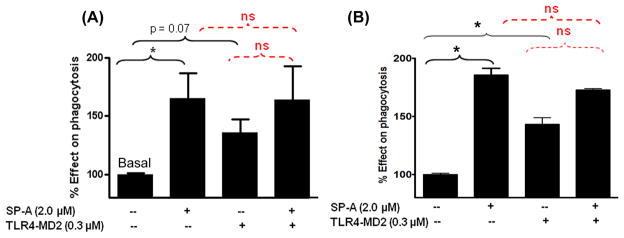
SP-A reduces the TLR4-MD2 protein-induced TNF-α release against E. coli
We measured the TNF-α levels in cell-free supernatants of primary adult baboon lung DCs and fetal baboon lung DC-precursor cells treated with SP-A ± TLR4-MD2 proteins after 3h of phagocytosis reaction. Addition of purified lung SP-A did not induce the secretion of TNF-α by DCs in response to E. coli, but pulsing with TLR4-MD2 protein increased the TNF-α secretion significantly (p<0.05). However, when the SP-A and TLR4-MD2 proteins were added together to the cells and incubated further for another 3h with E. coli, the TNF-α levels were equivalent to those incubated without any exogenous protein or with SP-A only (Figure 7). There was no major difference in responses elicited by DC-populations harvested from adult or fetal baboon lung (Figures 7A and 7B), except that the amounts of TNF-α were lower in fetal cells.
Figure 7.
Effect of purified native SP-A and recombinant TLR4-MD2 proteins on TNF-α secretion by DCs against E. coli. (A) Primary adult baboon lung DCs or (B) fetal baboon lung DC-precursor cells were incubated with effector molecules for an hour prior to addition of pHrodo-labeled E. coli bioparticles. After 3h incubation at 37°C in 5% CO2 incubator, the cell-free supernatants were collected and subjected to ELISA for measurement of TNF-α. The results are representative of two experiments performed separately in triplicate. * p<0.05, ** p<0.001, ns: not significant.
Discussion
The results of our earlier study on fetal baboon bone marrow-derived DCs [31] and others’ report with monocyte-derived DCs [32] provided evidence that the DC functions (phagocytosis and cytokine secretion) are impaired during prenatal and neonatal periods [31]. However, recent understanding indicates that the tissue-resident DCs are different from the circulating or bone marrow-derived DCs [33]. Thus, we isolated primary lung DC-population from fetal baboons [11]. Results from our lab also demonstrate that fetal baboon lung cells are at precursor stage, express negligible levels of DC-markers, and are functionally defective in responding to infectious stimuli [11, 31]. Although, the developmental stage of the fetal lung DC-population remains to be completely elucidated in fetal baboons, we identify them as DC-precursor cells because they convert into typical DCs when cultured in presence of DC-promoting cytokines (unpublished data).
One possibility is that since these cells are not fully equipped with TLR or other pathogen-pattern recognition receptors because of developmental immaturity, the DC-precursor cells are not capable of capturing the microorganisms. SP-A also serves as pathogen-pattern recognition receptor and is known to stimulate DC-maturation and phagocytic uptake of infectious organisms [34]. However, at 125 days of gestation, the SP-A is not detectable. NICU care and proper clinical management induces expression of both SP-A and TLR4 which reaches to optimal levels under normal conditions [16–17]. However, despite an advanced and sophisticated clinical care, preterm babies are more prone to infection, and infection and ventilator-associated lung injury remarkably perturb the expression of SP-A and TLR4. Specifically, lavage pools of SP-A are decreased, and tissue expression of TLR4 is increased. These published results suggest that the introduction of SP-A may help maintain the tissue homeostasis and exert anti-infective and anti-inflammatory effects [35–38]. This study was designed to examine if the introduction of SP-A will impact the DC functions in preterm babies’ lung.
We focused on studying selected immune functions of DCs: phagocytosis and cytokine secretion, against infectious challenge. We pulsed the primary cells with purified or recombinant protein preparations for two reasons: one that the genetic-transfection of primary DCs will require longer time for efficient protein expression, and longer incubation may induce phenotypic changes in DCs [39], and second that the protein-pulsing mimics the physiological scenario because both SP-A and TLR4 proteins are known to exist in soluble extracellular, cell surface as well as in intracellular forms under steady-state conditions [40–41]. We have also included MD2 in conjunction with TLR4, because it serves as an important adaptor molecule to TLR4 and binds to SP-A [24]. However, MD2 does not carry intracellular signaling TIR domain and does not affect the phagocytic function of DCs on its own (Figure 5). A few investigations have shown that SP-A and TLR4 proteins interact in vitro [24–25]. Although functional relevance of this interaction in fetal or neonatal lungs remains largely unexplored, the results of the present study indicate that SP-A reduces TLR4-MD2-induced cytokine release against infectious stimuli.
We found that an exogenous addition of SP-A and TLR4-MD2 proteins in DC population increases the phagocytic uptake of encapsulated E. coli (Figures 5 and 6). These findings are of clinical importance because encapsulated bacteria resist phagocytosis by antigen-presenting cells and mount an aggressive inflammatory response [42]. It is possible that purified SP-A can also directly kill some of the bacteria by increasing the membrane permeability as reported earlier [43]. However, we have focused on studying the functional aspects of lung DCs and DC-precursor cells and not the direct antimicrobial effects of SP-A. The SP-A-induced phagocytosis of E. coli bioparticles by DC-precursor cells point towards the importance of SP-A in improving immune functions in preterm babies. Interestingly, SP-A suppresses the TLR4-mediated cytokine release significantly in response to infectious stimuli (Figure 7). Similar results have been obtained in Ureaplasma infection models in an established macrophage cell line RAW 264.7 and in mice [44–45]. Our results presented here further support the role of SP-A in improving the innate immune functions in preterm babies.
The results of this study are of clinical importance because surfactant preparations currently-used in NICUs do not contain SP-A. Our results support the idea of reformulating the currently-available clinical surfactant preparations to contain SP-A and their clinical usage in NICU. However, more comprehensive studies are warranted to understand the mechanisms of SP-A-induced protective effects in suitable animal models of premature birth and lung diseases of early childhood.
Acknowledgments
Fresh lung tissue samples of fetal animals were obtained from Baboon Resources (Director: Dr. Gary white, OUHSC). Authors acknowledge technical help from Kevin Brown and Catherine King.
Source of Support
This work was supported by research grants from American Lung Association and Presbyterian Health Foundation. The project described was partially supported by Grant Number P40RR012317 (at OUHSC) from the National Center for Research Resources.
Abbreviations
- APC
Allophycocyanin
- DC(s)
Dendritic cell(s)
- dGA
days of gestation age
- D-PBS
Dulbecco’s phosphate buffered saline
- FACS
Fluorescence activated cell sorting
- FBS
Fetal bovine serum
- FITC
Fluorescien isothiocyanate
- HBSS
Hanks balanced salt solution
- HEPES
N-2-Hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- MFI
Mean Fluorescent Intensity
- PE
Phycoerythrinin
- SEM
standard error of measurement
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fajardo-Moser M, Berzel S, Moll H. Mechanisms of dendritic cell-based vaccination against infection. Int J Med Microbiol. 2008;298:11–20. doi: 10.1016/j.ijmm.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Ohteki T. The dynamics of dendritic cell: mediated innate immune regulation. Allergol Int. 2007;56:209–14. doi: 10.2332/allergolint.R-07-148. [DOI] [PubMed] [Google Scholar]
- 3.Sabatte J, Maggini J, Nahmod K, Amaral MM, Martinez D, Salamone G, Ceballos A, Giordano M, Vermeulen M, Geffner J. Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev. 2007;18:5–17. doi: 10.1016/j.cytogfr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.van Vliet SJ, Dunnen J, Gringhuis SI, Geijtenbeek TB, van Kooyk Y. Innate signaling and regulation of Dendritic cell immunity. Curr Opin Immunol. 2007;19:435–40. doi: 10.1016/j.coi.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Banks EM, Kyriakidou M, Little S, Hamblin AS. Epithelial lymphocyte and macrophage distribution in the adult and fetal equine lung. J Comp Pathol. 1999;120:1–13. doi: 10.1053/jcpa.1998.0250. [DOI] [PubMed] [Google Scholar]
- 6.Fach SJ, Meyerholz DK, Gallup JM, Ackermann MR, Lehmkuhl HD, Sacco RE. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 And IL-10 gene transcripts. Viral Immunol. 2007;20:119–30. doi: 10.1089/vim.2006.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt PG, Nelson DJ, McWilliam AS. Population dynamics and functions of respiratory tract dendritic cells in the rat. Adv Exp Med Biol. 1995;378:177–81. doi: 10.1007/978-1-4615-1971-3_39. [DOI] [PubMed] [Google Scholar]
- 8.Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, Virchow JC. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30:878–86. doi: 10.1183/09031936.00036307. [DOI] [PubMed] [Google Scholar]
- 9.Masten BJ, Olson GK, Tarleton CA, Rund C, Schuyler M, Mehran R, Archibeque T, Lipscomb MF. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol. 2006;177:7784–93. doi: 10.4049/jimmunol.177.11.7784. [DOI] [PubMed] [Google Scholar]
- 10.Webb TJ, Sumpter TL, Thiele AT, Swanson KA, Wilkes DS. The phenotype and function of lung dendritic cells. Crit Rev Immunol. 2005;25:465–91. doi: 10.1615/critrevimmunol.v25.i6.20. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi S, Wolf R, White G. Ontogeny and phagocytic function of baboon lung dendritic cells. Immunol Cell Biol. 2009;87:419–27. doi: 10.1038/icb.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinman RM. The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 2003;51:59–60. doi: 10.1016/s0369-8114(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 13.Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85:326–32. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- 14.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010. 2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling Networks: An Integration of Adaptor Molecules, Kinases, and Cross-talk. J Dent Res. 2010 doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awasthi S, Coalson JJ, Crouch E, Yang F, King RJ. Surfactant proteins A and D in premature baboons with chronic lung injury (Bronchopulmonary dysplasia). Evidence for an inhibition of secretion. Am J Respir Crit Care Med. 1999;160:942–9. doi: 10.1164/ajrccm.160.3.9806061. [DOI] [PubMed] [Google Scholar]
- 17.Awasthi S, Coalson JJ, Yoder BA, Crouch E, King RJ. Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am J Respir Crit Care Med. 2001;163:389–97. doi: 10.1164/ajrccm.163.2.2004168. [DOI] [PubMed] [Google Scholar]
- 18.Awasthi S, Cropper J, Brown KM. Developmental expression of Toll-like receptors-2 and -4 in preterm baboon lung. Dev Comp Immunol. 2008;32:1088–98. doi: 10.1016/j.dci.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signaling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–7. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- 20.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano H, Kuroki Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol Immunol. 2005;42:279–87. doi: 10.1016/j.molimm.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Blander JM. Phagocytosis and antigen presentation: a partnership initiated by Toll-like receptors. Ann Rheum Dis. 2008;67(Suppl 3):iii44–9. doi: 10.1136/ard.2008.097964. [DOI] [PubMed] [Google Scholar]
- 23.Blander JM. Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol. 2007;9:290–9. doi: 10.1111/j.1462-5822.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–80. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 25.Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry. 2006;45:8657–64. doi: 10.1021/bi060176z. [DOI] [PubMed] [Google Scholar]
- 26.Awasthi S, Brown K, King C, Awasthi V, Bondugula R. A novel TLR4-interacting Surfactant protein-A-derived peptide suppresses LPS-induced TLR4 expressiona dn TNF-α release. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.173765. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang CH, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Whitsett JA, Chroneos ZC. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem. 2005;280:34447–57. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–88. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 29.Bharadwaj U, Zhang R, Yang H, Li M, Doan LX, Chen C, Yao Q. Effects of cyclophilin A on myeloblastic cell line KG-1 derived dendritic like cells (DLC) through p38 MAP kinase activation. J Surg Res. 2005;127:29–38. doi: 10.1016/j.jss.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Hulette BC, Rowden G, Ryan CA, Lawson CM, Dawes SM, Ridder GM, Gerberick GF. Cytokine induction of a human acute myelogenous leukemia cell line (KG-1) to a CD1a+ dendritic cell phenotype. Arch Dermatol Res. 2001;293:147–58. doi: 10.1007/s004030000201. [DOI] [PubMed] [Google Scholar]
- 31.Awasthi S, Cropper J. Immunophenotype and functions of fetal baboon bone-marrow derived dendritic cells. Cell Immunol. 2006;240:31–40. doi: 10.1016/j.cellimm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–7. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 33.Diao J, Winter E, Cantin C, Chen W, Xu L, Kelvin D, Phillips J, Cattral MS. In situ replication of immediate dendritic cell (DC) precursors contributes to conventional DC homeostasis in lymphoid tissue. J Immunol. 2006;176:7196–206. doi: 10.4049/jimmunol.176.12.7196. [DOI] [PubMed] [Google Scholar]
- 34.Kingma PS, Whitsett JA. In defense of the lung: surfactant protein A and surfactant protein D. Curr Opin Pharmacol. 2006;6:277–83. doi: 10.1016/j.coph.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Lee DC, Romero R, Kim CJ, Chaiworapongsa T, Tarca AL, Lee J, Suh YL, Mazaki-Tovi S, Vaisbuch E, Mittal P, Draghici S, Erez O, Kusanovic JP, Hassan SS, Kim JS. Surfactant protein-A as an anti-inflammatory component in the amnion: implications for human pregnancy. J Immunol. 184:6479–91. doi: 10.4049/jimmunol.0903867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldmann T, Kahler D, Schultz H, Abdullah M, Lang DS, Stellmacher F, Vollmer E. On the significance of Surfactant Protein-A within the human lungs. Diagn Pathol. 2009;4:8. doi: 10.1186/1746-1596-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madan T, Reid KB, Clark H, Singh M, Nayak A, Sarma PU, Hawgood S, Kishore U. Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol. 47:1923–30. doi: 10.1016/j.molimm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Hartshorn KL. Role of surfactant protein A and D (SP-A and SP-D) in human antiviral host defense. Front Biosci. (Schol) 2:527–46. doi: 10.2741/s83. [DOI] [PubMed] [Google Scholar]
- 39.Brinker KG, Garner H, Wright JR. Surfactant protein A modulates the differentiation of murine bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L232–41. doi: 10.1152/ajplung.00187.2002. [DOI] [PubMed] [Google Scholar]
- 40.Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682–6. doi: 10.4049/jimmunol.165.12.6682. [DOI] [PubMed] [Google Scholar]
- 41.Ochs M, Johnen G, Muller KM, Wahlers T, Hawgood S, Richter J, Brasch F. Intracellular and intraalveolar localization of surfactant protein A (SP-A) in the parenchymal region of the human lung. Am J Respir Cell Mol Biol. 2002;26:91–8. doi: 10.1165/ajrcmb.26.1.4570. [DOI] [PubMed] [Google Scholar]
- 42.Metkar S, Awasthi S, Denamur E, Kim KS, Gangloff SC, Teichberg S, Haziot A, Silver J, Goyert SM. Role of CD14 in responses to clinical isolates of Escherichia coli: effects of K1 capsule expression. Infect Immun. 2007;75:5415–24. doi: 10.1128/IAI.00601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuzmenko AI, Wu H, Wan S, McCormack FX. Surfactant protein A is a principal and oxidation-sensitive microbial permeabilizing factor in the alveolar lining fluid. J Biol Chem. 2005;280:25913–9. doi: 10.1074/jbc.M411344200. [DOI] [PubMed] [Google Scholar]
- 44.Okogbule-Wonodi AC, Chesko KL, Famuyide ME, Viscardi RM. Surfactant protein-A enhances ureaplasmacidal activity in Vitro. Innate Immun. doi: 10.1177/1753425909360552. [DOI] [PubMed] [Google Scholar]
- 45.Famuyide ME, Hasday JD, Carter HC, Chesko KL, He JR, Viscardi RM. Surfactant protein-A limits Ureaplasma-mediated lung inflammation in a murine pneumonia model. Pediatr Res. 2009;66:162–7. doi: 10.1203/PDR.0b013e3181aabd66. [DOI] [PMC free article] [PubMed] [Google Scholar]